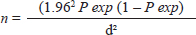| Research Article | ||
Open Vet. J.. 2024; 14(7): 1577-1584 Open Veterinary Journal, (2024), Vol. 14(7): 1577–1584 Research Article Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, EgyptSarah Gamal Yousef1, Nader Maher Sobhy1, Heba Gouda2 and Mahmoud Helmy Emam2*1Department of Animal Medicine, Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Animal Medicine, Internal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Mahmoud Helmy Emam. Department of Animal Medicine, Internal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: mh5378721 [at] gmail.com Submitted: 08/04/2024 Accepted: 15/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
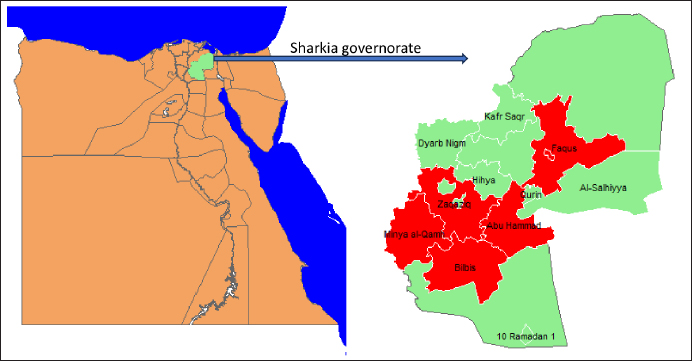
AbstractBackground: Bovine babesiosis represents a serious challenge for animal health, herd production, and profitability. Understanding the epidemiology and risk factors associated with babesiosis is critical to reduce their negative impacts. Aim: Investigation of the seroprevalence and risk factors associated with Babesia bigemina (B. bigemina) and Babesia bovis (B. bovis) in five districts in Sharkia governorate using ELISA. Methods: Across-sectional research was conducted to determine the seropositivity of babesiosis by collecting a total of 352 blood samples from 250 cattle and 102 buffaloes. A multivariate logistic regression model was implemented to evaluate the strength of the risk factors associated with both Babesia species infection. Results: The seroprevalence of B. bigemina and B. bovis was 42.6% and 17.0 %, respectively. The prevalence of babesiosis in cattle was found to be 48.8% for B. bigemina and 16.8% for B. bovis. Inclusive, in buffaloes, the prevalence was 27.5% for B. bigemina and 17.6% for B. bovis. Adult animals were more vulnerable to infection with babesia than young animals by 3–5 times, respectively. Males were more susceptible to B. bigemina and B. bovis than females by 3.7 and 3.5 times. Similarly, the odds of infection in infested animals with ticks were 2–4 times higher than in animals without ticks. Conclusion: The obtained results revealed that age, sex of the animal, and tick infestation were major risk factors for the seropositivity of both Babesia species. Inclusive, there was no evidence to support the premise that seroprevalence of babesiosis is correlated with the season and species. Keywords: Babesia bigemina, Babesia bovis, Egypt, Epidemiology, Risk factors. IntroductionTicks and tick-borne diseases are considered a global problem because of their detrimental effects on livestock productivity (Guswanto et al., 2017). Bovine babesiosis is considered the most prevalent infection detected in cattle and buffaloes, particularly in subtropical and in tropical areas (Fakhar et al., 2012; Jaimes-Dueñez et al., 2017). The clinical findings of bovine babesiosis vary from acute to subacute form (Góes et al., 2007). The sub-acute form usually occurs in endemic areas with previous exposure and an abundance of tick population; it is manifested only by a slight rise in body temperature (Terkawi et al., 2011). Babesia bovis and Babesia bigemina are the most popular species in Egypt because of the abundance of their vector ticks (Rhipicephalus microplus and Rhipicephalus annulatus) (Adham et al., 2009; Hassan et al., 2017). In Egypt, Babesia is the most endemic blood parasite in cattle and buffaloes and has a significant impact on milk, meat production, and herd management (Adham et al., 2009; Mahmoud et al., 2024). Previous studies recorded different prevalence rates of bovine babesiosis among cattle and buffaloes in Egypt (Ibrahim et al., 2013; Fereig et al., 2017; El-Bahy et al., 2018). The variation in the infection rate in Egypt is attributed to animal management, climatic changes, immune status, and study place (Menshawy et al., 2020). Molecular and serological assays are more sensitive in detection and differentiation between babesia species in carrier animals. Furthermore, Nucleic acid-based techniques are capable of identifying one parasite per 1 million RBCs (Criado-Fornelio, 2007). Serological tests such as ELISA and IFA are widely used in large-scale surveillances (Gul et al., 2015). ELISA is an adequate serological method as it is less laborious than IFA and has greater sensitivity and specificity for detection and differentiation between B. bovis and B. bigemina (Sharma et al., 2013). Nowadays, understanding the epidemiological features of babesiosis is a critical step toward the managemental control of the disease and improving our ability to detect and predict this health disorder (Aziz et al., 2014; Mahmoud et al., 2015). To the best of authors knowledge, there is limited information regarding bovine babesiosis in Sharkia governorate, Egypt. Therefore, our study focused on seroprevalence of Babesia species in large ruminants. Moreover, evaluation of the risk factors including seasonal variations, age, species, sex, and tick infestation associated with bovine babesiosis in five districts in the Sharkia governorate. Material and MethodsStudy design and sample size calculationOur investigation was carried out in Sharkia governorate, particularly in five districts (Zaqaziq, Abu Hammad, Bilbis, Minya Al-Qamh, and Faqus) since April 2023 up to March 2024. Sharkia governorate is located in the northern part of Egypt in the Eastern Nile Delta (Fig. 1). A cross-sectional study has been conducted in five different districts to investigate the prevalence of babesiosis in the Sharkia governorate. In our investigation, each district was subject to a random selection of smallholder farms. These farms were for meat or milk production. Additionally, the cattle and buffaloes that were used for sampling were chosen at random from each farm (Table 1). The minimum sample size was calculated presuming a 95% confidence interval, 50% expected true population and a 0.05 margin of error (α) regarding the following formula (Thrusfield, 2018).
Where, n=Sample size, P exp=Expected true population, d=Desired margin of error. Samples and data collectionA total of 352 blood samples were collected from apparently healthy 250 cattle and 102 buffaloes for diagnosis of babesiosis infection (B. bigemina and B. bovis) in Sharkia governorate. The blood samples were collected from jugular vein in sterile vacutainer tubes without anticoagulant for separation of serum. All samples were transported in an ice box to the laboratory of the Animal Medicine Department, Zagazig University, Egypt. The serum samples were separated and frozen at –20°C until further examination. The data regarding animals' species, age, sex, housing condition, and feeding management were recorded during sample collection. Moreover, all animals were inspected for the presence of ticks. Sandwich ELISABabesia bigemina and B. bovis antibody test kits were obtained from Sinogeneclon Biotech company (Hangzhou, China). The tests were performed according to manufacturer instructions. Briefly, the antigen-coated microtiter plates were incubated with 50 µl of diluted serum samples (10:40) for 30 minutes at 37°C. The plates were washed five times with diluted washing solution and incubated with 50 µl of horseradish peroxidase conjugate for 30 minutes at 37°C. The plates were washed again five times then, and 100 µl of substrate solution was added to each well for 15 minutes. Finally, 50 µl of stop solution was added to stop the reaction. The optical density was measured by a Microtiter plate reader (Biotech 808) at a wavelength of 450 nm. The cut-off value was calculated as the average of negative control well +0.15.
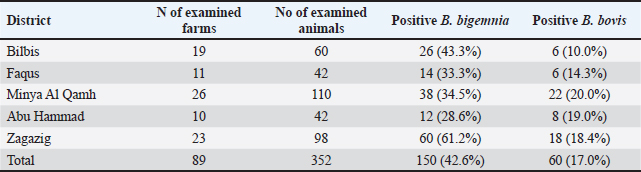
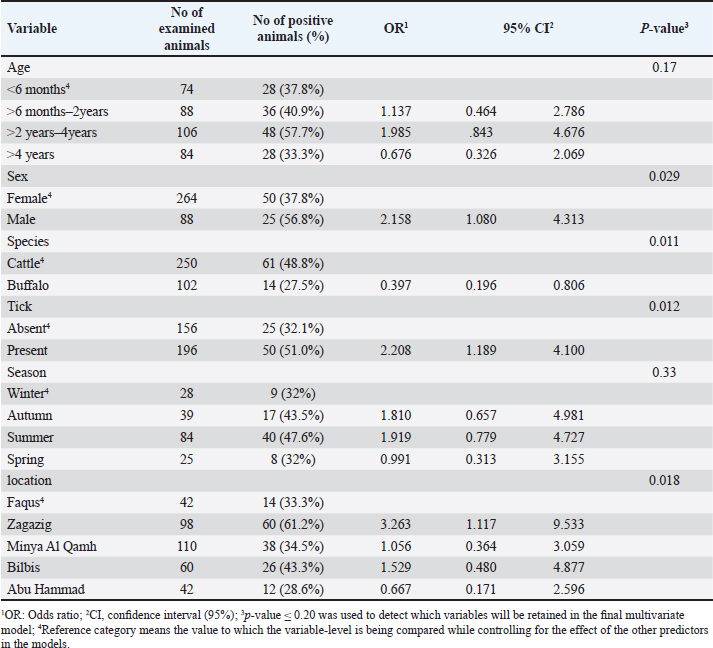
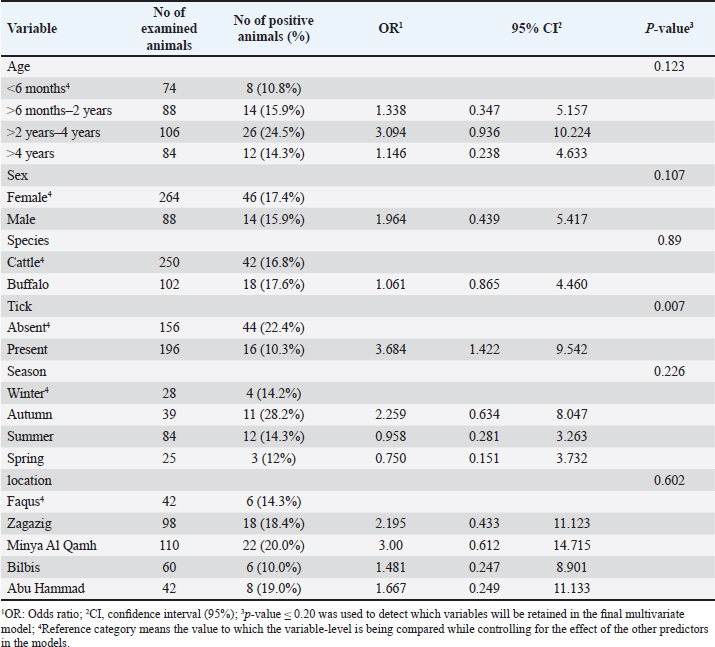
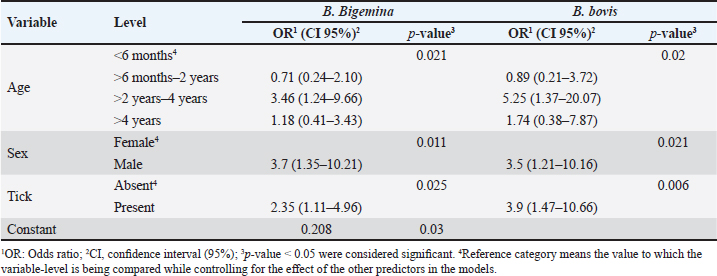
Fig. 1. Map of sampling areas in Sharkia governorate, Egypt. The five districts were Zagazig, Abu Hammad, Bilbis, Faqus and Minya Al Qamh. Statistical analysisThe statistical analysis was performed with the SPSS program (SPSS 25 for Windows, SPSS Inc., Chicago, IL, USA). The objected explanatory variables were age, sex and species of the animals, season, location, and tick infestation. The response variable was the serological status of the examined animals (either seronegative or seropositive) for both types of Babesia species. The Univariate logistic regression model was used firstly to evaluate the association between each independent variable and the serological status of B. bigemina and B. bovis. The predictors with p values ≤ 0.20 were retained in the final multivariate logistic regression model. The predictors with a p value < 0.05 were considered significant. Ethical approvalThe protocol of animal handling was approved by the Institutional Animal Care and Use Committee at Zagazig University with protocol number (ZU-IACUC/2/F/74/2024). ResultsSeroprevalence of bovine babesiosisThe prevalence of babesiosis in the Sharkia governorate was 42.6% and 17.0 % for B. bigemina and B. bovis, respectively. The number of examined animals and the prevalence of the infection in each district were documented in (Table 1). In total, 250 cattle and 102 buffaloes were tested for B. bigemina and B. bovis antibodies. The seroprevalence of B. bigemina and B. bovis in cattle was 48.8% and 16.8%, respectively. In buffaloes, the seroprevalence of B. bigemina and B. bovis was 27.5% and 17.6%, respectively (Tables 2 and 3). The seroprevalence of B. bigemina was (56.8%, 37.8%) while B. bovis was (15.9%, 17.4%) for males and females, respectively. The seroprevalence of both Babesia species was higher in the presence of ticks compared to the absence of ticks. Regarding the animal's age, the category (>2–4 years) represented the highest prevalence for B. bigemina and B. bovis with 57.7% and 24.5%, respectively. Babesia bigemina had the highest seroprevalence throughout the summer, with a rate of 47.3%, whereas B. bovis had the highest seroprevalence during autumn, with a rate of 28.2% (Tables 2 and 3). The animal-seroprevalence among the studied districts ranged from (28% to 43% for B. bigemina) and (10%–20% for B. bovis) except for Zagazig that had higher seroprevalence for B. bigemina (61%) (Table 1). Univariate regression analysisThe explanatory variables were evaluated separately for their association with the seropositivity of Babesia species (B. bigemina and B. bovis). As shown in Table 2, age, sex, species, existence of ticks, and locality were considered significant risk factors for infection with B. bigemina (p ≤ 0.20). Both yearling and adult animals (up to 4 years) were more susceptible to infection compared to calves. Females and buffaloes were at a lower level of risk for infection relative to males and cattle. The presence of tick infestation heightened the risk of infection compared to the absence of ticks. Animals from Minya al-Qamh had the highest risk of exposure compared with other districts. Regarding B. bovis, the seropositivity was significantly associated with age, sex, and tick infestation (Table 3). Animals younger than 6 months were more resistant to infection compared to older ages. Males were more vulnerable than females, and infested animals with ticks were more exhibited to infection than animals free from ticks. Multivariate regression analysisThe final models (Table 4) revealed that the presence of ticks beside age and sex was significantly associated with babesiosis prevalence (p < 0.05). The probability of infection increased with age. Adult animals were more exposed to infection with B. bigemina and B. bovis than young animals by 3–5 times, respectively. The odds of infection in infested animals with ticks were 2–4 times higher than in animals without ticks. The risk of exposure to both infections was lower in females than in males. The odds of exposure to B. bigemina and B. bovis in males were 3.7 and 3.5 times higher than in females. Table 1. The number of farms and animals selected for the study and subjected to ELISA testing from Sharkia governorate, Egypt, for B. bigemina and bovis antibodies.
Table 2. The univariate logistic regression model for risk factors associated with B. bigemina infection.
DiscussionThe objective of this study was to evaluate the prevalence and potential causes of bovine babesiosis in various regions within the Sharkia governorate, Egypt, using ELISA. Sharkia is the second governorate at the level of the Republic in terms of agricultural area after the Al-Behera Governorate in Egypt. This investigation was objected to this area because it is one of the most famous governorates for breeding livestock, and there is a lack of available data regarding the infection with bovine babesiosis. In current investigation, the overall seroprevalence for babesiosis in the Sharkia governorate was 42.6% and 17.0 % for B. bigemina and B. bovis, respectively. The higher occurrence of B. bigemina may be due to the abundance of their main vector tick (Rhipicephalus annulatus) in the Sharkia governorate (Yousef, 2020) in addition to the diversity of tick species that transmit B. bigemina counter to B. bovis that is transmitted mainly by R. microplus (Bock et al., 2004). Our results agreed with (Ibrahim et al., 2013), who reported a higher prevalence of B. bigemina compared with B. bovis in the Behiera and Faiyum governorates, with percentages of 12.5% and 10.12%, respectively. Similarly, Mahmoud et al. (2015) and Fereig et al. (2017) reported a higher frequency of B. bigemina compared with B. bovis in different districts in lower and upper Egypt. On the other hand, a Vietnamese study recorded a higher seroprevalence of B. bovis (37.4%) than B. bigemina (9.3%) in the studied buffaloes (Li et al., 2014). This fluctuation may be attributed to the environmental and managemental differences between Egyptian and other foreign districts and the geographic distribution of tick vectors. Table 3. The univariate logistic regression model for risk factors associated with Babesia bovis infection.
The investigated cattle had a higher serological prevalence for B. bigemina than water buffaloes, with percentages of 42.6% and 27.5%, respectively. Meanwhile, both animal species were nearly similar in their seropositivity to B. bovis. The variation may be attributed to the thinner skin of cattle compared with buffaloes. Thus, cattle are more preferable to tick vectors and more susceptible to infection with blood parasites than buffaloes Siddique et al., (2020). These findings coincide with what was mentioned by Jacob et al. (2020) in their meta-analysis; they reported that cattle are more susceptible to babesiosis than buffaloes. In the univariable analysis, cattle were more vulnerable to infection with B. bigemina compared to buffaloes. These results came in accordance with (Ibrahim et al., 2021), who found that water buffaloes revealed a lower percentage of infection with B. bigemina (35.6%) compared with cattle (41.6%). On the other hand, Abas et al. (2021) recorded a higher prevalence of babesiosis in buffaloes compared to cattle. The seroprevalence of B. bigemina and B. bovis was higher in males than females. The univariable analysis revealed that the odds of infection in males were 2.15 and 1.9 times higher than in females for B. bigemina and B. bovis, respectively. The obtained results agreed with Fereig et al. (2017), who found that the investigated males had a higher prevalence of B. bovis compared to females, with an odds ratio of 4.4. On the contrary, Hamsho et al. (2015) and Siddique et al. (2020) reported that females had a higher risk for infection with bovine babesiosis than males. However, another Egyptian study mentioned that sex did not represent any risk for babesia infection (Rizk et al., 2017). Table 4. The final multivariate logistic regression model for risk factors associated with B. bigemina and B. bovis infection.
In both infections, the animals aged from 2 to 4 years had the highest seroprevalence for B. bigemina and B. bovis with percentages of 57.7% and 24.5%, respectively, followed by the age group (>6 months–2 years). In the univariate analysis, the animal's age was a significant risk factor associated with exposure to the infection (p < 0.20). These findings were in the same line with Wesonga et al. (2017), who found that the seroprevalence of B. bigemina was higher in adult animals. Other studies in Pakistan and Sudan by Atif (2012) and Shuaib et al. (2015) didn’t detect any association between age and babesiosis. On the other hand, Fereig et al. (2017) mentioned that younger ages had a higher risk of B. bovis infection. Regarding tick infestation, the risk of infection to Babesia parasites extended with the presence of ticks. The odds of exposure to both Babesia species were 2.2 and 1.4 times in infested animals related to free animals for both Babesia species. In agreement with our results, previous reports in different governorates in Egypt reported that ticks exaggerated the presence of babesiosis (Fadly, 2012). In the current study, the highest seroprevalence of B. bigemina and B. bovis was in summer and autumn, respectively. However, there weren’t any significant seasonal variations regarding the seroprevalence of both Babesia species. These results coincided with El-Moghazy et al. (2014), who reported a higher prevalence of bovine babesiosis in summer and autumn without statistical differences between different seasons. In contrast, several studies reported that the risk of exposure to babesia parasites increased significantly during the summer season (Siddique et al., 2020; Ibrahim et al., 2021). The close seroprevalence of babesiosis among different seasons may be due to the humid, hot climate in the studied area and the availability of tick vectors most of the year. Moreover, Bock et al. (2004) mentioned that the antibodies of both Babesia species may be detected for up to 7 months post- natural infection. The highest seroprevalence of B. bigemina and B. bovis was recorded in Zagazig and Minya al-Qamh, respectively. There was no significant difference among the studied districts for the distribution of babesiosis except for Zagazig, which had higher seroprevalence for B. bigemina (61%). The high prevalence in our investigation may be due to the nature of animal housing in the studied cities, which contributes to tick infestation and impedes effective tick control. The final multivariate logistic analysis was used to visualize the combined effect of the significant variables in each babesia species. Interestingly, age, sex, and tick infestation are significant risk factors for the seropositivity of both Babesia species. It is clear from the obtained results that the prevalence increased with age. Adult animals are more exposed to infection with Babesia bigemina and B. bovis than young animals by 3–5 times, respectively. This observation suggests the accumulated parasite exposure by getting older. Furthermore, the sex and existence of tick vectors are an influential factor in the seropositivity of both babesia species. Males were 3.7 and 3.5 times more likely to be exposed to B. bovis and B. bigemina, respectively, than females. Similarly, the odds of infection in infested animals with ticks were from 2 to 4 times higher than in animals without ticks. In current investigation, most males are fattened and reared in closed houses made from unshelled bricks. Inclusive, these types of houses besides high humidity, are considered a suitable environment for the tick population. Thus, males have a higher risk of exposure to tick infestation and, accordingly, babesia parasites than females which are reared in an open housing system. ConclusionIn this study, age, and sex, besides tick infestation, are considerable independent factors that could be used for the prediction of the occurrence of bovine babesiosis in the investigated population. Understanding babesiosis epidemiology can inform future management and control strategies for large ruminants, reducing negative impacts on animal production and profitability. More future studies are recommended for a better understanding of the epidemiological status of bovine babesiosis in Sharkia governorate, Egypt. AcknowledgmentsWe thank all the animal owners in Sharika governorate for granting access to their animals. Conflict of interestThe Authors declare that there is no conflict of interest. FundingThis work didn’t receive any external funding. Data availabilityAll data findings during our investigation are available within the manuscript. Author contributionsSGY and MHE were involved in data curation, samples collection, formal analysis, discussion of results and prepared the initial draft. NMS and HG were involved in samples collection, discussion of results and review. All authors approved the submitted form. ReferencesAbas, O., Abd-Elrahman, A., Saleh, A. and Bessat, M. 2021. Prevalence of tick-borne haemoparasites and their perceived co-occurrences with viral outbreaks of FMD and LSD and their associated factors. Heliyon. 7(3), 64–79. Adham, F.K., Abd-El-Samie, E.M., Gabre, R.M. and Hussein, H.E. 2009. Detection of tick blood parasites in Egypt using PCR assay I—Babesia bovis and Babesia bigemina. Parasitol. Res. 105, 721–730. Atif, F.A. 2012. Prevalence of tick-borne diseases in Punjab (Pakistan) and hematological profile of Anaplasma marginale infection in indigenous and crossbred cattle. Pakistan J. Sci. 64(1), 11–15. Aziz, K.A., Khalil, W.K.B., Mahmoud, M.S., Hassan, N.H.A., Mabrouk, D.M. and Suarez, C.E. 2014. Molecular characterization of babesiosis infected cattle: improvement of diagnosis and profiling of the immune response genes expression. Glob. Vet. 12, 197–206. Bock, R., Jackson, L., De Vos, A. and Jorgensen, W. 2004. Babesiosis of cattle. Parasitology 129(S1), S247–S269. Criado-Fornelio, A. 2007. A review of nucleic acid-based diagnostic tests for Babesia and Theileria, with emphasis on bovine piroplasms. Parassitologia 49, 39–44. El-Bahy, N.M., Menshawy, S.M., Goda, W.M., Nasr, S.M., AbouLaila, M.R., Bazh, E.K. and Abou-Rwash, A.A. 2018. Molecular detection of Babesia bigemina and Babesia bovis in cattle in Behaira Governorate. Ejpmr 5(12), 441–446. El Moghazy, H.M., Ebied, M.M., Abdelwahab, M.G., El Sayed, A.A. 2014. Epidemiological studies on bovine babesiosis and theileriosis in Qalubia governorate. Banha Vet. Med. J. 27, 36–48. Fadly, R.S. 2012. Prevalence of blood parasites of some farm animals in Behera province. Assiut Vet. Med. J. 58(134), 1–7. Fakhar, M., Hajihasani, A., Maroufi, S., Alizadeh, H., Shirzad, H., Piri, F. and Pagheh, A.S. 2012. An epidemiological survey on bovine and ovine babesiosis in Kurdistan Province, western Iran. Trop. Anim. Health Prod. 44, 319–322. Fereig, R.M., Mohamed, S.G., Mahmoud, H.Y., AbouLaila, M.R., Guswanto, A., Nguyen, T.T. and Nishikawa, Y. 2017. Seroprevalence of Babesia bovis, B. bigemina, Trypanosoma evansi, and Anaplasma marginale antibodies in cattle in southern Egypt. Ticks Tick-Borne Dis. 8(1), 125–131. Góes, T.S., Góes, V.S., Ribeiro, M.F.B. and Gontijo, C.M. 2007. Bovine babesiosis: anti-erythrocyte antibodies purification from the sera of naturally infected cattle. Vet. Immunol. Immunopathol. 116(3-4), 215–218. Gul, N., Ayaz, S., Gul, I., Adnan, M., Shams, S. and Akbar, N. 2015. Tropical theileriosis and east coast fever in cattle: present, past, and future perspective. Int. J. Curr. Microbiol. Appl. Sci. 4(8), 1000–1018. Guswanto, A., Allamanda, P., Mariamah, E.S., Sodirun, S., Wibowo, P.E., Indrayani, L. and Igarashi, I. 2017. Molecular and serological detection of bovine babesiosis in Indonesia. Parasit. Vectors, 10(1), 1–13. Hamsho, A., Tesfamarym, G., Megersa, G. and Megersa, M. 2015. A cross-sectional study of bovine babesiosis in Teltele District, Borena Zone, Southern Ethiopia. J. Vet. Sci. Technol. 6(230), 2. Hassan, M.I., Gabr, H.S., Abdel-Shafy, S.O., Hammad, K.M. and Mokhtar, M.M. 2017. Molecular detection of Borrelia sp. in ornithodorids savignyi and Rhipicephalus annulatus by Flab gene and Babesia bigemina in R. annulatus by 18S rRNA gene. J. Egypt. Soc. Parasitol. 47(2), 403–414. Ibrahim, H.M., Galon, E.M.S., Tumwebaze, M.A., Byamukama, B., Liu, M., Mohammed-Geba, K. and Xuan, X. 2021. Serological Survey of Babesia bigemina and Babesia bovis in cattle and water buffaloes from Menoufia Province, Egypt. Acta Parasitol. 66(4), 1458–1465. Ibrahim, H.M., Moumouni, P.F.A., Mohammed-Geba, K., Sheir, S.K., Hashem, I.S., Cao, S. and Xuan, X. 2013. Molecular and serological prevalence of Babesia bigemina and Babesia bovis in cattle and water buffalos under small-scale dairy farming in Beheira and Faiyum Provinces, Egypt. Vet. Parasitol. 198(1-2), 187–192. Jacob, S.S., Sengupta, P.P., Paramanandham, K., Suresh, K.P., Chamuah, J.K., Rudramurthy, G. R. and Roy, P. 2020. Bovine babesiosis: an insight into the global perspective on the disease distribution by systematic review and meta-analysis. Vet. Parasitol. 283, 109–136. Jaimes-Dueñez, J., Triana-Chávez, O. and Mejía-Jaramillo, A.M. 2017. Parasitological and molecular surveys reveal high rates of infection with vector-borne pathogens and clinical anemia signs associated with infection in cattle from two important livestock areas in Colombia. Ticks Tick-Borne Dis. 8(2), 290–299. Li, Y., Luo, Y., Cao, S., Terkawi, M.A., Lan, D.T., Long, P.T., Yu, L., Zhou, M., Gong, H., Zhang, H., Zhou, J., Yokoyama, N., Suzuki, H. and Xuan, X. 2014. Molecular and sero epidemiological survey of Babesia bovis and Babesia bigemina infections in cattle and water buffaloes in the central region of Vietnam. Trop. Biomed. 31(3), 406–413. Mahmoud, H.Y., Rady, A.A. and Tanaka, T. 2024. Molecular detection and characterization of Theileria annulata, Babesia bovis, and Babesia bigemina infecting cattle and buffalo in southern Egypt. Parasit. Epidemiol. Control 25, 340. Mahmoud, M.S., Kandil, O.M., Nasr, S.M., Hendawy, S.H., Habeeb, S.M., Mabrouk, D.M. and Suarez, C.E. 2015. Serological and molecular diagnostic surveys combined with examining hematological profiles suggests increased levels of infection and hematological response of cattle to babesiosis infections compared to native buffaloes in Egypt. Parasit. Vectors 8(1), 1–15. Menshawy, S.M., AbouLail, M.R., Bessat, M.N. and Beder, N.A. 2020. A review on Bovine Babesiosis in Egypt. Egypt. Vet. Med. Soc. Parasitol. J. 16(1), 8–19. Rizk, M.A., Salama, A., El-Sayed, S.A.E.S., Elsify, A., El-Ashkar, M., Ibrahim, H. and El-Khodery, S. 2017. Animal level risk factors associated with Babesia and Theileria infections in cattle in Egypt. Acta Parasitol. 62(4), 796–804. Sharma, A., Singla, L.D., Tuli, A., Kaur, P., Batth, B.K., Javed, M. and Juyal, P.D. 2013. Molecular prevalence of Babesia bigemina and Trypanosoma evansi in dairy animals from Punjab, India, by duplex PCR: a step forward to the detection and management of concurrent latent infections. BioMed Res. Int. 2013, 893862. Shuaib, Y.A., Osman, H.M., Hussein, M.O., Bakhiet, M.A., Omer, R.A., Al-Nahas, A. and Ismail, A.A. 2015. Seroprevalence of Babesia bigemina antibodies in cattle in North Kordofan state, the Sudan. ARC J. Anim. Vet. Sci. 1, 1–11. Siddique, R.M., Sajid, M.S., Iqbal, Z. and Saqib, M. 2020. Association of different risk factors with the prevalence of babesiosis in cattle and buffaloes. Pak. J. Agri. Sci. 57(2), 517–524. Terkawi, M.A., Huyen, N.X., Shinuo, C., Inpankaew, T., Maklon, K., Aboulaila, M. and Igarashi, I. 2011. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet. Parasitol. 178(3-4), 201–207. Thrusfield, M. 2018. Veterinary epidemiology, 4th ed. Hoboken, NJ: John Wiley & Sons Ltd. Wesonga, F.D., Gachohi, J.M., Kitala, P.M., Gathuma, J.M. and Njenga, M.J. 2017. Seroprevalence of Anaplasma marginale and Babesia bigemina infections and associated risk factors in Machakos County, Kenya. Trop. Anim. Health Prod. 49, 265–272. Yousef, S.G. 2020. Studies on bovine theileriosis at Sharkia governorate. PhD thesis, Zagazig Uni., Zagazig, Egypt. | ||
| How to Cite this Article |
| Pubmed Style Yousef SG, Sobhy NM, Gouda H, Emam MH. Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. Open Vet. J.. 2024; 14(7): 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 Web Style Yousef SG, Sobhy NM, Gouda H, Emam MH. Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. https://www.openveterinaryjournal.com/?mno=197107 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i7.7 AMA (American Medical Association) Style Yousef SG, Sobhy NM, Gouda H, Emam MH. Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. Open Vet. J.. 2024; 14(7): 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 Vancouver/ICMJE Style Yousef SG, Sobhy NM, Gouda H, Emam MH. Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. Open Vet. J.. (2024), [cited January 24, 2026]; 14(7): 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 Harvard Style Yousef, S. G., Sobhy, . N. M., Gouda, . H. & Emam, . M. H. (2024) Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. Open Vet. J., 14 (7), 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 Turabian Style Yousef, Sara Gamal, Nader Maher Sobhy, Heba Gouda, and Mahmoud Helmy Emam. 2024. Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. Open Veterinary Journal, 14 (7), 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 Chicago Style Yousef, Sara Gamal, Nader Maher Sobhy, Heba Gouda, and Mahmoud Helmy Emam. "Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt." Open Veterinary Journal 14 (2024), 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 MLA (The Modern Language Association) Style Yousef, Sara Gamal, Nader Maher Sobhy, Heba Gouda, and Mahmoud Helmy Emam. "Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt." Open Veterinary Journal 14.7 (2024), 1577-1584. Print. doi:10.5455/OVJ.2024.v14.i7.7 APA (American Psychological Association) Style Yousef, S. G., Sobhy, . N. M., Gouda, . H. & Emam, . M. H. (2024) Sero epidemiological study on bovine babesiosis in cattle and buffaloes in Sharkia Governorate, Egypt. Open Veterinary Journal, 14 (7), 1577-1584. doi:10.5455/OVJ.2024.v14.i7.7 |