| Research Article | ||
Open Vet. J.. 2024; 14(7): 1607-1613 Open Veterinary Journal, (2024), Vol. 14(7): 1607–1613 Research Article The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agentMilahayati Daulay1*, Muhammad Syahputra2, Mutiara Indah Sari2, Tri Widyawati3 and Dwi Rita Anggraini41Department of Physiology, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia 2Department of Biochemistry, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia 3Department of Pharmacology, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia 4Department of Anatomy, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia *Corresponding Author: Milahayati Daulay. Department of Physiology, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia. Email: milahayati.daulay [at] usu.ac.id Submitted: 16/04/2024 Accepted: 19/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
AbstractBackground: Hyperglycemia in diabetes mellitus (DM) can lead to dyslipidemia, which is a risk factor for macrovascular complications such as heart disease and stroke. Aside from administering antidiabetic medications, DM treatment can also be achieved through the use of natural components, such as Myrmecodia pendans, commonly known as the ant nest plant (ANP). Aim: This study aimed to investigate the impact of administering the ANP on the lipid profile of Wistar rats. Methods: A group of 20 rats was divided into two categories: 6 rats served as healthy controls (H), while the remaining 14 rats were subjected to a high-lipid diet and streptozotocin to generate a model of type 2 diabetes mellitus (T2DM). The diabetic rats were divided into two groups: the DM group consisted of rats that did not receive any treatment, while the ANP group was administered the herb orally. Results: The results revealed significant variations in triglyceride, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels among the three groups (p < 0.05). The post hoc test revealed disparities in triglyceride and LDL between those in the DM group and the ANP group (p < 0.05). Conclusion: Myrmecodia pendans demonstrated the ability to decrease triglyceride and LDL, while increasing HDL levels in rats with T2DM. Keywords: Myrmecodia pendans, Ant nest plant, Diabetes mellitus, Cholesterol. IntroductionThe International Diabetes Federation reports that over 415 million individuals globally were afflicted with diabetes mellitus (DM) in 2015, and it is projected that this number will rise to 640 million by the year 2040 (International Diabetes Federation 2021). Similarly, there is a significant number of individuals in Asia who suffer from DM, totaling approximately 3.4 million (South East Asia Regional Office 2019). Inadequate management of suboptimal DM or uncontrolled diabetes will result in a range of consequences, specifically acute and chronic problems. The long-term effects of DM are significant contributors to vision loss, renal failure, myocardial infarctions, cerebrovascular accidents, and lower extremity amputations. The study demonstrates that those with diabetes face a heightened risk of heart attack and stroke, with a two to threefold increase in this risk (Setiyorini and Wulandari 2017). The pathogenesis of microvascular and macrovascular problems in type 2 diabetes mellitus (T2DM) initiates with an elevation in oxidative stress triggered by high blood glucose levels. Hyperglycemia leads to dysregulation of fat metabolism, which is characterized by an increase in cholesterol levels (hypercholesterolemia). Hypercholesterolemia is a contributing factor to the development of coronary heart disease. Elevated cholesterol can initiate the progression of atherosclerosis, leading to the formation of atheroma plaques that can obstruct the coronary arteries in the heart. Hence, it is crucial to address the issue of hypercholesterolemia to prevent cardiovascular diseases, including coronary heart disease (von Eckardstein et al., 2023). Pharmacological therapy is a treatment method used to manage T2DM. The government enforces regulations on the utilization of traditional medicines, which hold significant cultural value and have been employed by numerous individuals for generations. Nevertheless, the safety and efficacy of it have not been sufficiently proven by adequate studies. The ant nest plant (ANP) (Fig. 1) is one of the plants employed in the treatment of diabetes. The ant nests are classified into a collective of 26 distinct species, all of which are found exclusively on Irian Island in Papua, Indonesia. They belong to the Hydnophytinae subfamily, which is a part of the Rubiaceae family. The Myrmecodia pendans species is responsible for the production of the therapeutic chemical. This plant exhibits epiphytic growth, commonly seen attached to specific tree species such as whitewood, mountaineering, kaka, and beech trees. A single species of ant typically inhabits ANPs (Engida et al., 2013; Gartika et al., 2018). The indigenous people of Papua are familiar with ants as a medicinal plant used to heal many ailments. A study found that ant nest decoctions and ethanol extracts at 25% and 50% concentrations could kill Escherichia coli bacteria (Roslizawaty et al., 2013). In addition, it has been suggested that it possesses potential anti-cancer properties (Supriatno, 2014; Soeksmanto et al., 2010; Achmad et al., 2016;), antimicrobial effects against bacteria (Crisnaningtyas and Rachmadi 2010; Gartika et al., 2018), and the ability to modulate the immune system (Hertiani et al., 2010). The ANP is believed to possess antidiabetic effects. Using a glucose-induced hyperglycemic paradigm, a study found that administering ant nest ethanol extract at a concentration of 8.4% b/v effectively decreased blood glucose levels (Taebe et al., 2012). A study conducted on Sprague-Dawley diabetes rats discovered that the injection of ant nest water extract at a dosage of 360 mg per 200 g of body weight, resulted in a decrease in fasting blood glucose (Raya et al., 2016). In previous studies, researchers used water as a solvent to make ant nest extracts. The study only examined the decrease in fasting blood glucose levels following ant nest administration. This study employs 70% ethanol as the solvent. Ethanol is used as a solvent because the secondary metabolites of the plant will be identified and isolated. Furthermore, the study examined not only the reduction in blood glucose levels, but also the effects of ant nest ethanol extract in treating dyslipidemia, a risk factor for macrovascular complications in T2DM. Nevertheless, there have been no reports on the efficacy of the ANP in preventing complications of T2DM. As a result, the goal of this study was to examine the effect of ant-nest ethanol extract administration on the lipid profile of model rats with T2DM.
Fig. 1. The ant nest plant. Materials and MethodsAnimal careThe research was carried out under the authorization of the Health Research Ethics Committee of the Medical Faculty of the Universitas Sumatera Utara, following the 3R principle, which focuses on replacement, reduction, and refinement. The animal endeavors to get compassionate care, uphold its welfare, prevent injury, and minimize distressing procedures to ensure its well-being during the duration of the study. The principles of animal welfare should be grounded in the five freedoms (5F): freedom from hunger and thirst, freedom from discomfort, freedom from pain, damage, or sickness, freedom from fear and distress, and freedom to engage in natural behavior. Professionals skilled in minimizing or eradicating animal distress conduct executions in a compassionate manner. Competent, trained, and experienced staff conduct all procedures to treat animals, with the aim of minimizing stress. Animal modellingThe animal modeling procedures involving high-fat meals and STZ injections were derived from earlier studies (Srinivasan et al., 2005; Zhang et al., 2008; Machrina et al., 2018). The rats were acclimatized for a period of 7 days, during which they were provided with food and water ad libitum. The typical diet includes water (up to 13% content), protein (18.5%–20.5%), fat (at least 4%), fiber (up to 6%), ash (up to 0.8%), calcium (at least 0.9%), and phosphorus (at least 0.7%). Following a period of 3 days, all of the rats were provided with a diet that was high in fat for a duration of 5 weeks in order to induce obesity (Yuniarto et al., 2015; Shiyan et al., 2017). The components required for preparing a high-fat meal include 8 kg of standard pellets, 15 duck egg yolks, 2.5 kg of grated flour, 0.25 kg of crushed coconut oil, 1 kg of goat fat, 0.5 kg of sand sugar, and a sufficient amount of hot water. Following a period of 5 weeks, the rats underwent a 24-hour fasting period, after which they were intraperitoneally given a low dose of streptozotocin (STZ) (30 mg/kg body weight in 0.1 citrate buffer, pH 4.5). Streptozotocin has diabetogenic effects with toxic properties that can decrease the function of pancreatic β cells (Skovsø 2014; Ghasemi and Jeddi 2023). The administration of STZ was repeated after a week, using a dosage of 45 mg/kg body weight. One week following the second STZ administration, blood glucose levels are measured, and if the reading shows a value higher than 200 mg/dl, it indicates the presence of DM. The evaluation involved the application of xylocaine spray to the rat's tail, followed by a brief waiting period, the dissection of the tail, and its subsequent placement onto the glucose strip. Extraction technique of ant nestThe ant nest is thoroughly cleansed and finely diced, followed by a process of sun-drying, but not in direct exposure. A total of 500 g of ant nest powder (Fig. 2) is obtained through the process of maceration, employing 70% ethanol for two consecutive periods of 24 hours each, followed by two further rounds of maceration. The ant nest powder is placed into the macerator and combined with the ethanol solvent in a ratio of 1 part powder to 12 parts solvent. It is then left to infuse for a total of 48 hours (Fig. 3). The obtained filter is subsequently subjected to further filtration, and the remaining substances are soaked in a fresh solvent to achieve a transparent filter. The extract is connected to a vacuum rotary evaporator and maintained at a temperature of 64°C (Roslizawaty et al., 2013; Sudiono et al., 2015). Phytochemical screeningA qualitative phytochemical screening was done on the extract to find a secondary metabolite molecule that might be an antioxidant (Agustina et al., 2017). The phytochemical assays include the presence of tannins, flavonoids, alkaloids, saponins, and steroids. Tannin testingTannin testing involves the activation of the extract using a 1% solution of FeCl3. A change in color—which could be green, purple, blue, or black—indicates a positive response. Flavonoid testingFlavonoid testing involves inserting an extract into a reaction tube, adding ethanol, heating until boiling, filtering, and then adding magnesium powder and dropping HCl. The presence of a red hue indicates a positive test result.
Fig. 2. Ant nest powder.
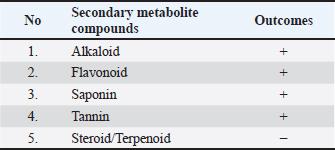
Fig. 3. Maceration of ant nest. Alkaloid testingAlkaloid testing is performed with Bouchardat, Dragendorff, and Mayer reagents, resulting in the production of a white or cream-colored endpoint, indicating a positive result for alkaloids. Saponin testingSaponin testing is performed by adding heated and filtered aquades. The development of a foam layer indicates the presence of saponins. Steroid/Terpenoid testingSteroid/Terpenoid testing is conducted using the Liebermann-Buchard method, which involves the addition of acetic acid followed by the addition of concentrated sulfuric acid. The presence of terpenoid is confirmed by the manifestation of a stunted or violet hue, while the presence of steroid is confirmed by the development of a blue hue. Research design and blood analysisThe control group consisted of healthy (H group) rats that were not fed a high-fat diet and were not stimulated with STZ. The remaining two groups consisted of rats that were administered a high-fat diet and injected with low-dose STZ to produce a T2DM model. One of the two groups of rats with diabetes (DM group) received an oral dose of 400 mg/kg body weight of ethanol extract of ANP. The extract is dissolved in a suspension solution of 0.5% sodium methyl cellulose carboxyl (CMC-Na) and delivered via the nasogastric canal. After a duration of 8 weeks, the animal was euthanized while under sedation using ketamine anesthesia administered intraperitoneally. A sample of blood was extracted directly from the heart for analysis. The varied examination procedures involved testing blood glucose levels using a glucometer (Autocheck 3in1®, Taiwan). The Cholesterol Quantitation Kit (Meril®, India) was used to examine the fat profiles. The examination process for both variables adheres to the protocol specified on each device. Statistical analysisThe data is analyzed using statistical tools. To assess the disparity in the average value of each measured variable across different groups, the one-way ANOVA test will be employed if the data adheres to a normal distribution. If there is a statistically significant difference (p < 0.05), then the test proceeds with Bonferroni's post hoc analysis. The Kruskal-Wallis test was conducted for non-normally distributed data. If there is a statistically significant difference (p < 0.05), the analysis proceeds with a post hoc Mann-Whitney test to determine which groups exhibit significant differences. Test findings with a p-value less than 0.05 were deemed significant. Ethical approvalThe research conducted at the Universitas Sumatera Utara has been granted approval by the Research Ethics Committee, as stated in letter No. 345/KEP/USU/2020. ResultsPhytochemical test findingsThe phytochemical analysis of the ant nest ethanol extract, as shown in Table 1, revealed positive results (+) for alkaloid, flavonoid, saponin, and tannin tests while yielding negative results (−) for triterpene and steroid tests. Analysis of the findings from examining research variablesTable 2 contains the calculated disparities in the mean initial body weight and initial blood glucose for each group. The statistical tests revealed that there was no statistically significant distinction between the two variables across the three groups (p=0.487 and 0.610, respectively). By the conclusion of the study period, there were disparities in the mean blood glucose among each group. The statistical analysis revealed substantial variations across groups in terms of blood glucose (p < 0.001). Post hoc analysis reveals the H group had the lowest blood glucose values, which were considerably different from the values observed in the DM and ANP groups (p < 0.001). The ANP group exhibited reduced blood glucose in comparison to the DM group (p < 0.001). Table 3 shows that the average total cholesterol (TC) varied across each group, but there was no meaningful statistical difference (p=0.203). Triglyceride varied among the different groups (p=0.020). The post hoc tests revealed substantial differences across groups, with the highest triglyceride observed in the DM group, which was significantly different from the H and ANP groups (p=0.038 and 0.039, respectively). This also applies to low-density lipoprotein (LDL) levels. The highest LDL was observed in the DM group, which was significantly different from the H and ANP groups (p=0.017 and 0.022, respectively). Meanwhile, H had the highest high-density lipoprotein (HDL), which was statistically different from DM (p=0.048), but not significantly different from ANP (p=0.110). HDL levels were higher in the ANP group compared to the DM group, but this was not statistically significant (p=0.621). DiscussionDyslipidemia, which refers to irregularities in lipid metabolism, is commonly observed in people with T2DM. Dyslipidemia is a condition that affects the way lipids are processed in the body, resulting in either an increase or reduction in the amount of lipids present in the blood plasma. The primary lipid fraction abnormalities consist of elevated levels of TC, triglycerides, LDL, and reduced levels of HDL. The dyslipidemia produced by DM is classified as secondary dyslipidemia. Lipid peroxidation, which involves the oxidation of LDL by free radicals, is identified as a risk factor for cardiovascular complications in individuals with T2DM (Katakami 2018). The elevated levels of LDL cholesterol in people with DM are strongly associated with the development of atherosclerosis. The tiny size and weak binding to LDL receptors make LDL cholesterol prone to being transported to the subendothelial region and more susceptible to oxidation or degradation due to its limited antioxidant capacity (Katakami 2018). Table 1. Phytochemical test results for ethanol extracts of ant nests.
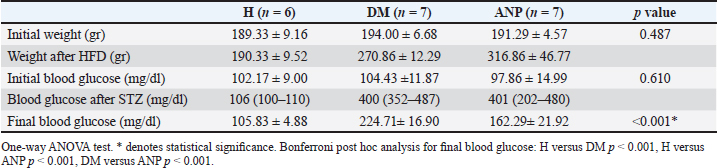
Table 2. Comparison of initial weight, initial blood glucose, and final blood glucose.
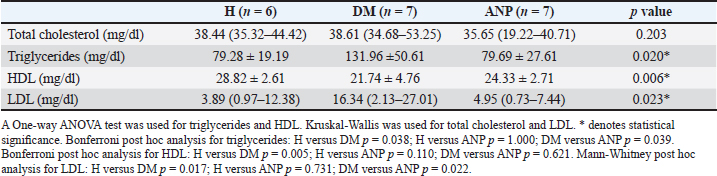
Table 3. Analysis of the lipid profile.
There was no statistically significant disparity observed in the mean initial body weight and mean initial blood glucose level among the three groups in this study. This indicates that the sample's original state prior to the investigation was uniform throughout. The group of T2DM rats that received ethanol extract from ant nests had the lowest blood glucose levels, which were significantly distinct from the levels observed in the other two groups. These findings demonstrate that the ethanol extract derived from ant nests has the ability to lower blood glucose in rats with T2DM. This is in line with the findings of Raya et al.'s (2016) research. Despite using different methods in animal testing models of diabetic mellitus, the two studies obtained similar results, showing a significant decrease in fasting blood glucose levels after administering ant nest. Ant nests contain flavonoids, which are compounds with antioxidant and antihyperglycemic properties. These flavonoids have the ability to reduce blood glucose and decrease problems associated with T2DM. Administration of ants nest ethanol extract leads to a decrease in fasting blood glucose. The reason for this is that ethanol extracts from ants' nests include flavonoids, which have the ability to reduce blood glucose by inhibiting the enzymes α glucosidase, maltase, and amylase. Flavonoids have the ability to reduce blood glucose by enhancing the uptake of glucose through the control of GLUT4 and by reducing the absorption of glucose in the intestines (Anggraini 2020). Tannin facilitates the body's absorption of glucose by interacting with the insulin signalling system, GLUT-4 translocation, and phosphoinositide 3-kinase. Tannin possesses the ability to stimulate the regeneration of pancreatic beta cells, enhance insulin secretion in adipocytes, and inhibit the activity of amylase and glucosidase enzymes, hence preventing postprandial elevation in blood glucose (Kumari and Jain 2012). Significant disparities in triglyceride and LDL were seen between the group with T2DM and the group administered ethanol extract from ant nests. Unlike other studies, the reduction in triglycerides and LDL levels was insignificant (Hartono et al., 2019). These differences are likely due to differences in the methods used. Previously, researchers conducted an animal trial using a hypercholesterolemia model and a high-fat diet, whereas this study used a T2DM model trial animal with a high-fat diet and STZ injections. Thus, the mechanisms of dyslipidemia in these two conditions have different pathophysiologies. In addition, this study used ethanol as the solvent, whereas previous studies used water to manufacture ant nest extracts. In the previous study, the duration of herbal administration was relatively short at 15 days, but in this study, it lasted for 8 weeks. It affects the activity of the flavonoids contained in the ant nest as an antihypercholesterolemia. The ethanol extract of M. pendans demonstrated a reduction in the quantity of foam cells in the aorta, indicating potential advantages for treating early atherosclerotic lesions in individuals with T2DM (Daulay et al., 2022). Phytochemical tests conducted on ant nests have revealed the presence of chemical compounds belonging to the flavonoid and tannin groups (Widyawati et al., 2020a,b). Flavonoids act as antioxidants by inhibiting the aldose reductase enzyme, which is involved in the polyol pathway of glucose metabolism during hyperglycemic conditions, thus mitigating oxidative stress (Mohan and Nandhakumar 2014). Flavonoids can potentially decrease the development of atherosclerosis by stimulating the PPARγ-LXRα-ABCA1/ABCG1 pathway, which promotes the removal of cholesterol from cells (Chen et al., 2023). Flavonoids have antioxidant properties that can protect LDL cholesterol from oxidation. Furthermore, flavonoids can also affect the activity of enzymes involved in cholesterol metabolism, such as inhibiting the HMG-CoA reductase enzyme involved in liver cholesterol production (Tang et al., 2021). By employing these various pathways, the flavonoids in the ethanol extract from the ant nest can contribute to the mitigation of cardiovascular disease by modulating cholesterol levels within the body. ConclusionThe ethanol extract of M. pendans has demonstrated the capacity to reduce blood glucose, triglyceride, and LDL levels while simultaneously increasing HDL levels in rats with a model of T2DM. Therefore, the extract derived from this herb demonstrates notable effectiveness in preventing the advancement of dyslipidemia., a well-established risk factor for macrovascular complications in T2DM. AcknowledgmentsThe authors express their gratitude to all the participants for their diligent cooperation in this study. Conflicts of interestThe author of this paper does not have any conflicting interests. FundingThe research is funded by Universitas Sumatera Utara (USU) through the TALENTA USU research grant for the year 2021 (Grant number. 340/UN5.2.3.1/PPM/SPP-TALENTA/2021). Data availabilityAll data generated and analyzed are included in this research article. Author contributionsMilahayati Daulay, Muhammad Syahputra, and Mutiara Indah Sari carried out the concept, design, and drafting of the article. The acquisition of data, interpretation of data, statistical analysis, critical revision, supervision, and final approval were carried out by Milahayati Daulay, Mutiara Indah Sari, Tri Widyawati, and Dwi Rita Anggraini. ReferencesAchmad, H., Supriatno, Singgih, M.F., and Hendrastuti, H. 2016. Akt signal transduction pathways and nuclear factor-kappa B (NF-KB) transcription as a molecular target of oral tongue squamous cell carcinoma (SP-C1) using Papua’s Anthill Plant (Myrmecodia pendans). Pakistan J. Biol. Sci. 19, 323–330. Agustina, W., Nurhamidah, and Handayani, D. 2017. Skrining Fitokimia Dan Aktivitas Antioksidan Beberapa Fraksi Dari Kulit Batang Jarak ( Ricinus Communis L.). Alotrop 1, 117–122. Anggraini, A. 2020. Manfaat Antioksidan Daun Salam Terhadap Kadar Glukosa Darah Dan Penurunan Apoptosis Neuron Di Hippocampus Otak Tikus Yang Mengalami Diabetes. J. Med. Hutama 2, 349–355. Chen, Y., Zhang, F., Sun, J., and Zhang, L. 2023. Identifying the natural products in the treatment of atherosclerosis by increasing HDL-C level based on bioinformatics analysis, molecular docking, and in vitro experiment. J. Transl. Med. 21, 1–14. Crisnaningtyas, F., and Rachmadi, A.T. 2010. Pemanfaatan Sarang Semut (Myrmecodia Pendens) Asal Kalimantan Selatan Sebagai Antibakteri. J. Ris. Ind. Has. Hutan 2, 31–35. Daulay, M., Lindarto, D., Sembiring, R.J., Machrina, Y., Purba, A., Munir, D., Wahyuni, A. S., and Yamamoto, Z. 2022. Slow-type interval training and ethanol extract of Sarang Semut (Myrmecodia pendans) can improve the early lesions of atherosclerosis in type-2 diabetes mellitus rats. Open Access Maced. J. Med. Sci. 10, 1079–1081. von Eckardstein, A., Nordestgaard, B.G., Remaley, A.T., and Catapano, A.L. 2023. High-density lipoprotein revisited: biological functions and clinical relevance. Eur. Heart J. 44, 1394–1407. Engida, A.M., Kasim, N.S., Tsigie, Y.A., Ismadji, S., Huynh, L.H., and Ju, Y.H. 2013. Extraction, identification and quantitative HPLC analysis of flavonoids from Sarang Semut (Myrmecodia Pendan). Ind. Crops Prod. 41, 392–396. Gartika, M., Pramesti, H.T., Kurnia, D., and Satari, M.H. 2018. A terpenoid isolated from Sarang Semut (Myrmecodia pendans) bulb and its potential for the inhibition and eradication of Streptococcus mutans biofilm. BMC Complement. Altern. Med. 18, 1–8. Ghasemi, A., and Jeddi, S. 2023. Streptozotocin As a tool for induction of rat models of diabetes: a practical guide. EXCLI J. 22, 274–294. Hartono, H., Setiawan, I., and Saktiningsih, H. 2019. Potensi Anti Hiperkolesterolemia Ekstrak Tumbuhan Sarang Semut (Myrmecodia pendans Merr. & Perry). J. Farm. 3, 1. Hertiani, T., Sasmito, E., Sumardi, and Ulfah, M. 2010. Preliminary study on immunomodulatory effect of Sarang-Semut tubers Myrmecodia tuberosa and Myrmecodia pendens. Online J. Biol. Sci. 10, 136–141. International Diabetes Federation. 2021. IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation. Katakami, N. 2018. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler. Thromb. 25, 27–39. Kumari, M., and Jain, S. 2012. Tannins: an antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. 1, 1–8. Machrina, Y., Damanik, H.A.R., Purba, A., and Lindarto, D. 2018. Effect various type of exercise to insr gene expression, skeletal muscle insulin receptor and insulin resistance on diabetes mellitus type-2 model rats. Int. J. Heal. Sci. 6, 50–56. Mohan, S., and Nandhakumar, L. 2014. Role of various flavonoids: hypotheses on novel approach to treat diabetes. J. Med. Hypotheses Ideas. 8, 1–6. Raya, M.K., Legowo, A.M., and Wijayahadi, N. 2016. Efektivitas Ekstrak Umbi Sarang Semut (Myrmecodia Pendens Merr.& Perry) Sebagai Penurun Kadar Glukosa Darah Tikus Sprague Dawley Yang Diabetes Mellitus. J. Gizi Indones. 4, 138–144. Roslizawaty, Budiman, H., Laila, H., and Herrialfian. 2013. Pengaruh Ekstrak Etanol Sarang Semut (Myrmecodia Sp.) Terhadap Gambaran Histopatologi Ginjal Mencit (Mus Musculus) Jantan Yang Hiperurisemia. J. Med. Vet. 7, 116–120. Setiyorini, E., and Wulandari, N.A. 2017. Hubungan Lama Menderita Dan Komplikasi diabetes melitus Dengan Kualitas Hidup Lansia Penderita Diabetes Melitus Tipe 2: In Seminar Nasional dan Gelar Produk, pp: 75–82. Shiyan, S., Herlina, H., and Bella, A.M. 2017. Antiobesity and antihypercholesterolemic effects of white tea (Camellia Sinensis) infusion on high-fat diet induced obese rats. Pharmaciana 7, 278–288. Skovsø, S. 2014. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 5, 349–358. Soeksmanto, A., Subroto, M., Wijaya, H., and Simanjuntak, P. 2010. Anticancer activity test for extracts of Sarang Semut plant (Myrmecodya Pendens) to HeLa and MCM-B2 Cells. Pakistan J. Biol. Sci. 13, 148–151. South East Asia Regional Office. 2019. Diabetes Fact Sheet, Geneva, Switzerland. Srinivasan, K., Viswanad, B., Asrat, L., Kaul, C.L., and Ramarao, P. 2005. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 52, 313–320. Sudiono, J., Oka, C., and Trisfilha, P. 2015. The scientific base of Myrmecodia pendans as herbal remedies. Br. J. Med. Med. Res. 8, 230–237. Supriatno. 2014. Antitumor activity of Papua’s Myrmecodia pendans in human oral tongue squamous cell carcinoma cell line through induction of cyclin-dependent kinase inhibitor P27Kip1 and suppression of cyclin E. J. Cancer Res. Ther. 2, 48–53. Taebe, B., Randalinggi, E.A., Manggau, M.A., and Usmar. 2012. Uji Efek Hipoglikemik Kombinasi Ekstrak Etanol Propolis Dan Ekstrak Etanol Sarang Semut (Myrmecodia Pendens Merr & Perry) Pada Mencit (Mus Musculus). Maj. Farm. Dan Farmakol. 16, 151–158. Tang, G., Li, S., Zhang, C., Chen, H., Wang, N., and Feng, Y. 2021. Clinical efficacies, underlying mechanisms and molecular targets of chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 11, 2749–2767. Widyawati, T., Pase, M.A., Daulay, M., and Sumantri, I.B. 2020. Standardization and phytochemoical screening of syzygium polyanthum wight leaf and Myrmecodia pendans simplicia. In International Conference of Science, Technology, Engineering, Environmental and Ramification Researches, pp: 114–116. Widyawati, T., Pase, M.A., Daulay, M., Sumantri, I.B., and Yusoff, N.A. 2020. Evaluation of Myrmecodia pendans water extracts on hematology profiles, liver, kidney function and malondialdehyde level in healthy volunteer. Pharmacogn. J. 12, 1489–1493. Yuniarto, A., Kurnia, I., and Ramadhan, M. 2015. Antiobesity effect of ethanolic xtract of jasmine flowers ( Jasminumsambac (l) Ait ) in high fat diet induced mice : potent inhibitor of pancreatic lipase enzyme. Int. J. Adv. Pharmachy, Biol. Chem. 4, 18–22. Zhang, M., Lv, X.Y., Li, J., Xu, Z.G., and Chen, L. 2008. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008, 704045. | ||
| How to Cite this Article |
| Pubmed Style Daulay M, Syahputra M, Sari MI, Widyawati T, Anggraini DR. The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. Open Vet. J.. 2024; 14(7): 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 Web Style Daulay M, Syahputra M, Sari MI, Widyawati T, Anggraini DR. The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. https://www.openveterinaryjournal.com/?mno=197971 [Access: September 15, 2025]. doi:10.5455/OVJ.2024.v14.i7.10 AMA (American Medical Association) Style Daulay M, Syahputra M, Sari MI, Widyawati T, Anggraini DR. The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. Open Vet. J.. 2024; 14(7): 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 Vancouver/ICMJE Style Daulay M, Syahputra M, Sari MI, Widyawati T, Anggraini DR. The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. Open Vet. J.. (2024), [cited September 15, 2025]; 14(7): 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 Harvard Style Daulay, M., Syahputra, . M., Sari, . M. I., Widyawati, . T. & Anggraini, . D. R. (2024) The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. Open Vet. J., 14 (7), 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 Turabian Style Daulay, Milahayati, Muhammad Syahputra, Mutiara Indah Sari, Tri Widyawati, and Dwi Rita Anggraini. 2024. The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. Open Veterinary Journal, 14 (7), 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 Chicago Style Daulay, Milahayati, Muhammad Syahputra, Mutiara Indah Sari, Tri Widyawati, and Dwi Rita Anggraini. "The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent." Open Veterinary Journal 14 (2024), 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 MLA (The Modern Language Association) Style Daulay, Milahayati, Muhammad Syahputra, Mutiara Indah Sari, Tri Widyawati, and Dwi Rita Anggraini. "The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent." Open Veterinary Journal 14.7 (2024), 1607-1613. Print. doi:10.5455/OVJ.2024.v14.i7.10 APA (American Psychological Association) Style Daulay, M., Syahputra, . M., Sari, . M. I., Widyawati, . T. & Anggraini, . D. R. (2024) The potential of Myrmecodia pendans in preventing complications of diabetes mellitus as an antidiabetic and antihyperlipidemic agent. Open Veterinary Journal, 14 (7), 1607-1613. doi:10.5455/OVJ.2024.v14.i7.10 |