| Research Article | ||
Open Vet. J.. 2024; 14(8): 1850-1857 Open Veterinary Journal, (2024), Vol. 14(8): 1850–1857 Research Article Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combinationWinda Kusuma Dewi1, Bondan Sigit Purnomo Aji1, Faisal Fikri1, Agus Purnomo2, Salipudin Tasil Maslamama3, Hakan Çalışkan4 and Muhammad Thohawi Elziyad Purnama1,3*1Division of Veterinary Medicine, Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 2Department of Veterinary Surgery and Radiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 3Department of Biology, Graduate School of Natural and Applied Sciences, Eskişehir Osmangazi Üniversitesi, Eskişehir, Turkey 4Department of Biology, Faculty of Science, Eskişehir Osmangazi Üniversitesi, Eskişehir, Türkiye *Corresponding Author: Muhammad Thohawi Elziyad Purnama. Division of Veterinary Medicine, Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia. Email: thohawi [at] fkh.unair.ac.id Submitted: 21/04/2024 Accepted: 22/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Due to their efficient insulation, lack of sweat glands, relatively quick metabolic rate, and heightened sensitivity to heat, the poultry industry faces a serious problem with heat stress. Combining vitamins has been demonstrated to be more effective than implementing a single vitamin in reducing the effects of heat stress. Aim: This study aimed to investigate the efficacy of the multivitamin combination in feed on the growth performance, egg quality, and antioxidant enzymes in laying hens exposed to heat stress. Methods: A total of 28 Isa Brown strains aged 18 weeks were randomly designated into seven groups with four replications, i.e., (C-) normal temperature group, (C+) heat stress group, and the others with the administration of vitamin A and E (AE), vitamin K and C (KC), vitamin C and E (CE), vitamin E and selenium (ESE), and vitamin C and folic acid (CAF). Feed intake, feed efficiency, eggshell thickness, shape index, haugh unit (HU), yolk, and albumen index were evaluated at 22, 23, 24, and 25 weeks. Meanwhile, antioxidant enzymes were quantified at 22 and 25 weeks. Results: As a result, feed intake was reported a significant improvement in the AE and CE groups compared to the C+ group. Meanwhile, the feed efficiency was reported to be efficient in the CE and ESE groups. Based on egg quality evaluation, we reported significant shell thickness in the CE, ESE, and CAF groups compared to the C+; yolk index was reported slightly significant results in the AE and CAF groups; albumen index and HU were reported to increase significantly in the CAF group. Meanwhile, superoxide dismutase (SOD), malondialdehyde (MDA), and GPx activity were ameliorated significantly in the ESE and CAF groups. Conclusion: Combinations of multivitamins can thereby enhance feed intake, feed efficiency, egg quality, and antioxidant activity. The CE, ESE, and CAF groups were found to have made equivalent improvements in the eggshell thickness, shape index, HU, yolk, and albumen index. Keywords: Antioxidant activity, Egg quality, Environmental stress, Laying performance, Multivitamin. IntroductionIn tropical countries, extreme heat is a critical issue for poultry due to it is associated with global warming (Vandana et al., 2021). Due to their effective insulation, absence of sweat glands, relatively fast metabolic rate, and high deep body temperature, chickens are particularly sensitive to heat (Collier and Gebremedhin, 2015). As a tropical nation, Indonesia experiences scorching summer weather from March to August, when ambient temperatures range from 32 to 48°C (Lestari et al., 2014). During the thermoneutral range, laying hens use practical heat loss mechanisms to regulate body temperature when ambient temperatures rise, with little to no effect on egg production (Kilic and Simsek, 2013). Heat stress can have negative impacts such as decreased feed consumption, growth rate, body weight, egg quality, and egg production (Lara and Rostagno, 2013). In addition, any circumstance that has the potential to result in physiological issues can be regarded as stress. Stress reactions can result in the production of reactive oxygen species (ROS). High ROS levels can lead to lipid peroxidation and oxidative disorders in proteins and DNA by upsetting the equilibrium between oxidation processes and antioxidant activity (Hidayatik et al., 2021). One of the primary defense mechanisms against oxidative stress is the production of the enzymes superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GPx) (Sejian et al., 2018). Due to their efficacy in reducing free radicals and stopping lipid peroxidation, antioxidants play a crucial role in regulating ROS (Panda and Cherian, 2014). Mitigating the negative effects of heat stress is more focused on manipulating feed formulas due to reconstructing hencoops is considered unaffordable (Moritz et al., 2020). Consumption of vitamins such as α-tochoferol (vitamin E) (Sinkalu and Ayo, 2018), ascorbic acid (vitamin C) (Rhoads et al., 2013), folic acid (Gouda et al., 2020), and minerals such as zinc (Zn) and selenium (Se) that have been shown to be able to positively transform in the oxidation chain can all be used to boost the poor antioxidant activity in serum (Rao et al., 2016). However, studies on the efficacy of multivitamin combinations on egg quality and antioxidant activity during heat-challenged levels are limited and need to be expanded. The present study was demonstrated to investigate the multiple efficacies of vitamins A and E, vitamins K and C, vitamins C and E, vitamins E and selenium, and vitamins C and folic acid on growth performance, egg quality, and antioxidant enzymes in Isa Brown layer hens exposed to heat stress. Materials and MethodsAnimals and experimental designHeat stress induction was carried out by regulating the ambient in the installed coop at 43°C ± 3°C for 12 hours per day with extended irradiation of 16 hours per day. Heaters were positioned 2 m above the litter surface in each corner of the coop. An exhaust was installed on the coop’s back side to ensure proper ventilation. Temperature and relative humidity were evaluated every 6 hours (Table 1) to control the heat stress fluctuations during the study period. The sample size in each group was determined using Federer’s formula, which is (t–1) (n–1) > 15. In this formula, (t=7) denotes the number of groups, and (n ≥ 4) denotes the replication sample size. Two related issues led to the consideration of this formula design: in order to offer an accurate estimate of the error variance of a contrast, replication and randomization are required. A total of 28 Isa Brown layer hens kept in a battery cage system (50 × 46 × 45 cm) with an open-sided house were randomly assigned into seven groups with four replication, i.e., (C-) normal temperature group, (C+) heat stress group, and the others with the administration of (AE) 4.5 mg/kg diet vitamin A (IPI, Supra Ferbindo Farma, Indonesia) (Kucuk et al., 2003) + 150 mg/kg diet vitamin E (IPI, Supra Ferbindo Farma, Indonesia) (Ajakaiye et al., 2011), (KC) 3.1 mg/kg diet vitamin K (Kf, Erela, Indonesia) (Dangi et al., 2015) + 150 mg/kg diet vitamin C (C-San, Sanbe Farma, Indonesia) (Ajakaiye et al., 2011), (CE) 150 mg/kg diet vitamin C + 150 mg/kg diet vitamin E, vitamin E and selenium (ESE) 150 mg/kg diet vitamin E + 1.5 mg/kg diet selenium (GNC Live Well, Indonesia) (Habibian et al., 2015), and vitamin C and folic acid (CAF) 150 mg/kg diet vitamin C + 5 mg/kg diet folic acid (Marin Liza Farmasi, Indonesia) (Nouri et al., 2018), respectively. These multivitamin supplements were mixed into the diet and delivered by including them in the pellet form. During the experimental period, laying hens were fed a basal diet (Table 2) and drinking water ad libitum from acclimation to 25 weeks of age. Egg quality evaluationDaily feed intake, egg production, and egg morphological parameters were all documented for evaluation at 22, 23, 24, and 25 weeks. Total feed consumption was divided by the weight of all the eggs to get feed efficiency. A Vernier caliper was used to measure the thickness of the shell by calculating the average of the anterior, posterior, and lateral sites of eggshells. The shape index was calculated using the following equation: SI=W × L−1 × 100, where SI stands for shape index (%), W for egg width (mm), and L for egg length (mm). A tripod micrometer was used to measure the height and diameter of the albumen in each egg, which was halfway between the yolk and the albumen’s edge. The yolk index was calculated using the following equation: YI=H × D−1, where YI stands for yolk index, H for yolk height (mm), and D for yolk diameter (mm). This equation can also be applied to calculate the albumen index. Meanwhile, the Haugh unit (HU) was computed using the following equation: HU=100 log (H − 1.7W0.37 + 7.6), where H stands for albumen height (mm) and W for egg weight (g) (Rayan et al., 2013). Table 1. Ambient temperature and relative humidity at the hencoop area during the study period.
Table 2. Dietary composition of the experimental basal diet.
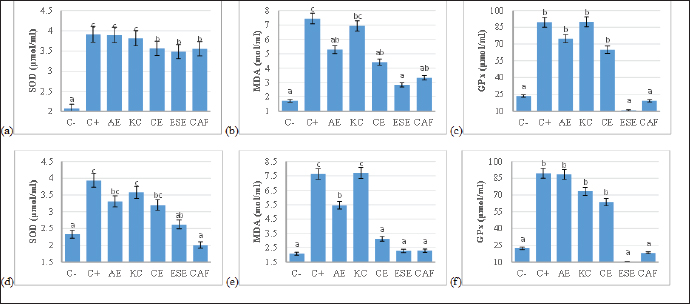
Antioxidant activity quantificationBlood samples totaling 5 ml were drawn from the brachial vein at week 22, the initial of the treatment, and week 25, the end of the treatment. The serum supernatant that resulted was gently aspirated into a microtube and kept at 4°C for SOD, MDA, and GPx levels quantification using the assay kits (Cat.SKT-214, StressMarq, Canada) with precision criteria for average inter-assay CV=6.3%, and average intra-assay CV=2.8% (Dewi et al., 2023). Statistical analysisEach set of data was evaluated using one-way ANOVA, followed by a post hoc Tukey multiple comparison test (p<0.05), and then the results were presented as the mean and standard error. This analysis was not only used to compare the diet control group with the multivitamin group, but also to compare between the multivitamin groups. Meanwhile, ambient temperature and relative humidity at the hencoop area were analyzed using an independent T-test. The software SPSS v.25 (IBM, USA) was utilized for the statistical analysis. Ethical approvalThe ethical approval (No.443/HRECC.FODM/VII/2021) from The Ethical and Research Committee, Universitas Airlangga, was considered to avoid animal abuse during the study. ResultsLaying hens performanceFeed intake in all combination multivitamin groups was reported to improve at 23 and 25 weeks. However, in comparison to the C+ group, we emphasized a highly significant increase in the AE and CE groups. The feed efficiency was reported to be efficient in the CE and ESE groups at 22 and 23 weeks, respectively. Meanwhile, we highlighted that all vitamin combinations can increase the laying rate compared to the C+ group (Table 3). As a result, even though there was no significant difference in egg weight production, we reported that the vitamin AE, CE, and ESE groups had the most significant efficacy on feed intake and feed efficiency. Egg qualityDuring the study period, in comparison to the C+ group, we reported significant shell thickness in the CE, ESE, and CAF groups at 22 weeks. For the yolk index variable, we reported slightly significant results in the AE and CAF groups at 25 weeks. Meanwhile, we reported a significant albumen index in the CAF group at 22 and 24 weeks. On the other hand, the HU value was also reported to increase significantly in the CAF group at 25 weeks (Table 4). Thus, we highlight that the CAF group can improve egg quality most significantly in general although it looks similar when compared to the AE, CE, and ESE groups. Antioxidant activityWe highlighted the finding that the ESE and CAF groups were able to gradually ameliorate SOD, MDA, and GPx activity at 22 and 25 weeks. Meanwhile, we also reported that the CE group also slightly significantly ameliorated SOD and MDA activities, although GPx showed insignificant results (Fig. 1). Despite showing fluctuating results, we specifically interpreted the efficacy of the ESE and CAF groups in ameliorating antioxidant activity during the study period. Table 3. Evaluation of feed intake, egg weight, and feed conversion ratio in the laying period at 22, 23, 24, and 25 weeks.
Table 4. Evaluation of egg quality in the laying period at 22, 23, 24, and 25 weeks.
Fig. 1. The activities of SOD, MDA, and GPx in the laying period at (a)–(c) 22 weeks and (d)–(f) 25 weeks. Values are expressed in mean ± standard deviation (n=4 laying hens for each seven groups). Values are represented statistically a,b,c when compared with C-group value. SOD=Superoxide dismutase, MDA=Malondialdehyde, GPx=Glutathione peroxidase. DiscussionExtreme ambient temperatures are the most significant impediments to the poultry industry in tropical areas, presumably due to hens cannot release the heat produced during feeds adequately, resulting in decreased feed intake and reduced weight gain or laying of eggs (He et al., 2018; Dameanti et al., 2020). Stress responses are thought to be primarily adaptive or protective, and hence should prevent or mitigate the negative effects of the stressor inflicted on the animal. Extreme ambient temperatures not only alter performance indicators but also necessitate a variety of physiological and immunological changes in hens (Pawar et al., 2016). Temperatures above the thermoneutral zone raise the internal temperature, triggering a series of reactions that lead to the neutralization of heat-induced physiological alterations (Agustin and Ningtyas, 2021; Hosseindoust et al., 2022). Hens are able to regulate their body temperature throughout the year due to their being homeothermic. However, their thermoregulatory systems only function well between 27.5°C and 37.7°C, or in the thermoneutral zone (Mascarenhas et al., 2021). In general, hens’ thermoregulation is similar to that of other avians; hens utilize salt glands, fat insulation, and plumage in this process. Additionally, due to their endotherms, hens may control their body temperature by producing heat inside. Some trimmed hens tried to compensate for their decreased ability to dissipate heat by vasodilating the superficial capillaries in their comb and wattle by increasing their panting and wing-spreading behaviors (Hidanah et al., 2023). The Krebs cycle, the pentose phosphate shunt pathway, the glycolysis pathway, and muscle activity are all catabolic mechanisms that help the hens body produce heat (Przybylski et al., 2022). The production of heat in hens is affected by enzyme, vitamin, and hormone concentrations, physical activity, oxygen intake, ambient temperature, and circadian rhythms. Extra heat is released into the environment by cellular transmission and vascular circulation to regulate the internal temperature and prevent hyperthermia (Taylor et al., 2014). This study demonstrated that dietary vitamin supplementation increases egg production during the laying phase. During the heat stress condition, there was a considerable decrease in egg production as well as feed conversion ratio (FCR) value (Torki et al., 2015). The negative effects on production performance caused by heat stress circumstances can be lessened by combining vitamins C and E into the diet. According to a previous study (Attia et al., 2016), vitamins E (125 IU/kg) and C (200 mg/kg) each individually increased egg production and FCR. In the present study, vitamin ESE and CE increased feed efficiency in egg production with ratio scores of 1.6 and 1.8, respectively. According to a previous study (Clark et al., 2019), laying hens with an FCR of < 1.80 ± 0.01 were classified as having high feed efficiency (HFE), whereas those with an FCR of < 2.02 ± 0.01 and < 2.31 ± 0.01 were classified as having medium feed efficiency (MFE) and low feed efficiency (LFE), respectively. It has been suggested that vitamin C contributes to bone maturation by enhancing the hydroxyproline synthesis necessary for collagen formation. Vitamin C supplements may be advantageous for preserving egg quality at extremes of temperature. Inferring that vitamin C is essential for the formation of eggshells, it is postulated that vitamin C increases 1,25-dihydroxycholecalciferol and accelerates calcium mobilization from bone (Garcia et al., 2013). In addition to neutralizing free radicals and producing dehydroascorbyl weak radicals, vitamin C also regenerates reduced glutathione in the cytoplasm and promotes the immune system (Kaźmierczak-Barańska et al., 2020). Additionally, vitamin C improves vitamin E’s functionality by diminishing tochoperoxyl radicals and recovering vitamin E (Chambial et al., 2013). The conversion of homocysteine to methionine, which is necessary for DNA repair and amino acid synthesis, requires folic acid. Folic acid can also eliminate free radicals from the body (Goossens et al., 2021). It has been demonstrated that administering a combination of vitamins rather than a single vitamin has a superior impact on reducing heat stress. It is known that vitamin C reduces the receptors for corticosteroids produced during stressful circumstances and performs an essential role in the reaction to stress (Hajati et al., 2015). In this study, laying hens subjected to heat stress were given dietary supplements of vitamin C and vitamin E in combination with selenium and folic acid. The yolk yield and total solid content had been improved by selenium supplementation, and selenium deposition grew with the age of the white layers (Muhammad et al., 2021). In the previous study, dietary supplements of 4 mg/kg diet of folic acid were used to reliably increase the amount of folate in laying hens’ eggs throughout their egg-laying period (Mas’ad et al., 2020; Sun et al., 2021). Using data compiled from many experiments, researchers examined the effects of dietary folic acid addition to basal and purified diets (Li et al., 2021). The ratios of plasma and yolk folates are proportional over the range of dietary folic acid levels examined (0 to 7 mg/kg), the saturation level of egg folate content is not an indication of limitations in transport pathways from the plasma to the egg yolk (He et al., 2023). The egg weight, eggshell thickness, albumen index, yolk index, and HU quality parameters were all significantly enhanced as a result. The addition of vitamin E to the diet seems to be more beneficial for laying hens under heat stress due to its concurrent function as a reproductive component (Karami et al., 2018; Pratama et al., 2021). The alternative pathway involves the oxidation of oxygen-derived free radicals, such as superoxides (O2-), mono-oxygen (O-), and hydroxyl (OH-), as well as the transport of radical analogs from lipid components to an aqueous segment (Radi, 2018). In order to carry out this activity, the vitamin interacts in alignment with other protective enzymes such as GPx, SOD, MDA, and catalase (CAT). Vitamin C supplementation significantly decreased the degree of SOD oxidation and hydrogen peroxide’s (H2O2) stimulation of proteolytic activity (Min et al., 2018). The vitamin performs a non-enzymatic process as part of its function as a scavenger of free radicals in cellular membranes to assist in the conversion of vitamin E’s oxidized form to its stabilized component. Vitamin E has also been demonstrated to be an antioxidant, similar to vitamin C, which neutralizes free radicals formed in cellular membranes that cause tissue deterioration. The vitamin engages in a three-way interaction with selenium, acting as the protagonist in the enzyme GPx, meanwhile, polyunsaturated fatty acids act as the antagonist (Savio et al., 2021). ConclusionIn particular, AE, CE, and ESE as multivitamin combinations can improve feed intake, FCR, egg quality, and antioxidant activity. The eggshell thickness, yolk index, albumen index, and HU were shown to have improved similarly in the CE, ESE, and CAF groups. Additionally, it should be noted that the ESE and CAF groups ameliorated SOD, MDA, and GPx levels more gradually than the other groups. Depending on the dose and period of peak production, multivitamin combinations might be applied on laying hens under heat stress at 40.9oC ± 1.04oC. AcknowledgmentThe authors acknowledge the Dean of the Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, and Mr. Suyanto, owner of the private pullet breeding farm for providing facilities during the study. Conflict of interestThe authors declare that they have no competing interests. FundingThis study received no specific grant from funding agencies, the government, and the public community. Authors contributionFF and MTEP: Conceptualized and designed the study. WKD, BSPA, AP, and STM: Reared the laying hens and observed egg qualities. MTEP, FF, and STM: Designed the hencoop condition. FF, MTEP, and WKD: Quantified the antioxidant activities. AP and STM: Helped in the visualization and validation of tables and figures. FF, MTEP, and HÇ: Helped in data curation and analysis. MTEP and HÇ: Drafted the manuscript. FF, MTEP, and HÇ: Revised and submitted the manuscript. All authors read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAgustin, A.L.D. and Ningtyas, N.S.I.I. 2021. Titer antibody of newcastle disease in layer chicken in Narmada District, West Lombok. J. Med. Vet. 4(1), 98–109. Ajakaiye, J.J., Cuesta-Mazorra, M. and Garcia-Diaz, J.R. 2011. Vitamins C and E can alleviate adverse effects of heat stress on live weight and some egg quality profiles of layer hens. Pak. Vet. J. 31(1), 45–49. Attia, Y.A., El-Hamid, A.B.D., Abedalla, A.A., Berika, M.A., Al-Harthi, M.A., Kucuk, O., Sahin, K. and Abou-Shehema, B.M. 2016. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. SpringerPlus 5(1), 1–12. Chambial, S., Dwivedi, S., Shukla, K.K., John, P.J. and Sharma, P. 2013. Vitamin C in disease prevention and cure: an overview. Ind. J. Clin. Biochem. 28(1), 314–328. Clark, C.E., Akter, Y., Hungerford, A., Thomson, P., Islam, M.R., Groves, P.J., O’Shea and C.J. 2019. The intake pattern and feed preference of layer hens selected for high or low feed conversion ratio. PLoS One 14(9), e0222304. Collier, R.J. and Gebremedhin, K.G. 2015. Thermal biology of domestic animals. Ann. Rev. Anim. Biosci. 3(1), 513– 532. Dameanti, F.N.A.E.P., Firdaus, M.A., Titisari, N., Aditya, S. and Guritno, I. 2020. The Effect of environmental factors on the productivity of kampong chicken eggs Balitbangtan (KUB) layer phase. J. Med. Vet. 3(2), 166–172. Dangi, S.S., Gupta, M., Dangi, S.K., Chouhan, V.S., Maurya, V.P., Kumar, P., Singh, G. and Sarkar, M. 2015. Expression of HSPs: an adaptive mechanism during long-term heat stress in goats (Capra hircus). Int. J. Biometeorol. 59(1), 1095–1106. Dewi, W.K., Fikri, F., Purnomo, A., Chhetri, S., Maslamama, S.T. and Purnama, M.T.E. 2023. Efficacy of vitamin C and E on the level of antioxidative stress and steroid hormones in Albino rats with environmental stress. Ind. Vet. J. 100(1), 16–19. Garcia, A.F.Q.M., Murakami, A.E., do Amaral Duarte, C.R., Rojas, I.C.O., Picoli, K.P. and Puzotti, M.M. 2013. Use of vitamin D3 and its metabolites in broiler chicken feed on performance, bone parameters and meat quality. Asian-Australas. J. Anim. Sci., 26(3), 408. Goossens, J.F., Thuru, X. and Bailly, C. 2021. Properties and reactivity of the folic acid and folate photoproduct 6-formylpterin. Free Radical Biol. Med. 171(1), 1–10. Gouda, A., Amer, S.A., Gabr, S. and Tolba, S.A. 2020. Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop. Anim. Health Prod. 52(1), 2987–2996. Habibian, M., Sadeghi, G., Ghazi, S. and Moeini, M.M. 2015. Selenium as a feed supplement for heat-stressed poultry: a review. Biol. Trace Elem. Res. 165(1), 183–193. Hajati, H., Hassanabadi, A., Golian, A., Nassiri-Moghaddam, H. and Nassiri, M.R. 2015. The effect of grape seed extract and vitamin C feed supplementation on some blood parameters and HSP70 gene expression of broiler chickens suffering from chronic heat stress. Ital. J. Anim. Sci. 14(3), 3273. He, S.P., Arowolo, M.A., Medrano, R.F., Li, S., Yu, Q.F., Chen, J.Y. and He, J.H. 2018. Impact of heat stress and nutritional interventions on poultry production. World’s Poult. Sci. J. 74(4), 647–664. He, X., Wang, J., Wang, Y., Wang, B., Zhang, J. and Geng, F. 2023. Quantitative lipidomic analysis of egg yolk, yolk granule, and yolk plasma. J. Food Compost. Anal. 115(1), 104880. Hidanah, S., Sabdoningrum, E.K., Chusniati, S., Nurliyani, Khairullah, A.R. and Nayan, N. 2023. Effectiveness of Phyllanthus niruri and Andrographis paniculata Extracts on Egg Quality in Laying Hens with Avian Pathogenic Escherichia coli. J. Med. Vet. 6(3), 48–54. Hidayatik, N., Purnomo, A., Fikri, F. and Purnama, M.T.E. 2021. Amelioration on oxidative stress, testosterone, and cortisol levels after administration of Vitamins C and E in albino rats with chronic variable stress. Vet. World. 14(1), 137–143. Hosseindoust, A., Kang, H.K. and Kim, J.S. 2022. Quantifying heat stress; the roles on metabolic status and intestinal integrity in poultry, a review. Domest. Anim. Endocrinol. 81(1), 106745. Karami, M., Torki, M. and Mohammadi, H. 2018. Effects of dietary supplemental chromium methionine, zinc oxide, and ascorbic acid on performance, egg quality traits, and blood parameters of laying hens subjected to heat stress. J. Appl. Anim. Res. 46(1), 1174–1184. Kaźmierczak-Barańska, J., Boguszewska, K., Adamus-Grabicka, A. and Karwowski, B.T. 2020. Two faces of vitamin C—antioxidative and pro-oxidative agent. Nutr. 12(5), 1501. Kilic, I. and Simsek, E. 2013. The effects of heat stress on egg production and quality of laying hens. J. Anim. Vet. Adv. 12(1), 42–47. Kucuk, O., Sahin, N. and Sahin, K. 2003. Supplemental zinc and vitamin A can alleviate negative effects of heat stress in broiler chickens. Biol. Trace Elem. Res. 94(1), 225–235. Lara, L.J. and Rostagno, M.H. 2013. Impact of heat stress on poultry production. Anim. 3(2), 356–369. Lestari, R.K., Watanabe, M., Imada, Y., Shiogama, H., Field, R.D., Takemura, T. and Kimoto, M. 2014. Increasing potential of biomass burning over Sumatra, Indonesia induced by anthropogenic tropical warming. Environ. Res. Lett. 9(10), 104010. Li, X., Zhang, Y., Jing, W., Tang, W., Xing, J. and Zhang, Y. 2021. Effects of folic acid supplementation to basal diets of broilers on growth performance, slaughter performance, IGF2 gene expression and methylation. Czech J. Anim. Sci. 66(12), 504–512. Mas’ad, K., Lokapirnasari, W.P., Arif, M.A.A., Soeharsono, S., Kurnijasanti, R. and Harijani, N. 2020. Potency of Probiotics on Feed Efficiency and Egg Mass in Laying Hens. J. Med. Vet. 3(2), 203–207. Mascarenhas, N.M.H., Furtado, D.A., de Souza, B.B., de Oliveira, A.G., da Costa, A.N.L., Feitosa, J.V., Calvacanti, C.R., Dornelas, K.C., Silva, R.S., and Rodrigues, R.C.M. 2021. Thermal environment characterization of laying hen-housing systems. J. Anim. Behav. Biometeorol. 10(2), 2208. Min, Y.N., Niu, Z.Y., Sun, T.T., Wang, Z.P., Jiao, P.X., Zi, B.B., Chen, P.P., Tian, D.L., and Liu, F.Z. 2018. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 97(4), 1238–1244. Moritz, B., Schmitz, A.E., Rodrigues, A.L.S., Dafre, A.L., and Cunha, M.P. 2020. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 85(1), 108459. Muhammad, A.I., Dalia, A.M., Loh, T.C., Akit, H., and Samsudin, A.A. 2021. Effect of organic and inorganic dietary selenium supplementation on gene expression in oviduct tissues and Selenoproteins gene expression in Lohman Brown-classic laying hens. BMC Vet. Res. 17(1), 1–15. Nouri, S., Ghalehkandi, J.G., Hassanpour, S., and Aghdam-Shahryar, H. 2018. Effect of in ovo feeding of folic acid on subsequent growth performance and blood constituents levels in broilers. Int. J. Pept. Res. Ther. 24(1), 463–470. Panda, A.K. and Cherian, G. 2014. Role of vitamin E in counteracting oxidative stress in poultry. J. Poult. Sci. 51(2), 109–117. Pawar, S.S., Sajjanar, B., Lonkar, V.D., Kurade, N.P., Kadam, A.S., Nirmal, A.V., Pandit, B.M. and Bal, S.K. 2016. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 4(6), 332–341. Pratama, H.S., Lokapirnasari, W.P., Soeharsono, S., Al-Arif, M.A., Harijani, N. and Hidanah, S. 2021. Effect of probiotics Bacillus subtilis on feed efficiency and egg mass of laying hens. J. Med. Vet. 4(1), 37–41. Przybylski, W., Sałek, P., Kozłowska, L., Jaworska, D. and Stańczuk, J. 2022. Metabolomic analysis indicates that higher drip loss may be related to the production of methylglyoxal as a by-product of glycolysis. Poult. Sci. 101(2), 101608. Radi, R. 2018. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proceed. Nat. Acad. Sci. 115(23), 5839–5848. Rao, S.R., Prakash, B., Raju, M.V.L.N., Panda, A.K., Kumari, R.K. and Reddy, E.P.K. 2016. Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. Biol. Trace Elem. Res. 172(1), 511–520. Rayan, G.N., Mahrous, M.Y., Galal, A. and El-Attar, A.H. 2013. Study of some productive performance and egg quality traits in two commercial layer strains. Egypt. Poult. Sci. J. 33(2), 357–369. Rhoads, R.P., Baumgard, L.H., Suagee, J.K. and Sanders, S.R. 2013. Nutritional interventions to alleviate the negative consequences of heat stress. Adv. Nutr. 4(3), 267–276. Savio, L.E.B., Leite-Aguiar, R., Alves, V.S., Coutinho-Silva, R. and Wyse, A.T. 2021. Purinergic signaling in the modulation of redox biology. Redox Biol. 47(1), 102137. Sejian, V., Bhatta, R., Gaughan, J.B., Dunshea, F.R. and Lacetera, N. 2018. Adaptation of animals to heat stress. Anim. 12(2), 431–444. Sinkalu, V.O. and Ayo, J.O. 2018. Combined effects of retinol, ascorbic acid and α-tocopherol on diurnal variations in rectal temperature of Black Harco pullets subjected to heat stress. Int. J. Biometeorol. 62(1), 9–15. Sun, D., Jin, Y., Zhao, Q., Tang, C., Li, Y., Wang, H., Qin, Y. and Zhang, J. 2021. Modified EMR-lipid method combined with HPLC-MS/MS to determine folates in egg yolks from laying hens supplemented with different amounts of folic acid. Food Chem. 337(1), 127767. Taylor, N.A., Tipton, M.J. and Kenny, G.P. 2014. Considerations for the measurement of core, skin and mean body temperatures. J. Therm. Biol. 46(1), 72–101. Torki, M., Akbari, M. and Kaviani, K. 2015. Single and combined effects of zinc and cinnamon essential oil in diet on productive performance, egg quality traits, and blood parameters of laying hens reared under cold stress condition. Int. J. Biometeorol. 59(1), 1169–1177. Vandana, G.D., Sejian, V., Lees, A.M., Pragna, P., Silpa, M.V. and Maloney, S.K. 2021. Heat stress and poultry production: impact and amelioration. Int. J. Biometeorol. 65(2), 163–179. | ||
| How to Cite this Article |
| Pubmed Style Dewi WK, Aji BSP, Fikri F, Purnomo A, Maslamama ST, Calışkan H, Purnama MTE. Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. Open Vet. J.. 2024; 14(8): 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 Web Style Dewi WK, Aji BSP, Fikri F, Purnomo A, Maslamama ST, Calışkan H, Purnama MTE. Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. https://www.openveterinaryjournal.com/?mno=198554 [Access: January 10, 2026]. doi:10.5455/OVJ.2024.v14.i8.13 AMA (American Medical Association) Style Dewi WK, Aji BSP, Fikri F, Purnomo A, Maslamama ST, Calışkan H, Purnama MTE. Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. Open Vet. J.. 2024; 14(8): 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 Vancouver/ICMJE Style Dewi WK, Aji BSP, Fikri F, Purnomo A, Maslamama ST, Calışkan H, Purnama MTE. Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. Open Vet. J.. (2024), [cited January 10, 2026]; 14(8): 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 Harvard Style Dewi, W. K., Aji, . B. S. P., Fikri, . F., Purnomo, . A., Maslamama, . S. T., Calışkan, . H. & Purnama, . M. T. E. (2024) Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. Open Vet. J., 14 (8), 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 Turabian Style Dewi, Winda Kusuma, Bondan Sigit Purnomo Aji, Faisal Fikri, Agus Purnomo, Salipudin Tasil Maslamama, Hakan Calışkan, and Muhammad Thohawi Elziyad Purnama. 2024. Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. Open Veterinary Journal, 14 (8), 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 Chicago Style Dewi, Winda Kusuma, Bondan Sigit Purnomo Aji, Faisal Fikri, Agus Purnomo, Salipudin Tasil Maslamama, Hakan Calışkan, and Muhammad Thohawi Elziyad Purnama. "Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination." Open Veterinary Journal 14 (2024), 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 MLA (The Modern Language Association) Style Dewi, Winda Kusuma, Bondan Sigit Purnomo Aji, Faisal Fikri, Agus Purnomo, Salipudin Tasil Maslamama, Hakan Calışkan, and Muhammad Thohawi Elziyad Purnama. "Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination." Open Veterinary Journal 14.8 (2024), 1850-1857. Print. doi:10.5455/OVJ.2024.v14.i8.13 APA (American Psychological Association) Style Dewi, W. K., Aji, . B. S. P., Fikri, . F., Purnomo, . A., Maslamama, . S. T., Calışkan, . H. & Purnama, . M. T. E. (2024) Strategies to combat heat stress in Isa Brown layer hens: Unveiling the roles of vitamin A, vitamin E, vitamin K, vitamin C, selenium, folic acid, and in combination. Open Veterinary Journal, 14 (8), 1850-1857. doi:10.5455/OVJ.2024.v14.i8.13 |