| Research Article | ||
Open Vet. J.. 2024; 14(5): 1243-1250 Open Veterinary Journal, (2024), Vol. 14(5): 1243–1250 Original Research Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickensMostafa Abbas Shalaby1*, Hamed Yahya Saifan1, Khaled Abo-EL-Sooud1, Mohamed A. Tony2 and Aya Mohye Yassin31Pharmacology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 2Nutrition and Clinical Nutrition Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 3Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt *Corresponding Author: Mostafa Abbas Shalaby. Pharmacology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. Email: mostafapharmacology [at] cu.edu.eg Submitted: 06/03/2024 Accepted: 25/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
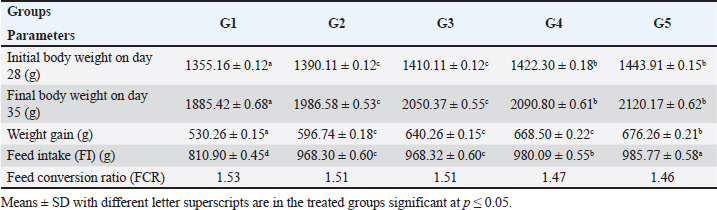
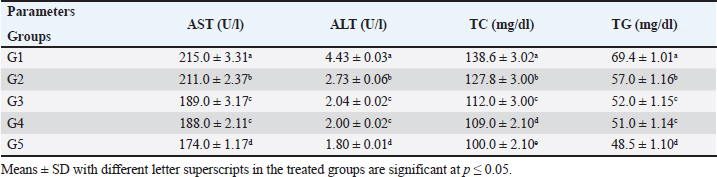
AbstractBackground: Feed additives are products used in poultry nutrition to improve the quality of feed and the safety of food byproducts from animal origin. They are promising antibiotic alternatives for the production of broilers. Aim: This study aimed to investigate the effect of sodium butyrate (SB) and RL on growth performance, biochemical profile, immunity, and carcass traits of broilers. Methods: Five hundred-one-day-old chicks of the Hubbard breed were reared on floor pens in a privet farm, Giza. The chicks were weighed on arrival (each chick weighted 43–45 gm) and randomly assigned into five equal groups, with four replicates each (25 chicks/replicate). Group 1 was fed on a broiler diet without any additions (control). The diets of groups 2 and 3 were supplemented with 500 g/ton SB and 4 kg/ton RL, respectively. In group 4, the diet was enriched with 250 g/ton SB plus 2 kg/ton RL. Chicks in group 5 were fed on a diet fortified with 500 g/ton SB plus 4 kg/ton RL. Results: Supplementation of broiler diet with 500 g/ton SB plus 4 kg /ton RL increased body weight gain (BWG) and feed efficiency ratio (FER) of birds. It decreased serum levels of aspartate aminotransferase, alanine aminotransferase, total cholesterol triglycerides, and malondialdehyde, but increased superoxide dismutase, catalase, and immunoglobulins, phagocytic activity, lysozyme activity, and nitric oxide concentrations. Antibody titers against the Newcastle disease virus were also elevated. Conclusion: Supplementation of broiler diet with 500 g/ton SB plus 4 kg/ton RL gives the best result regarding productive efficiency and immunity of broiler chickens. Keywords: Sodium butyrate, Rosemary herb, Broiler performance, Biochemistry, Immunity. IntrdoductionPoultry meat is a staple diet for most Egyptians; however, there is a serious shortage in Egypt. The majority of Egyptians prefer chicken white meat over red beef meat because of its lower cost. By 2023, Egypt is expected to produce 1.59 million tons of chicken meat (Abdelli et al., 2021). Feed components, including cereal grains such as barley, sorghum, and maize, are becoming more expensive due to price fluctuations and challenges associated with the importing process. Antibiotics have long been employed as traditional additives to support the health and growth performance of chickens. The use of antibiotics in poultry diets has been discontinued since 2006, due to the hazards to human health posed by the development of bacterial resistance and the presence of antibiotic residues. Feed additives are used in chicken nutrition to increase feed safety, improve growth and carcass quality, and raise immunity. The commonly used feed additives in poultry diets include antimicrobial agents (Alagawany et al., 2020); acidifiers (Ricke et al., 2020); anti-oxidants (Hashemi and Davoodi, 2011); antimycotoxins (Olivera et al., 2015); prebiotics, probiotics, and phytogenic additives (Al-Khalaifah, 2018). One common short-chain fatty acid is sodium butyrate (SB), which is the sodium salt of butyric acid. Feeding broilers a basal diet supplemented with SB improved growth performance, liver function, antioxidant capacity, carcass characteristics, and meat quality. According to a previous study, SB may be considered as a useful strategy to support intestinal health, especially for hens undergoing coccidiosis treatments (Lan et al., 2020). Protected sodium butyrate (CSB) at a concentration of 1,000 mg/kg improved the growth performance of broilers and regulated the intestinal flora (Zhao et al., 2022). Combining SB with the antibiotic bacitracin methylene disalicylate resulted in a significant improvement in body weight, feed intake (FL), and feed conversion ratio (FCR). Both chemically CSB and xylo-oligosaccharide improved broiler growth performance and produced anti-inflammatory and antioxidant effects when used alone or in combination (Deng et al., 2023). It is well known that rosemary (Rosmarinus officinalis) has high concentrations of tannin, resin, and saponin. Antibacterial and antioxidant properties of rosemary plants have been identified (Moreno et al., 2006). Broiler hens’ immune systems, meat quality, and productivity have all been demonstrated to benefit from the inclusion of rosemary in their diet (Ghazalah and Ali, 2008). However, according to Loetscher et al., (2013), adding rosemary to chicken diets had no appreciable effect on carcass characteristics. Materials and MethodsFeed additivesSB, a commercial feed additive known as CM3000®, contains 30% spherical granules coated in SB which is the sodium salt of butyric acid. It is a short-chain fatty acid that enters the chicken’s small and large intestines gradually and continually. It was purchased from China’s Hangzhou King Techina Feed Co. Ltd., which is the manufacturer of CM3000®. According to Sikandar et al. (2017), it was added to the broiler diet at a concentration of 500 g/ton feed. Based on a natural source, rosemary, Rosmarinus officinalis leaves powder RL, Family Lamiaceae, is used as a supplement in chicken feed. Rosemary is a popular spice for culinary and traditional medicine. It serves as a beneficial nutritional supplement for poultry to strengthen immunity. Rosemary essential oils are a potent growth enhancer for broiler chickens. Dried leaves of rosemary plants were bought from a nearby market in Cairo, Egypt, called Haras for Herbs, Medicinal Plants, Spices, and Natural Oils. The dried leaves were finely powdered in an electrical mill and added to the basal diet at a concentration of 4 kg/ton feed according to Al-Kassie et al. (2008). Chicks and dietsFive hundred one-day-old chicks of the Hubbard breed (unsexed) were procured from Al-Ahram Company for Poultry in Giza, Egypt. The chicks were weighed upon arrival (Each chick weight weight was 43–45 gm) and then randomly assigned into five equal groups, each consisting of four replicates (25 birds per replicate). As a control, group 1 was given only the broiler basal diet without any supplements. The basal feed of groups 2 and 3 was supplemented with 500 g/ton of SB and 4 kg/ton of RL, respectively. In group 4, the chicks were fed a basal diet supplemented with 250 g/tom plus 2 kg/ton. Chicks in group 5 were fed a basal diet fortified with 500 g/ton SB plus 4 kg/ton RL. On day 35 of broiler age, the growth performance, biochemical profile, immune status, and carcass traits were determined. The experiment was conducted on floor pens at a private farm in Giza, Egypt. Vaccination program for all experimental groups of birds included protection against Newcastle disease (ND), infectious bursal disease (IBD, Gumboro), and infectious bronchitis (IB). To meet the nutrient requirements of Hubbard broilers, diets comprising the corn-soybean meal and basal components were prepared according to the Hubbard manual catalog (2022). During the experimental period (35 days) supplemented diets in the form of mash-type for three stages: starter, grower, and finisher were provided and water was offered ad labium. Growth performanceThe chicks were weighed and their daily FI was reported throughout the experiment period (g). Body weight gain (BWG) and FCR were weekly computed (g). BWG (g on period)=BW (g) at the end period—BW (g) on the first day. FCR is calculated by dividing kilograms of feed by the weight of the chicken (kg). Collection of bloodOn the 35th day, 30 chickens from each group were randomly selected and 5 ml of blood was withdrawn from the brachial wing vein into dry plain tubes. Blood was allowed to clot at room temperature. To obtain clear serum, the clots were removed by centrifugation at 2,000–3,000 X g for 15 minutes in a refrigerated centrifuge. The serum samples were poured into Eppendorf tubes and kept in a refrigerator until biochemical analysis. Biochemical analysisSerum samples were collected to determine aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities according to the method of Bergmeyer et al. (1978). Total protein (TP) was determined using the biuret method according to Zheng et al. (2017). TP (g/l) was calculated using commercially accessible diagnostic kits and an automated biochemical analyzer (Alizé). Serum uric acid (UA) and creatinine levels were estimated as described by Lorentz and Brendt (1967). Serum total cholesterol (TC) was determined calorimetrically according to Allain et al. (1974) and triglycerides (TG) according to Wahlefeld (1974). The level of serum malondialdehyde (MDA) was measured according to the method described by Ohkawa et al. (1979). The activities of serum superoxide dismutase (SOD) and catalase (CAT) enzymes were respectively assessed according to Nishikimi et al. (1972) and Aebi (1984) using a spectrophotometer. The zone electrophoresis method was used to separate the serum protein fractions on an agarose gel plate according to Tothova et al. (2019). Immune statusThe phagocytosis test was carried out in accordance with the procedure of Bos and de Souza (2000). The percentage of phagocytic cells that have been engulfed by Candida albicans yeast cells is known as phagocytic activity (PA). The number of yeast cells phagocytized divided by the number of macrophage phagocytic cells is known as the phagocytic index (PI). Serum samples were taken from the brachial wing vein on day 7 and day 21 after the ND virus immunization. The lysozyme assay (LA) method was adopted using the lytic activity of lysozyme against the cell wall of Micrococcus lysodeikticus as a substrate using agarose gel plate lysate method, following the protocol outlined by Peeters and Vantrappen (1977). The lysozyme concentration was determined by generating a logarithmic curve with a standard lysozyme solution. Nitric oxide (NO) assay was accomplished in accordance with Sun et al. (2003) using Griess reaction assay after removing protein via a mixture of ZnSO4 and NaOH. The absorbance at 540 nm exhibits a linear correlation with the concentration of NO present in the sample. Antibody titers against the ND virus were measured using the hemagglutination inhibition (HI) test according to Beard (1989). The enzyme-linked immunosorbent assay technique, as outlined by Engvall and Perlmann (1971) was used to determine serum levels of IgG and IgM concentrations. Carcass traitsThirty chickens were randomly selected from each group at the end of the experimental period and prepared for slaughter. The birds were slaughtered by bleeding of the jugular vein after a 12-hour fasting. After being killed, the birds were defeathered. The heart, liver, spleen, thymus, bursa, and belly fat were weighed using a digital scale. After removing the head and offals, the carcasses that remained were weighed to determine the weight at which they were ready to be cooked. The carcass dressing percentage (dressing %) was also computed using this weight in accordance with Rosa et al. (2007). Statistical analysisData were recorded as means ± SEM. To analyze the data, IBM SPSS® version 19 software was utilized on a personal computer (2010). The means ± SEM were compared with a one-way ANOVA test, with a significance level of p < 0.05, and the Post Hoc Duncan test was then applied. Ethical approvalThe current study was approved according to the Institutional Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Cairo, University, Egypt, with reference number: Vet CU 09092023791, dated 9/ 9/ 2023. ResultsGrowth performanceThe present results revealed that supplementation of a basal diet with SB and rosemary leaves (RLs), alone and in combination, increased BWG and FCR on day 35 of the age of broilers as recorded in Table 1. Biochemical profileTable 2 shows that fortification of a basal diet with SB and RL alone and in combination significantly decreased AST, ALT, TC, and TG in the serum of broiler chickens. Table 1. Effect of SB and RL on the growth performance of broiler chickens.
Table 2. Impact of SB and RL alone and in combination on serum concentration of liver enzymes (AST and ALT), TC, and TG on day 35 of age of broiler chickens.
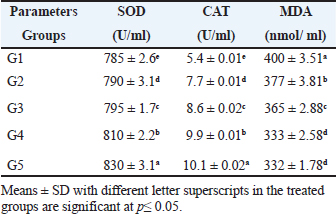
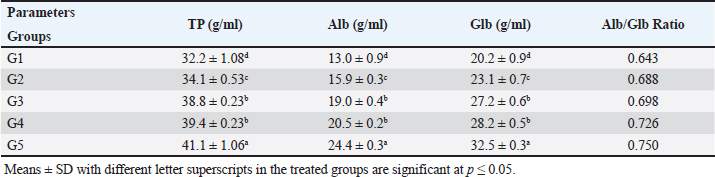
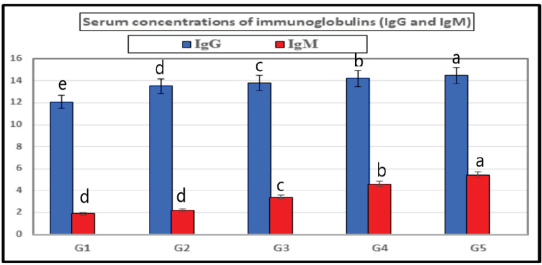
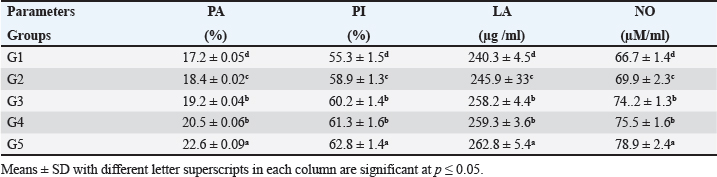
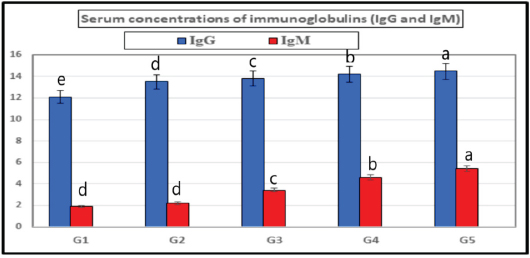
The present results indicated that the addition of SB and RL, alone and in combination, to the broilers’ basal diet caused significant increases in serum levels of SOD and CAT antioxidant enzymes and a significant decrease in MDA serum level as recorded in Table 3. The results of this study indicated that supplementation of the basal diet with SB and RL alone and in combination significantly increased TP, albumin (Alb), globulin (Glb), and albumin/globulin ratio on day 35 of the age of broilers as shown in Table 4. As elucidated in Figure 1, the addition of SB and RL alone and in combination with the basal diet elevated serum immunoglobulin IgG and IgM concentrations in broiler chickens on day 35 of age. Immuine statusAs recorded in Table 5, the addition of SB and RL, alone and in combination, increased macrophage PA, PI, lysozyme activity (LA), and serum NO concentration in broiler chickens. Results of the current study revealed that HI antibody titers against NDV were significantly increased in sera collected on day 7 post vaccination with Hitchner B1 strains and on day 21 post vaccination with Lasota strains. It was found that the Lasota vaccine had a good potency against ND in broilers as demonstrated in Figure 2. Table 3. Impact of SB and RL alone and in combination on serum SOD, CAT, and MDA levels on day 35 of the age of broiler chickens.
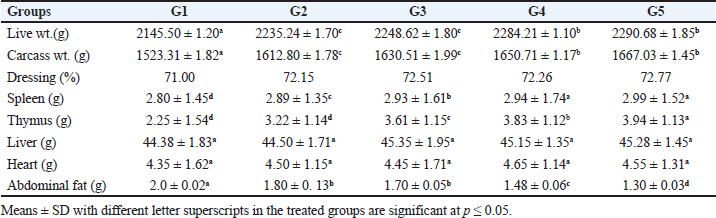
Carcass traitsAs depicted in Table (6), the addition of SB and RL, alone and in combination, increased live and carcass weights. The dressing percentage (DP %) ranged from 71% to 72.77%. There were significant increases in the weights of the spleen, thymus, and a significant decrease in abdominal fat. Non-significant changes were reported in the weights of the heart and liver. DiscussionThe current research shows that feedig basal diets supplemented with SB increased BWG and FCR The findings of Sikandar et al. (2017), Lan et al. (2020), Zhao et al. (2022), and Deng et al. (2023) were in agreement with our findings. The prior authors claimed that feeding a basal diet supplemented with SB improved the BWG and FER of broilers. On the contrary, dietary SB had no effect on the growth performance of broilers, according to Zhang et al. (2011). The discrepancy between the two studies could be due to variations in added SB concentrations. While in this study a large quantity (500 g/ton) was added, the earlier authors used lower concentrations (200 and 400 g/ton) of SB. The results of this investigation showed that the rosemary herb improved the growth performance of broilers. BWG and FCR were both enhanced. The results of Al-Kassie et al. (2008) revealed that RL increased growth performance in broilers. The findings of this study also agreed with those of Loetscher et al. (2013). Additionally, Ghazalah and Ali (2008) reported a markedly higher growth rate and better FCR when using rosemary powder at a 0.5% concentration in the feed. According to Petricevic et al. (2018), a broiler diet supplemented with 0.4% dried and finely powdered RL had a good impact on feed conversion and weight gain. The beneficial effects of utilizing various concentrations of rosemary powder in the feed were also confirmed by Norouzi et al. (2015). Table 4. Effect of SB and RL alone and in combination on serum concentrations of TP, albumin (Alb), globulin (Glb), and albumin/globulin ratio in serum of broilers on day 35 of age of broiler chickens.
Fig. 1. Showing the effect of SB and RL, alone and in combination, on serum concentrations of IgG and IgM on day 35 of age of broiler chickens in the treated groups. Table 5. Effect of SB and RL alone or in a mixture on PA, PI, LA, and serum NO concentration on day 35 of age of broiler chickens.
Fig. 2. Showing the effect of SB and RL alone and in combination on HI antibody titers against NDV on day 7 and day 21 post-vaccination in the treated groups. According to biochemical tests, adding SB and RL to broiler basal diets elevated serum levels of TP, albumin, globulin, SOD, CAT, IgG, and IgM but lowered levels of TC, TG, MDA, AST, and ALT. These results concurred with those of Lan et al. (2020) when using SB and with those of Ghazalah and Ali (2008) when adding RL to feeds. Table 6. Effect of SB and RL alone and in combination on carcass traits on 35 days of age of broiler chickens (n=30 birds).
According to this study, adding SB and RL to the basal diet significantly raised macrophage PA PI, IA, and serum NO concentration in broiler chickens. The findings of Sikandar et al. (2017) and Lan et al. (2020), were comparable to our findings. The previous authors came to the conclusion that supplementing basal diets with SB and RL at various concentrations enhanced immunoglobulin IgG and IgM and improved immunity in broiler chickens. Additionally, Nafaa et al. (2023) investigated how SB affected the intestinal immune response to Eimeria maxima infection and the histomorphological structure of the intestine of broiler chickens. The results showed that in the treated groups the intestinal villi and crypt depth were much longer than those of the control group. According to Zhang et al. (2023), SB and RL exhibited considerably higher gut immunity than control birds against E. maxima infection. The previous authors concluded that adding RL to the basal diet raised the immunity of the ducks and broilers, respectively. The current findings reported that adding SB and RL to basal diets considerably improved carcass traits by increasing live weight, carcass weight, and dressing percent. Additionally, it decreased the weight of abdominal fat, while significantly increasing spleen and thymus weights. There were non-significant changes in the weight of the heart and liver. These findings were in line with those of Petricevic et al. (2018) and Lan et al. (2020) who reported that adding SB and RL to the basal diet had a positive impact on carcass traits of broiler chickens. ConclusionThe optimum addition is to supplement the basal diet with 500 g/ton SB and 4 kg/ton RL which gives the best result. The diet supplementation with these feed additives (SB and RL) produces growth-promoting, hypolipidemic, antioxidant, and immunostimulant effects. More research to understand the mechanisms of action underlying these effects in broiler chickens is necessary. AcknowledgmentsMany thanks and appreciation to Dr Aya Mohye Yassin, Assistant Professor of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Cairo University, for estimating the parameters of the biochemical profile. Conflict of interestAll authors declare that there are no conflicts of interest. FundingThe authors declare that there are no funds were offered to complete this work. Authors’ contributionsHamed Yahya Saifan conducted the experiments and collected data. Mostafa Abbas Shalaby, Khaled Abo-El-Sooud, and Mohamed Ahmed Tony designed and supervised the work. Khaled Abo-El-Sooud performed statistical analysis. Mostafa Abbas Shalaby and Mohamed Ahmed Tony wrote the original draft of the article and prepared the figures. Aya Mohye Yassin performed the biochemical analyses. Mostafa Abbas Shalaby wrote and revised the final manuscript before submission Data availabilityThe participants of this study do not give written consent for their data to be shared publicly, because of the sensitive nature of the research supporting data are not available. ReferencesAbdelli, N., Sola-Oriol, D. And Perez, J.F. 2021. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animal (Basel) 11(12), 347–4381. Aebi, H.E. 1984. Catalase in vitro. Methods Enzymol. 105, 121–126. Alagawany, M., Elnesr, S.S., Farag, M.R., Tiwari, R., Yatoo, M.I., Karthik, K., Michalak, I. and Dhama, K. 2020. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—a comprehensive review. Vet. Q. 41(1), 1–29. Al-Kassie, G.A.M., Mohammed, M.F. and Hamood, M.F. 2008. The effect of anise and rosemary on the microbial balance in gastro intestinal tract of broiler chicks. Int. J. Poult. Sci. 7, 610–612. Al-Khalaifah, H.S. 2018. Benefits of probiotics and/or prebiotics for antibiotic reduced poultry. Poult. Sci. 97, 3807–3815. Allain, C.C., Poon, L.S. and Chan, C.S. 1974. Enzymatic determination of serum total cholesterol. Clin. Chem. 20, 470–475. Beard, C.W. 1989. Serological procedures: In a Laboratory manual for the isolation and identification of avian pathogens, Edited by American Association of Avian Pathologists. Dubuque, IA: Kendall/Hunt Publishing Company, pp: 192–200. Bergmeyer, H.U., Schreiber, P. and Wahlefeld, A.W. 1978. Optimization of methods for aspartate and alanine aminotransferases. Clin. Chem. 24, 58–61. Bos, H. and de Souza, W. 2000. Phagocytosis of yeast: a method for concurrent quantification of binding and internalization using differential interference on contrast microscopy. J. Immunol. Methods. 238(1-2), 29–43. Deng, F., Tang, S., Zhao, H., Zhong, R., Liu, L., Meng, Q., Zhang, H. and Chen, L. 2023. Combined effects of sodium butyrate and xylo-oligosaccharide on growth performance, anti-inflammatory and antioxidant capacity, intestinal morphology and microbiota of broilers at early stage. Poult. Sci. 102(5), 102585. Engvall, E. and Perlmann, P. 1971. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochem 8(9), 871–874. Ghazalah, A.A. and Ali, A.M. 2008. Rosemary leaves as a dietary supplement for growth in broiler chickens. Int. J. Poult. Sci. 7(3), 25–33. Hashemi, S.R. and Davoodi, H. 2011. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 35, 169–180. Lan, R., Zhao, Z., Siqi, L.S. and An, L. 2020. Sodium butyrate as an effective feed additive to improve performance, liver function, and meat quality in broilers under hot climatic conditions. Poult. Sci. 99(11), 5491–5500. Loetscher, Y., Kreuzer, M. and Messikommer, R.E. 2013. Oxidative stability of the meat of broilers supplemented with rosemary leaves, rosehip fruits, chokeberry pomace, and entire nettle on performance and meat quality. Poult. Sci. 92, 2938–2948. Lorentz, K. and Berndt, W. 1967. Enzymic determination of uric acid by a colorimetric method. Anal. Biochem. 18(1), 58–63. Moreno, S., Scheyer, T., Romano, C.S. and Vojnov, A.A. 2006. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 40(2), 223–231. Nafaa, R.E., Gad, F.A. and El-Mahmoudy, A.M. 2023. Effects of dietary sodium butyrate on innate immunity and gut health of broiler chickens challenged with Eimeria maxima. Benha Vet. Med. J. 43(2), 1–5. Nishikimi, M., Appaji, N. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem. Biophys. Res. Commun. 46(2), 849–854. Norouzi, B., Ahmad, A.A.Q., Seidavi, A.S. and Marin, A.L.M. 2015. Effect of different dietary levels of rosemary (Rosmarinus officinalis) and yarrow (Achillea millefolium) on the growth performance, carcass traits and ileal microbiota of broilers. Italian J. Anim. Sci. 14, 448–453. Olivera, A.A., Keller, K.M., Devesa, M.V. and Rosa, C.A.R. 2015. Effect of three different anti-mycotoxin additives on broiler chickens exposed to aflatoxin B1. Arch. Vet. Med. 47, 175–183. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2), 351–358. Peeters, T.L. and Vantrappen, G.R. 1977. Factors influencing lysozyme determination by lysoplatemethod. Clin. Chem. Acta 74, 217–255. Petricevic, V., Lukic, M., Skrbic, Z., Rakonjak, S. and Doskovic, V. 2018. The effect of using rosemary (Rosmarinus officinalis) in broiler nutrition on production parameters, slaughter characteristics, and gut microbiological population. Turkish J. Vet. Anim. Sci. 42, 628–664. Ricke, S.C., Dittoe, D.K. and Richardson, K.E. 2020. Formic acid as an antimicrobial for poultry production: a review. Front. Vet. Sci. 7, 563. Rosa, P.S., Filho, D.E., Dahlke, F., Vieira, B.S. Macari, M. and Furlan, R.L. 2007. Performance and carcass characteristics of broiler chickens with different growth potential and submitted to heat stress. Brazilian J. Poult. Sci. 9(3), 50–59. Sikandar, A., Zaneb, H. and Younus, M. 2017. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australian J. Anim. Sci. 30(5), 690–699. Sun, J., Zhang, X., Broderick, M. and Fein, H. 2003. Measurement of nitric oxide production in biological systems by using Griess Reaction Assay. Sensory 3(8), 276–284. Tothova, C., Sesztáková, E., Bielik, B. and Nagy, O. 2019. Changes of total protein and protein fractions in broiler chickens during the fattening period. Vet. World. 12(4), 598–604. Wahlefeld, A.W. 1974. Triglycerides determination after enzymatic hydrolysis, In: methods of enzymatic analysis. Ed. H. U. Bergmeyer, 2nd English Ed. New York, USA: Academic Press. Zhao, H., Bai, H., Deng, F., Zhong, R., Liu, L., Chen, L. and Zhang, H. 2022. Chemically protected sodium butyrate improves growth performance and early development and function of small intestine in broilers as one effective substitute for antibiotics. Antibiotics (Basel). 11(2), 132. Zhang, S., Zhu, C., Xie, H., Wang, L. and Hu, J. 2022. Effect of Gan Cao (Glycyrrhiza uralensis Fisch) polysaccharide on growth performance, immune function, and gut microflora of broiler chickens. Poult. Sci. 101(10), 1–6. Zhang, W.H., Jiang, Y., Zhu, Q.F., Gao, F., Dai, S.F., Chen, J. and Zhou, G.H. 2011. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 52(3), 292–301. Zheng, K., Wu, L., He, Z., Yang, B. and Yang, Y. 2017. Measurement of the total protein in serum by biuret method with uncertainty evaluation. Measurements 112, 16–21. | ||
| How to Cite this Article |
| Pubmed Style Shalaby MA, Saifan H, Abo-el-sooud K, Tony MA, Yassin AM. Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. Open Vet. J.. 2024; 14(5): 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 Web Style Shalaby MA, Saifan H, Abo-el-sooud K, Tony MA, Yassin AM. Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. https://www.openveterinaryjournal.com/?mno=197516 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.19 AMA (American Medical Association) Style Shalaby MA, Saifan H, Abo-el-sooud K, Tony MA, Yassin AM. Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. Open Vet. J.. 2024; 14(5): 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 Vancouver/ICMJE Style Shalaby MA, Saifan H, Abo-el-sooud K, Tony MA, Yassin AM. Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 Harvard Style Shalaby, M. A., Saifan, . H., Abo-el-sooud, . K., Tony, . M. A. & Yassin, . A. M. (2024) Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. Open Vet. J., 14 (5), 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 Turabian Style Shalaby, Mostafa Abbas, Hamed Saifan, Khaled Abo-el-sooud, Mohamed Ahmed Tony, and Aya Mohye Yassin. 2024. Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. Open Veterinary Journal, 14 (5), 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 Chicago Style Shalaby, Mostafa Abbas, Hamed Saifan, Khaled Abo-el-sooud, Mohamed Ahmed Tony, and Aya Mohye Yassin. "Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens." Open Veterinary Journal 14 (2024), 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 MLA (The Modern Language Association) Style Shalaby, Mostafa Abbas, Hamed Saifan, Khaled Abo-el-sooud, Mohamed Ahmed Tony, and Aya Mohye Yassin. "Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens." Open Veterinary Journal 14.5 (2024), 1243-1250. Print. doi:10.5455/OVJ.2024.v14.i5.19 APA (American Psychological Association) Style Shalaby, M. A., Saifan, . H., Abo-el-sooud, . K., Tony, . M. A. & Yassin, . A. M. (2024) Sodium butyrate and rosemary herb improve growth performance, biochemical profile, immunity, and carcass traits in broiler chickens. Open Veterinary Journal, 14 (5), 1243-1250. doi:10.5455/OVJ.2024.v14.i5.19 |