| Research Article | ||
Open Vet. J.. 2024; 14(8): 1905-1911 Open Veterinary Journal, (2024), Vol. 14(8): 1905–1911 Research Article Epidemiological and pathomorphologic investigation of peste des petits ruminants in Western Algeria: A comprehensive study of clinical and histopathological findingsKhaldia Merdja1,2, Houari Hemida1* and Assia Boumezrag1,21Institute of Veterinary Sciences; University of Tiaret, Tiaret, Algeria 2Laboratory for Improvement and Valorization of Local Animal Production, University of Tiaret, Tiaret, Algeria *Corresponding Author: Houari Hemida. Institute of Veterinary Sciences, University of Tiaret, Tiaret, Algeria. Email: hemida.houari [at] univ-tiaret.dz Submitted: 24/04/2024 Accepted: 27/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: This study delves into the epidemiology and pathomorphologic characteristics of peste des petits ruminants (PPR) in western Algeria, a viral disease that constantly threatens small animals in Africa, the Middle East, and Asia. Aim: The purpose of this investigation was to evaluate the epidemiology of PPR in western Algeria and to understand the pathomorphological lesions in naturally infected small ruminants. Methods: An online survey conducted via google forms and shared with veterinarians in the wilaya of Tiaret, provided insights into the prevalence and clinical manifestations of PPR. A comprehensive examination of organs was conducted and representative tissue samples from the lungs, trachea, thymus, spleen, liver, kidney, heart, tongue, stomach, different parts of the small and large intestine, and mesenteric lymph nodes were collected and the specimen was fixed in a 10% neutral buffer formalin solution. Results: Among 2,200 small ruminants managed by expert veterinarians, 192 small ruminants exhibited clinical signs compatible with PPR, and 79 dead animals. Among the 31 sick young small ruminants, eight were confirmed to be infected with the PPR virus. Necropsies of affected animals revealed significant gross lesions in organs such as the lungs, intestines, spleen, and lymph nodes. Histopathological analysis further illuminated the severity of lesions, including interstitial pneumonia, syncytial cell formation, and severe gastroenteritis. Conclusion: The study’s comprehensive approach, encompassing epidemiological data, necropsy findings, and histopathological insights, contributes valuable knowledge for understanding and managing PPR outbreaks. The pathological lesions observed in this study exhibited consistency with those previously documented in experimental studies, thereby providing support for the diagnosis based on clinical signs and disease history. Keywords: Peste des petits ruminant, Epidemiology, Pathology, Lymphoid tissue, Algeria. IntroductionPeste des petits ruminants (PPRs) are a severe and highly contagious viral infection that affects goats and sheep, leading to significant morbidity (Fallahi, 2017). Furthermore, it continues the mortality of millions of sheep and goats each year and poses an ongoing risk to the livelihood of subsistence farmers in numerous African, Middle Eastern, and Asian nations (Dundon et al., 2020). The cause of this disease is the PPR virus (PPRV), which is a morbillivirus belonging to the Paramyxoviridae family. It is genetically similar to the rinderpest virus (RPV), measles virus (MV), and canine distemper virus (CDV) (Pastoret et al., 2006). The virus is present as a single serotype and can be classified into four separate lineages (I, II, III, and IV) based on sequence comparison of short segments of the F gene (Shaila et al., 1996) or N (Kwiatek et al., 2007). It is a very destructive viral illness that has severe economic impacts on tiny ruminants. The PPR Global Control and Eradication Strategy (PPR-GCES) was initiated by the Food and Agriculture Organization of the United Nations (FAO) and the World Organisation for Animal Health (WOAH) in April 2015. Its objective is to achieve worldwide eradication of PPRs by the year 2030 (FAO, 2015). There is a powerful and durable international agreement to eliminate the disease to safeguard the well-being of the most impoverished populations worldwide (Njeumi et al., 2020). PPR pathology is marked by regressive and necrotic alterations in the digestive and respiratory tract lymphoid tissues and epithelial cells (Balamurugan et al., 2014). In Algeria, PPR is considered endemic with nine (9) episodes reported over the last 13 years, including the most recent in 2022. The potential risk factors contributing to the spread and persistence of the disease are the movement of animals through 6,343 km of land borders shared with seven neighbor countries, especially in the absence of rigorous application of control measures. Moreover, there are many weekly livestock markets (Souk), where animals are bought and sold on local markets during the whole week without proper veterinary inspection, contributing to the rapid spread of PPR due to the close and repeated contact between animals from different locations and when infected animals are unwittingly introduced into new areas. Insufficient or infrequent vaccination programs (limited vaccine quantity and inappropriate application), can result in low levels of immunity in the livestock population. This increases the susceptibility of livestock to PPR outbreaks and allows the virus to persist in this region. This investigation aimed to evaluate the epidemiology of PPR in western Algeria and to understand the pathomorphologic lesions in naturally infected small ruminants. Materials and MethodsSurveyAn epidemiological study was realized through an electronic survey using Google Forms and shared with veterinarians in different areas of the wilaya (province, plural wilayas) of Tiaret. This survey examined the overall population of small ruminants on the farm, the age distribution of these animals, the specific symptoms of the disease, the number of sick animals showing clinical manifestations compatible with PPR, and the number of dead animals. Also, when an outbreak of PPR first appeared, we contacted government veterinarians to find out about the circumstances surrounding the outbreak. Subsequently, we contacted field veterinarians to facilitate the recovery of cadavers from small ruminants infected by these diseases. Macroscopical studyFollowing the recovery of the corpses of small ruminants infected by this disease. A comprehensive examination of all organs was conducted, with a special intention taken to the lung, trachea, different parts of the small and large intestine, and mesenteric lymph node (MLN). Representative tissue samples from the lungs, trachea, thymus, spleen, liver, kidney, heart, tongue, stomach, different parts of the small and large intestine, and MLNs were collected and the specimen was fixed in a 10% neutral buffer formalin solution. Histopathological studyFollowing fixation, the trimmed tissue is subjected to the standard processing procedures and finally embedded in paraffin wax. The sections were cut at a thickness of 5 μm and then stained using hematoxylin and eosin (H&E), according to the conventional method described by (Suvarna et al., 2018). The stained sections were observed using a light microscope equipped with a digital camera with a resolution of 16.1 megapixels, and images were subsequently recorded. Ethical approvalNot needed for this study. ResultsResults of the surveyThe obtained results of the epidemiological survey showed that most veterinarians practice on ruminants, and the majority of those who completed the survey have been practicing veterinary medicine for more than 5 years. All the veterinarians interviewed encountered cases of the disease through clinical suspicion. Major symptoms of disease observed by veterinarians are respiratory and digestive signs and the majority of PPR cases were in the acute stage. Based on the field veterinarian’s findings, younger animals between the ages of 4 and 12 months were more susceptible to PPRs. Also, it has been demonstrated that out of 2,200 small ruminants managed by twenty field veterinarians in different areas of the wilaya of Tiaret, 1,400 (64%) sheep and 800 (36%) goats, 192 small ruminants showed clinical signs compatible with PPR and the number of dead animals is 79. During this period, 31 sick young small ruminants (<6 months) showed clinical signs (fever (41°), oculo-nasal discharge, catarrhal conjunctivitis, dyspnea, and severe diarrhea) suspected to be infected with PPRV and 22 dead animals. The local veterinary services visited the outbreak and took eight blood samples and ocular discharge for subsequent confirmatory analysis. The results obtained from the central veterinary laboratory confirmed the presence of PPRV using Ag-ELISA in all analyses samples. Also, these results were reported by the WOAH. Necropsy findingsThe results of the clinical signs observed in the five lambs are shown in Table 1. PPR-affected animals showed high fever (41°C–42°C), and they exhibited symptoms of anorexia and depression, along with a dry muzzle. They showed also serous oculo-nasal discharge, that became mucopurulent in advanced stages, lacrimation and catarrhal conjunctivitis, dyspnea, and severe profuse watery to Muco-haemorrhagic diarrhea with dehydration, and finally death. Although necropsy of all dead animals was not possible, five PPR-affected lambs were subjected to a complete macroscopic examination, which revealed the following lesions: The oral cavity of lambs showed extensive erosion, ulcerative, necrosis, and fibrin deposits in the gingiva, tongue, and hard palate (Fig. 1). Important fibrin deposits have been also observed in the oesophageal mucosa. Diffuse congestion and hemorrhage have been observed in the intestines of examined lambs and are more severe in the large intestine. The corresponding MLNs showed hypertrophy with moderate congestion and hemorrhage (Fig. 2). Tracheal mucosa showed congestion and hemorrhage with foamy exudate (Fig. 3). The lungs showed red consolidated cranial lobes representing 25 % of areas of the lungs and mild congestion in the caudal lobes (Fig. 4). Additional lesions were: splenomegaly, brownish discoloration in the liver, and multifocal petechial hemorrhages in the myocardium. Table 1. Clinical history and distribution of lesions in PPR-infected lambs (n=5).
Histopathology findingsTracheal mucosa showed epithelial sloughing with hemorrhages and congestion in the layer of connective tissue, degeneration, and cystic lesions in the tracheal gland. The most important histopathological lesions were observed in the lungs, intestines, spleen, and lymph nodes. The lungs had the most severe histopathological abnormalities, which were characterized by edema in the alveoli combined with fibrinous exudate infiltration of mononuclear cells and thickening of the interalveolar septa (Fig. 5), presence of syncytial cells (Fig. 5). Intestines revealed ulcerations of the intestinal mucosa in some areas, loss of intestinal villi with the intestinal crypts appearing clear and loss of epithelium, the lamina propria was infiltrated with Macrophages and lymphocytes, the duodenum, jejunum, and ileum showed extensive effusion of the submucosa with progressive thickening of the submucosal layer (Fig. 6). Degeneration and necrosis of glandular epithelial cells were also seen. The lymphoid follicles in Peyer’s patches exhibited destruction, characterized by lympholysis (Fig. 6). MLNs showed severe congestion, hemorrhage, swelling, and depletion of lymphoid cells (Fig. 7). Additionally, other lesions are marked by the depletion of lymphocytes in the spleen, and histopathological changes in the heart, including congestion and hemorrhages. The thymus showed severe depletion of lymphocytes and severe congestion (Fig. 8). Furthermore, the liver showed severe congestion and hemorrhage with hepatocyte atrophy accompanied by the expansion of the sinusoidal spaces. The kidney exhibited slight glomerular atrophy, significant necrosis, and loss of epithelium in the renal tubules. DiscussionThe results of the veterinary survey indicate that animals in the younger age group, between 4 and 12 months, are more susceptible to PPRs. This finding is consistent with the findings of Chowdhury and his colleagues, who reported that young animals are more susceptible to PPR infection than older animals since maternally acquired antibodies only persist for up to four months (Chowdhury et al., 2014). In this study, the incidence of herd mortality was found to be in the range of 70%–100%. This is in contrast to the previously reported incidence of 23%–100%, by (Chowdhury et al., 2014) and is consistent with the incidence of 50%–80%, reported by Abu Elzein et al. (1990) and Taylor et al. (1990) (Abu Elzein et al., 1990; Taylor et al., 1990).
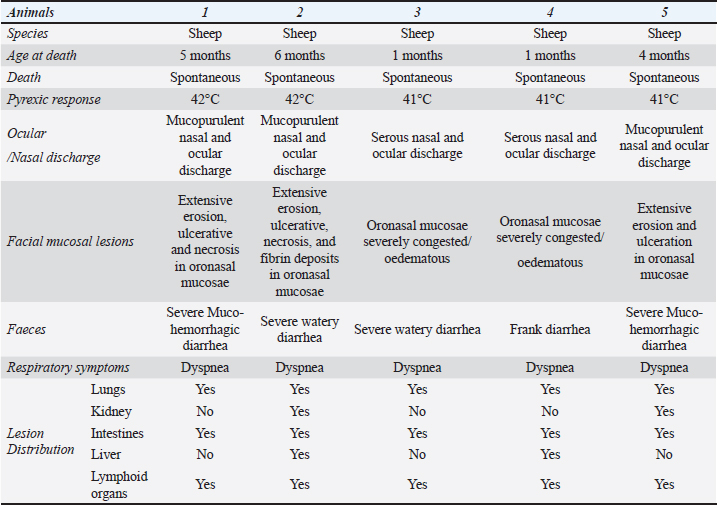
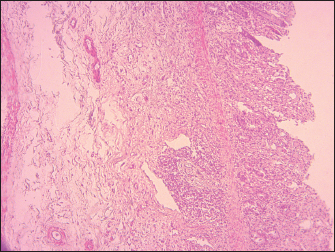
Fig. 1. Lamb. Oral cavity showing erosion, ulceration, necrosis, and fibrins deposit.
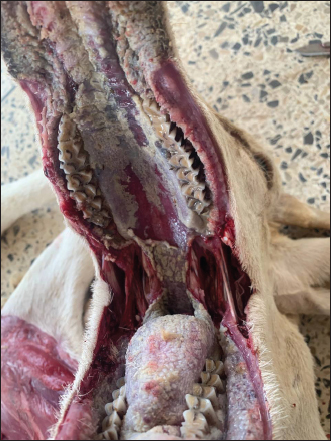
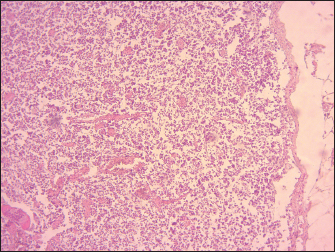
Fig. 2. Lamb. The MLN showing a mild congestion, hemorrhages, and swelling. Following the confirmation of a potential outbreak of pneumonia, a perifocal vaccination strategy was implemented to prevent further spread of the disease and delimit the outbreak. A global vaccination campaign subsequently took place across the entire wilaya, with active and regular surveillance efforts conducted to monitor the occurrence of further outbreaks. Algeria adheres to the recommendations of the World Organization for Animal Health regarding disease reporting and notification, animal movements, routine surveillance, and official mass vaccination. Given the circulation of the same virus in Algeria, Morocco, and Tunisia, it would be beneficial to implement a unified North African strategy to combat PPR. Our findings revealed significant macroscopic changes including extensive erosion, ulcerative, necrosis in the gingiva, tongue, and hard palate. After viremia, the virus reached the oral, nasal, and ocular mucosa, where it replicated extensively and shed virus into the environment, this led to necrosis and ulceration (Begum et al., 2021). Macroscopic findings of trachea, lungs, and small intestine found in this study indicated considerable macroscopic alterations including diffuse congestion, and hemorrhage found in the trachea and intestine, the lungs showed severe pneumonia. Our observation aligns closely with those reported by Wohlsein and Saliki (2006), who described necrotizing and ulcerative stomatitis and bronchointerstitial pneumonia (Wohlsein and Saliki, 2006). The extensive necrosis and ulceration indicate that the virus has directly caused cytopathic effects, resulting in significant epithelial damage, these lesions play a significant role in the clinical manifestations of the disease, including diarrhea, respiratory distress, mucopurulent nasal and ocular discharge, and severe ulcerations in the mouth and gastrointestinal tract. The results of the histopathological study revealed lympholysis and loss of lymphoid cells in Peyer’s patches, spleen, thymus, and lymph nodes of lambs affected by PPR.
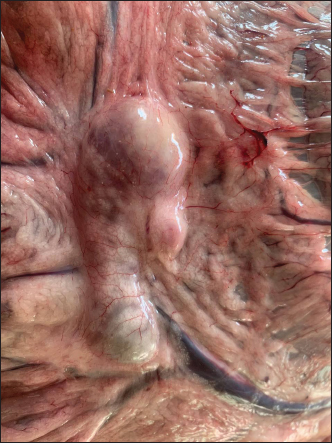
Fig. 3. Lamb. The tracheal mucosa showing foamy exudates and hemorrhages.
Fig. 4. Lamb. The lungs showing red and firm cranial lobes and emphysema, with mild congestion in the caudal lobes.
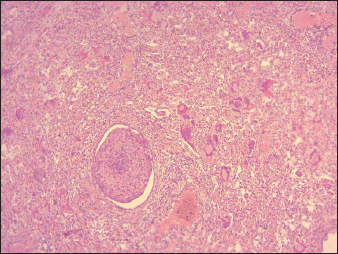
Fig. 5. Pulmonary parenchyma severely infiltrated with inflammatory cells (interstitial pneumonia) with a group of syncycial cells associated with severe bronchiolitis (40X, H&E). The lymphoid depletion observed in the present study can be attributed to a combination of direct viral cytopathic effects. These include the replication of PPRV within lymphocytes, which results in direct damage and death of these cells, and the immunosuppression resulting from lymphoid depletion predisposes the host to secondary infections, which can further destroy and deplete lymphoid tissues. These findings were consistent with a previous study (Pope et al., 2013; Rojas et al., 2016). According to Kul et al. (2007), there is a significant decrease (at least 25%) in the number of white blood cells in the bloodstream, known as leukopenia (Kul et al., 2007). It is well documented that the virus induces leukopenia by apoptosis in PBMCs in vitro (Mondal et al., 2001) and the viral proteins H, N, and P have a specialized role in inhibiting lymphoproliferation (Yokota et al., 2003). Additionally, they may induce autoimmunity by releasing nucleic acids and proteins from the cells (Cosby et al., 2006). Our study revealed the presence of severe broncho-pneumonia, characterized by thickening of the interalveolar septa and the presence of syncytial cells. These findings can be attributed to the extensive replication of the virus in the respiratory epithelium. The thickening of the alveolar septa indicates substantial tissue damage, which is likely attributable to direct viral cytopathic effects and the host’s immune response. Such damage may impair gas exchange, potentially leading to respiratory distress and in more severe cases, hypoxemia and death. PPRV is known to produce syncytia (fusion of cells) in Vero cells, a cell line derived from African green monkey kidney epithelial cells (Osman et al., 2019). Macroscopic findings of the lungs, trachea, and small intestine found in this study are mostly similar to those observed in previous studies reported by Begum et al. (2021) and Maina et al. (2015) in experimentally infected goats and sheep and Ugochukwu et al. (2019) in naturally infected sheep and goats.
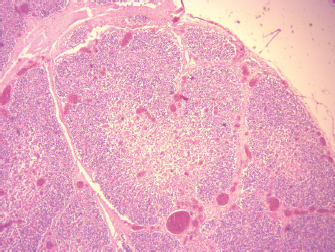
Fig. 6. Sheep, small intestine mucosa showing total loss of villi, ulceration, and inflammatory cell infiltration, thickening of submucosa by edema. Moderate depletion of Peyer’s patches (100X, H&E).
Fig. 7. Sheep, MLNs demonstrating severe lymphoid depletion mainly in the cortex with congestion and severe hemorrhage (100X, H&E).
Fig. 8. Sheep, thymus, severe congestion and depletion of lymphoid cells (40X, H&E). According to Rosemary et al., pneumonia and tracheal hemorrhage are likely a result of a downward upper respiratory infection of PPR, which has been further aggravated by a secondary bacterial infection. Additionally, the presence of lung congestion and “red hepatization” aroused suspicions of possible involvement of the virus (Kumar et al., 2004). In the intestine, particularly the ileum and colon, there was evidence of marked hemorrhagic and necrotic changes. The atrophy of intestinal villi could be attributed to the epitheliotropic characteristics of the PPRV (Maina et al., 2015) causing necrosis of epithelial cells. These lesions are a principal factor in the clinical presentation of severe muco-hemorrhagic diarrhea. The histopathological lesions of the intestine in the current study are in correlation with those reported by Begum et al. (2021) and Patel et al. (2017). The epidemiological survey highlighted several key findings regarding PPRs in the wilaya of Tiaret, Algeria. Notably, younger animals between 4 and 12 months old were found to be more susceptible to PPR, with major symptoms including respiratory and digestive signs. Necropsy findings revealed characteristic lesions in various organs, consistent with PPR infection, and histopathological examination further supported the diagnosis, with severe tissue damage observed in the lungs, intestines, spleen, and lymph nodes. The pathological lesions observed in this study exhibited consistency with those previously documented in experimental studies, thereby providing support for the diagnosis based on clinical signs and disease history. AcknowledgmentThe authors would like to thank all veterinarians who participated in the survey and all cooperating farmers. Authors’ contributionsHH, BA, and MK: contributed to the design of the study. HH and MK prepared, shared, and managed the survey and performed a pathological study. All authors drafted and revised the final version of the manuscript. Conflict of interestThe authors declare that there are no conflicts of interest related to this article. FundingThis research is a contribution to the project PRFU D01N01UN140120220003, funded by the DGRSTD-MESRS-ALGERIA. Data availabilityAll data are provided in the manuscript. ReferencesAbu Elzein, E.M., Hassanien, M.M., Al-Afaleq, A.I., Abd Elhadi, M.A., and Housawi, F.M. 1990. Isolation of peste des petits ruminants from goats in Saudi Arabia. Vet. Rec. 127(12), 309–310. Balamurugan, V., Hemadri, D., Gajendragad, M. R., Singh, R. K. and Rahman, H. 2014. Diagnosis and control of peste des petits ruminants: a comprehensive review. Virusdisease. 25(1), 39–56. Begum, S., Nooruzzaman. M., Islam, M. R. and Chowdhury, E. H. 2021. A sequential study on the pathology of peste des petits ruminants and tissue distribution of the virus following experimental infection of Black Bengal goats. Front. Vet. Sci. 8, 635671. Chowdhury, E.H., Bhuiyan, A.R., Rahman, M.M., Siddique, M.S. and Islam, M.R. 2014. Natural peste des petits ruminants virus infection in Black Bengal goats: virological, pathological and immunohistochemical investigation. BMC Vet. Res. 10(1), 1–10. Cosby, S.L., Kai, C. and Yamanouchi, K. 2006. Immunology of rinderpest—an immunosuppression but a lifelong vaccine protection. In Rinderpest and Peste des Petits Ruminants. Cambridge, MA: Academic Press, pp: 196–XI. Dundon, W.G., Diallo, A., and Cattoli, G. 2020. Peste des petits ruminants in Africa: a review of currently available molecular epidemiological data, 2020. Arch. Virol. 165(10), 2147–2163. Fallahi, R. 2017. Isolation and molecular diagnosis of Peste des petits ruminants (PPR) virus from contaminated areas in Iran. Iran. J. Vet. Sci. Technol. 9(1), 17–21. FAO, O. 2015. Global Strategy for the Control and Eradication of PPR. FAO and OIE. Kul, O., Kabakci, N., Atmaca, H.T. and Özkul, A. 2007. Natural peste des petits ruminants virus infection: novel pathologic findings resembling other morbillivirus infections. Vet. Pathol. 44(4), 479–486. Kumar, P., Tripathi, B.N., Sharma, A.K., Kumar, R., Sreenivasa, B.P., Singh, R.P., Dhar, P., and Bandyopadhyay, S.K. 2004. Pathological and immunohistochemical study of experimental peste des petits ruminants virus infection in goats. J. Vet. Med. B. Infect. Dis. Vet. Public Health 51(4), 153–159. Kwiatek, O., Minet, C., Grillet, C., Hurard, C., Carlsson, E., Karimov, B., Albina, E., Diallo, A. and Libeau, G. 2007. Peste des petits ruminants (PPR) outbreak in Tajikistan. J. Comp. Pathol. 136(2–3), 111–119. Maina, S., Gitao, C. and Gathumbi, P. 2015. Clinicopathological observations in sheep & goats exposed to lineage III peste des petits ruminants virus infection in kenya. JEBAS. 3(1),72–80. Mondal, B., Sreenivasa, B.P., Dhar, P., Singh, R.P. and Bandyopadhyay, S.K. 2001. Apoptosis induced by peste des petits ruminants virus in goat peripheral blood mononuclear cells. Virus Res. 73(2),113–119. Njeumi, F., Bailey, D., Soula, J.J., Diop, B. and Tekola, B.G. 2020. Eradicating the scourge of peste des petits ruminants from the world. Viruses. 12(3), 313. Osman, N.A., Portugal, R., Giesow, K. and Keil, G. M. 2019. Productive replication of peste des petits ruminants virus Nigeria 75/1 vaccine strain in vero cells correlates with inefficiency of maturation of the viral fusion protein. Virus Res. 269, 197634. Pastoret, P.P., Barrett, T. and Taylor, W.P. 2006. Rinderpest and peste des petits ruminants: virus plagues of large and small ruminants. Cambridge, MA: Academic Press. Patel, Y.R., Patel, J.M., Vihol, P.D., Kalyani, I., Solanki, J.B. and Panchal, P.P. 2017. Pathomorphological studies of peste des petits ruminants (ppr) in goats of Navsari and Valsad Districts, India. Int. J. Curr. Microbiol. Appl. Sci. 6(12), 238–244. Pope, R.A., Parida, S., Bailey, D., Brownlie, J., Barrett, T. and Banyard, A.C. 2013. Early events following experimental infection with peste-des-petits ruminants virus suggest immune cell targeting. PLoS One. 8(2), e55830. Rojas, J., Sevilla, N. and Martin, V. 2016. PPRV-induced immunosuppression at the interface of virus-host interaction. Br. J. Virol. 3(5), 140–160. Shaila, M., Shamaki, D., Forsyth, M.A., Diallo,A., Goatley, L., Kitching, R. and Barrett, T. 1996. Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 43(2), 149–153. Suvarna, K.S., Layton, C. and Bancroft, J.D. 2018. Bancroft’s theory and practice of histological techniques E-Book. New York, NY: Elsevier Health Sciences. Taylor, W., Al Busaidy, S. and Barrett, T. 1990. The epidemiology of peste des petits ruminants in the Sultanate of Oman. Vet. Microbiol. 22(4), 341–352. Ugochukwu, I.C., Ezeasor, C.K., Agina, O.A., Anyogu, D.C., Chukwudi, I.C., Idoko, S.I. and Ugochukwu, E.I. 2019. Peste des petits ruminants: aetiology, pathology, immunology, disease status in Africa, diagnosis, control, prevention and treatment: a review. Not. Sci. Bio. 11(1), 12. Wohlsein, P. and Saliki, J. 2006. Rinderpest and peste des petits ruminants—the diseases: Clinical signs and pathology. In Rinderpest and peste des petits ruminants. Cambridge, MA: Academic press, pp: 68-V. Yokota, Si., Saito, H., Kubota, T., Yokosawa, N., Amano, Ki. and Fujii, N. 2003. Measles virus suppresses interferon-α signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-α receptor complex. Virology 306(1), 135–146. | ||
| How to Cite this Article |
| Pubmed Style Merdja K, Hemida H, Boumezrag A. Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. Open Vet. J.. 2024; 14(8): 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 Web Style Merdja K, Hemida H, Boumezrag A. Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. https://www.openveterinaryjournal.com/?mno=198709 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.18 AMA (American Medical Association) Style Merdja K, Hemida H, Boumezrag A. Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. Open Vet. J.. 2024; 14(8): 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 Vancouver/ICMJE Style Merdja K, Hemida H, Boumezrag A. Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 Harvard Style Merdja, K., Hemida, . H. & Boumezrag, . A. (2024) Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. Open Vet. J., 14 (8), 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 Turabian Style Merdja, Khaldia, Houari Hemida, and Assia Boumezrag. 2024. Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. Open Veterinary Journal, 14 (8), 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 Chicago Style Merdja, Khaldia, Houari Hemida, and Assia Boumezrag. "Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings." Open Veterinary Journal 14 (2024), 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 MLA (The Modern Language Association) Style Merdja, Khaldia, Houari Hemida, and Assia Boumezrag. "Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings." Open Veterinary Journal 14.8 (2024), 1905-1911. Print. doi:10.5455/OVJ.2024.v14.i8.18 APA (American Psychological Association) Style Merdja, K., Hemida, . H. & Boumezrag, . A. (2024) Epidemiological and pathomorphologic investigation of peste des petits ruminants (PPR) in Western Algeria: A comprehensive study of clinical and histopathological findings. Open Veterinary Journal, 14 (8), 1905-1911. doi:10.5455/OVJ.2024.v14.i8.18 |