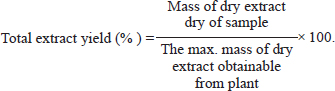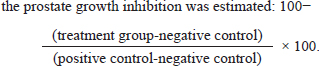| Research Article | ||
Open Vet. J.. 2024; 14(8): 1928-1935 Open Veterinary Journal, (2024), Vol. 14(8): 1928–1935 Research Article In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male ratsJamela K. Abd-Alhussen1, Masar J. Al-Kurdy2* and Suha A. Hussein11Basic Sciences Branch, College of Dentistry Medicine, University of Al-Qadisiyah, Diwaniyah, Iraq 2Community Health Technologies Department, Technical Institute of Al-Diwaniyah, AL-Furat AL Awsat Technical University, Diwaniyah, Iraq *Corresponding Author: Masar J. Al-Kurdy. Nursing Techniques Department, Technical Institute of Al-Diwaniyah, AL-Furat AL Awsat Technical University, Diwaniyah, Iraq. Email: dw.msr [at] atu.edu.iq Submitted: 08/05/2024 Accepted: 12/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: The most widespread condition that affected on primarily the male population is Benign hyperplasia of the prostate benign prostatic hyper-plasia (BPH). Flax seeds have been reported to have antiproliferation properties and exhibit antitumor. Aim: We assessed the impact of flax seeds ethanolic excerpt on BPH within a testosterone propionate (TP)-induced model of rats. Methods: A pre-3-week daily injection of TP (3 mg/kg BW) was used to induce BPH. Twenty male rats (200–240 gm) were randomly divided into 4 equal groups (n=5) negative Group under control was given PBS orally, corn oil S/C, BPH-induced rats received 3 mg/kg BW TP for 3 weeks, extract group received 50 mg/Kg extract twice daily for 2 weeks Finasteride group received standard drug 10 mg/Kg BW for 2 weeks. When the course of treatment is over, rats were sacrificed and the blood was collected and separated, the prostate of the rats was harvested for histological examination. Results: The results showed that flax seeds ethanolic extract could significantly (p < 0.05) reduce the prostate gland weight, prostate index, serum level of PAS, testosterone, and 5-a reductase enzyme in BPH-induced rats and improve the tissue morphology of the prostate. Conclusion: Based on our results, the extract suggested that have a promising role in the treatment of benign hyperplasia of the prostate. Keywords: Finasteride, Flax seeds, Prostatic hyperplasia, Testosterone, Tissue morphology. IntroductionProstate gland enlargement is one of the important and common urinary tract conditions affecting aging men, which may result in symptoms of the lower urinary tract (LUTS). This disease prevalence increases clearly with age (Csikós et al., 2021). Benign prostatic hyper-plasia (BPH) is a histological diagnosis indicating elevated in the size of noncancerous stromal, glandular tissue, and cell number, which is common in older men (Johnson and Mirk, 2024). It can be also associated with various other health outcomes, which affect more than 20% of American males between the ages of 30 and 79, or around 15 million men, and can have major detrimental effects on quality of life to varied degrees even though it is rarely fatal (Egan, 2016). Prostate enlargement typically affects the transitional zone, or periurethral zone, which includes both stromal and glandular tissue (Schweizer et al., 2019). Despite the fact that the etiology and the pathophysiological processes of BPH are still not entirely clear. It is widely known that the androgen system and androgen receptor (AR) play a significant function in the growth of BPE and LUTS, as well as how prostatic inflammation and certain metabolic factors may contribute to these conditions (Madersbacher et al., 2019). There are various methods for treating this issue, including surgery, with transurethral prostate resection being the most popular operation, and the use of drugs like finasteride and terazosin (Kortt and Bootman, 1996; Ghazi and Al-Bayati, 2020). Surgical procedures and 5-alpha-reductase inhibitors are two common therapies for prostatic enlargement, both of which have some evident negative effects. Contrarily, there is some data, albeit limited, that suggests alternate treatments are less dangerous and may also help BPH patients with their not only their sexual dysfunction but also their quality of life and LUTSs (Bhat et al., 2021). Linseeds, also known as one of the possible oil seeds contain high ratios of lignans, cyclic peptides, polysaccharides, alkaloids, cyanogenic glycosides, cadmium, and linolenic acid. Additionally, flax Seeds have nutritional and health-beneficial potential oil seeds (Shekhara et al., 2020). Flax is an annual plant in the linaceae family (Linum usitatissimum). Depending on the type of plant and the environmental factors it grew in, seeds have different chemical compositions. The seeds have about 40% lipids, 30% dietary fiber, and 20% proteins in them. The present work aimed to evaluate the effect of flax seed ethanolic extract on BPH in testosterone rat models triggered by propionate. Materials and MethodsExtraction methodThe flax seeds L. usitatissimum (Linn.) (1 kg) were purchased from local markets in Al-Diwaniya city. The flax seeds were dried and then ground into a coarse powder. Thereafter, using 500 ml of 99.6% ethanol as the extraction solvent for 72 hours, plant material (50 g) was packed into a Soxhlet apparatus thimble (Wisd, Korea). The extract was concentrated using a vacuum rotary evaporator (Labtech, Korea) and filtered through Whatman filter paper No. 1 at 90 rpm and 80°C. The extract was subsequently dried, and kept at 4°C in a refrigerator. To assess the total extract yield, five samples of the crude plant’s extract have been taken (Devi and Singh, 2017). Calculation of yieldThe total extract yield was calculated by the following formula.
Experimental animalsA total of twenty-four fully grown male Wister rats, eight weeks old, weighing between 200 and 240 g at birth, were utilized. They were acquired from the animal house of the College of Veterinary Medicine at Al-Qadisiyah University. Rats were placed in solid-bottomed plastic cages with easy access to regular laboratory food and water for seven days before the experiment began to help them acclimate. In our controlled room, the rats were kept in an air-conditioned environment with a temperature range of 23°C to 25°C, a 12-hour light/dark cycle, and a relative humidity of 40% to 60%. Ad libitum supplies of water and standard laboratory food were provided. BPH inductionBPH was induced in all experimental groups except the negative control group by subcutaneous injection in the inguinal region with 3 mg/Kg BW of exogenous testosterone propionate (TP) diluted with corn oil for 3 weeks, after those two rats were sacrificed and examined in order to examine the prostatic enlargement in detail. Experimental groupsThe rats were randomly divided into four equal groups of 6 animals each. The first group served as negative control and was given subcutaneous corn oil and oral PBS. The final three groups received subcutaneous injections of TP to cause BPH, which was further divided into Group II were given BPS orally as positive control (BPH group), and group III received extract 50 mg/kg twice daily for two weeks orally. group IV received finasteride drug 10 mg/Kg BW daily for two weeks orally. All treatment volumes of extract and standard drug were modified after weighing of each rat weekly. At the end of the experiments, all rats were weighed and then scarified via deeply anaesthetized with chloroform. The whole prostate was immediately separated, photographed, and weighed. A part of the ventral prostate lobes was embedded in paraffin and preserved with 10% neutral-buffered formalin for histomorphological analysis in each group. Using a microtome, the paraffin was divided into 4 µm thick sections and then stained with hematoxylin and eosin (H&E) solution. After that, tissues were covered with mounting media, mounted, and viewed under a microscope (Olympus, Tokyo, Japan). Three sections in each group were used for this purpose. Prostatic index (PI) and prostate growth inhibitionEach rat’s prostate index was calculated by multiplying the prostate weight (mg/g) by the total weight of the body. Using the following formula,
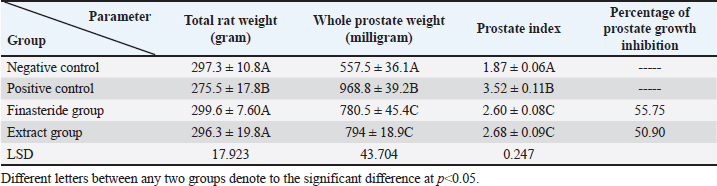
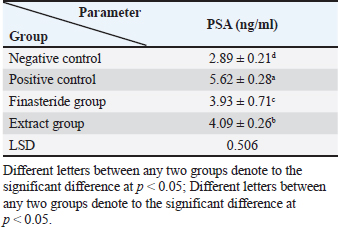
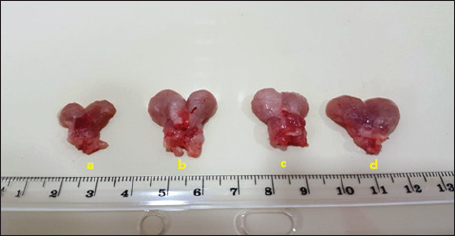
Serum analysisAt the time of sacrifice, entire blood samples were obtained by performing a cardiac puncture directly from the heart. Blood was placed into vacutainers without anticoagulants and left to clot for 30 minutes. Centrifugation at 1,500 g for 10 min was used to separate the serum. Assay of prostate-specific antigen (PSA), DHT, and 5-α-reductase enzymeThe enzyme immunoassay technique was used for the quantitative determination of PSA by using PSAspecific antigen ELISA kit (Ela Science Company Ltd., USA), Dihydrotestosterone (DHT) level by using rat DHT ELISA kit (Sunlong Biotech Company Ltd., China). 5-α-reductase enzyme by using rat steroid 5 alpha-reductase 2 (SRD5a2) ELISA kit (Sunlong Biotech Company Ltd., China). All ELISA procedures were performed according to the manufacturer’s protocols. Statistical analysisAll data were presented as means ± standard deviation (SD) values for six independent experiments. One-way ANOVA and the multiple comparison LSD test were used with SPSS (SPSS Inc., Chicago, IL) version 23 for Windows to determine the statistical significance between the groups. Differences were deemed significant when p<0.05 was reached. Ethical approvalThe Ethics Committee of the Thi-Qar Health Directorate, Al Habbobi Teaching Hospital has approved all treatments and experimental protocols. ResultsPlant extraction yieldThe yield percentage of flax seeds hydro-ethanolic extract of five crude material samples was 33.4% ± 5.35% at % a yield range of 28–41 for each 50 g of crude plant powder as shown in Table 1. Total body weight and prostate weightsThe total body weight changes of the rats in the control and the experimental groups are illustrated in Table 2. The final body weight decreases p < 0.05 significantly in the positive control group compared with other experimental groups. Other groups recorded no significant p > 0.05 differences in body weight among them. The whole prostate weight of the different studied groups is also illustrated in Table 2. Prostate weight of the PBH group (Positive groups) was significantly <0.05 increased (968.8 ± 39.2 g) compared to that of the normal group. At the same time, the prostate gland size in flax seed extract group and finasteride treatment group were significantly p<0.05 decreased than that of the BPH induction rats (Fig. 1). Prostate index significantly p < 0.05 increased in the BPH group (3.52 ± 0.11) when compared with negative control group (1.87 ± 0.06), whereas prostate index recorded (2.68 ± 0.09), (2.60 ± 0.08) in both flax seed extract and finasteride group, respectively. The percentage of prostate growth inhibition recorded 50.9, and 55.75 in both flax seed extract and finasteride treatment group, respectively, as shown in Table 2. PSA levelTable 3 shows the serum level of the PSA in the treated and control groups. There was marked significant p < 0.05 elevation in the serum PSA in the BPH induction rats 5.62 ± 0.28 ng/ml compared with negative control rats 2.89 ± 0.21 ng/ml. At the same time, finasteride and flax seed administrated rats exhibited a significant p < 0.05 reduction in PSA serum levels 3.93 ± 0.71 and 4.09 ± 0.26 ng/ml, respectively, as compared with the BPH induction group. However, the finasteride activity showed improved PSA better than flax seed extract activity. Table 1. Extraction ratio of flaxseeds extract.
Table 2. Effect of flaxseed ethanolic extract, finasteride on total body weight, prostate weight, prostate index, and percentage of prostate growth inhibition
Table 3. Effect of flaxseed ethanolic extract, finasteride on level of prostate-specific antigen in serum.
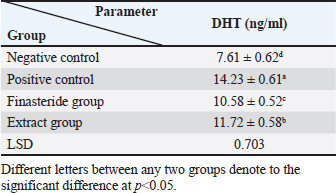
Table 4. Effect of flaxseed ethanolic extract, finasteride on level of DHT in serum
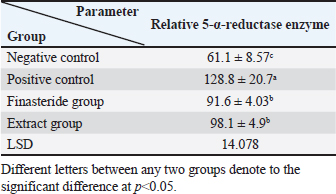
Table 5. Effect of flaxseed ethanolic extract, finasteride on level of 5-α-reductase enzyme in serum.
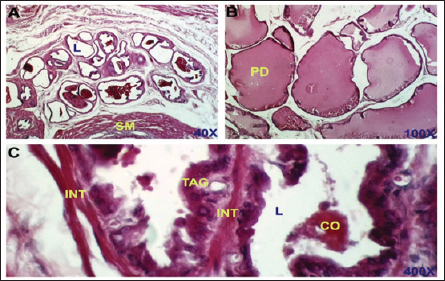
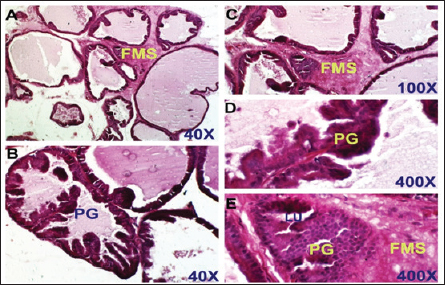
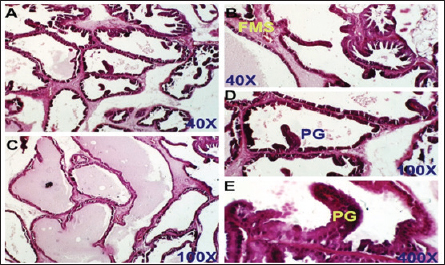
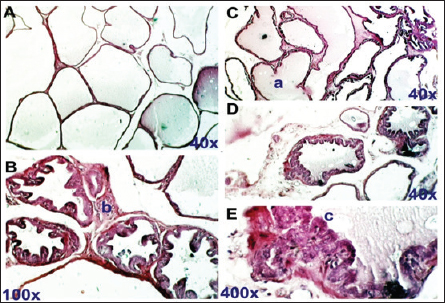
Dihydro-testosterone levelIn Table 4 the serum level of the DHT showed a significant p < 0.05 elevated in the serum DHT in the BPH group 14.23 ± 0.61 ng/ml when compared with negative control group 7.61 ± 0.62 ng/ml. At the same time, finasteride and flax seed administrated groups exhibited a significant p < 0.05 reduction in DHT serum levels (10.58 ± 0.52), (11.72 ± 0.58) ng/ml, respectively, as compared with the BPH group. However, the finasteride activity showed improved PSA better than flax seed extract activity. 5-α-reductase enzymeTable 5 showed significant p < 0.05 enzyme elevation in the serum level of the 5-α-reductase enzyme in the BPH induction rats 128.8 ± 20.7 when compared with negative control rats 61.1 ± 8.57. As well as, finasteride and flax seed administrated rats exhibited a significant p <0.05reduction in serum levels of the 5-α-reductase enzyme (91.6 ± 4.03), (98.1 ± 4.9), respectively, as compared with the BPH induction group. Microscopically changesTo verify the role of flax seeds, extract in mitigating prostatic hypertrophy, H&E staining was performed and histological changes were observed. Rats in the negative control group’s prostates displayed normal histological structure including tubule-alveolar gland (TAG), normal interstitial tissue (INT), corpora amylacea (CO), normal smooth muscle (SM), normal prostatic discharge (Fig. 2). As seen in Figure 3, the BPH-induced group’s prostates had hyperplastic epithelial cells, proliferating glands (PGs), thickened fibromuscular stroma (FMS), and destroyed lumen (LU). Finasteride treatment reduced the amount of epithelial cell hyperplasia in the prostates of the BPH-induced group and enhanced lacuna narrowing to the same degree as the control group (Fig. 4). In addition, showed normal prostate tissue at various zones, the amount of epithelial cell decreased in the treated group with flax seed extract. Figure 5 shows a typical prostatic epithelial lining, a normal LU filled with fluid, and a normal INT. DiscussionIn the last decades, phytochemical agents have been impacted signify as effective and safe remedial strategies for benign prostatic hyperplasia. The increment usage of nutraceuticals due to their no entirely effective treatment for the illness, this maneuver for overcoming the unwanted reactions linked with perilous hazardous synthetic drugs (Cai et al., 2018; Hwangbo et al., 2018). Conferred to the previous evidence, these natural source remedies can drive therapeutic effects not only by hormonal modulated mechanism but implicated as the anti-inflammatory/antioxidant effect, and it play a vital role in the ameliorate and amend degree severity of BPH symptomatic and consequences (Aryal et al., 2007; Morgia et al., 2013). The mismatch between cell division and apoptosis, inflammatory mediators, apoptotic processes, and adrenergic stimulation have been demonstrated to have significant involvement in the onset and development of BPH (Chen et al., 2016; Lee et al., 2017). In the current study, we aimed to detect the effects of using flax seed extract on male rats-induced BPH. The prostate grew larger in this induction BPH model as a result of the glandular and stromal prostatic tissue’s gradual hyperplasia. Afriyie et al. (2014) confirmed the influence of steroid hormones on prostate growth and the success of the establishment of the model and sharing common features with human and animal BPH (Afriyie et al., 2014). Most of the BPH models are induced by testosterone because the prostate gland is an androgen-dependent organ, and its growth and differentiation are controlled by androgen (Mori et al., 2009). These hormones when given in high doses and combination manner can induce changes in the prostate volume through autocrine and paracrine manner leading to the disturbance in both prostate cell proliferation and apoptosis (Choi et al., 2016). Through the data of the present study, it becomes clear that the administration of the flax seeds hydro-alcoholic extract and standard drug (finasteride) showed no significant elevation p < 0.05 in the prostate gland weight, prostate index, serum level of PAS, testosterone, 5-a reductase enzyme in BPH induced rats. Prostate gland volume is an important marker of BPH as well as PAP which serves as an important and clear indicator of BPH to reflect the prostate condition because its level is the highest in prostate tissue (Sarwar et al., 2017). DHT and testosterone, two male hormones, are directly linked to BPH. Testicular androgens are a well-known risk factor for the development of BPH; this is because these hormones are essential for controlling the proliferation and apoptosis of prostate gland cells (Lee et al., 2017). Steroid androgens influence cell signals through high affinity binds to AR, which translocate to the nucleus and bind to specific DNA binding sites, which results in increased transcription of androgen-dependent genes and ultimately stimulates of the protein synthesis and tissue involution (La Vignera et al.,2016). In addition to these direct effects, androgens play a role in regulating the expression/activity of several growth factors and their receptors as fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), and insulin-like growth factor (IGF-I) such action in the gland that has permissive effect in the transformation from normal tissue to the BPH or prostatic cancer (Ub Wijerathne et al.,2017). Although the precise mechanism of action of various flax seed extracts is still not fully understood, it has been reported that the therapeutic agents may also have pro-apoptotic, anti-estrogenic, and anti-inflammatory effects, as well as an inhibitory activity towards 5-reductase (Pal et al.,2019). The impact of flax seed or flax seed supplements on LUTS and BPH has been the subject of numerous investigations. After flax seed administration, Demark-Wahnefried et al. (2003) showed a significant decrease in total serum PSA and prostatic epithelial proliferation (Demark-Wahnefried et al., 2003). Another study showed that, in the rat BPH model, dietary flax seed supplementation for four weeks resulted in TP increased trend toward decreased prostate weights, decreased epithelial height, and decreased ventral prostatic epithelial cell proliferation (Said et al., 2015). Ren et al. demonstrated that Secoisolariciresinol diglucoside, a flax seed derivative, had an inhibitory effect on BPH. They showed that ENL, a mammalian metabolite of SDG, could bind to and activate GPER as well as one of its downstream substrates, ERK, which in turn prevented the proliferation of prostatic stromal WPMY-1 cells (Ren et al., 2016). Due to the up-regulation of cell cycle negative regulators like p21 and p53 and a decrease in the production of cyclin D1. One of the most crucial proteins involved in cell cycle progression, ENL prevented WPMY-1 cells from progressing past the G0/G1 phase (Ren et al., 2016). Ground flax seed and oils are rich in omega-3 fatty acids, which helps to reduce the influence of prostaglandins and leukotrienes on the inflammatory component of BPH. Whole flax seed seeds have the added benefit of lignan fibers, which help to bind estrogen in the gut and thereby promote estrogen removal. They consequently influence the development of BPH (Das et al., 2019).
Fig. 1. Prostate gland size in (a) control group, (b) positive control, (c) finasteride group, and (d) flaxseed extract group.
Fig. 2. Control negative group. section of the prostate gland shows normal TAG, normal INT, CO, normal SM, normal prostatic discharge (PD). H&E stain, (A)-40X. (B)-100X. (C)-400X.
Fig. 3. Control positive group. Section of prostate shows benign prostatic hyperplasia. There are PGs, thickening of the FMS, and obliterated LU. H&E stain, (A) and (B) 40X. (C) 100X. (D) and (E) 400X.
Fig. 4. Finasteride-treated group. section of the prostate gland shows almost normal prostatic epithelial layer, normal INT thickening, normal prostate fluid, and LU, but some fibro-mascularstroma (FMS), and few proliferated glands (PGs). H&E stain, (A) and (B) 40X. (C) and (D) 100X. (E) 400X.
Fig. 5. Flaxseeds treated group. Section of prostate gland shows normal prostate tissue at different zones. Normal LU fill with fluid (a), normal INT (b), and normal prostatic epithelial lining (c). H&E stain, (A), (C), and (D) 40X. (B) 100X. (E) 400X. ConclusionOur finding demonstrated that hydro-alcoholic flax seed extract can appreciably relieve the severity of BPH induced by high doses of TP compared to the finasteride treatment group and suggest a potential of flax seeds for a new pharmacotherapy treatment for BPH. AcknowledgmentThe authors extend their heartfelt gratitude to the Deanship of Scientific Research at the Technical Institute of Al-Diwaniyah, AL-Furat AL Awsat Technical University, for their unwavering support. FundingSelf-funded by authors. Authors’ contributionsJKA, MJA, and SAH were designed the experiments, and wrote the manuscript; JKA and MJA performed experiments and collected data, editing, and preparing the manuscript for journal submission. JKA, MJA, and SAH were checking the final approval of the version to be published. All authors read and approved the final manuscript. Conflict of interestThe authors declare that they have no competing interests. Data availabilityThe datasets used and analyzed during the current study are available from the corresponding author on reasonable request. ReferencesAfriyie, D.K., Asare, G.A., Bugyei, K., Adjei, S, Lin, J.M., Peng J and Hong, Z.F. 2014. Treatment of benign prostatic hyperplasia with Croton membranaceus in an experimental animal model. J. Ethnopharmacol. 18, 90–98. Aryal, M., Pandeya, A., Gautam, N., Baral, N., Lamsal, M., Majhi, S., Chandra, L., Pandit, R. and Das, B.K. 2007.Oxidative stress in benign prostate hyperplasia. Nepal Med. Coll. J. 9(4), 222–224. Bhat, S.A., Rather, S.A. and Islam, N. 2022. An overview of benign prostatic hyperplasia and its appreciation in Greco-Arab (Unani) system of medicine. Asian J. Urol. 9(2), 109–118. Cai, H., Zhang, G., Yan, Z. and Shang, X. 2018. The effect of Xialiqi capsule on testosterone-induced benign prostatic hyperplasia in rats. Evid. Based Complement Alternat. Med. 2018, 5367814. Chen, J., Zhang, H.F., Xiong, C.M. and Ruan, J.L. 2016. Inhibitory effect of diosgenin on experimentally induced benign prostatic hyperplasia in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 36(6), 806–810. Choi, H.M., Jung, Y., Park, J., Kim, H.L., Youn, D.H, Kang, J., Jeong, M.Y., Lee, J.H., Yang, W.M., Lee, S.G., Ahn, K.S. and Um, J.Y. 2016. Cinnamomi cortex (Cinnamomum verum) suppresses testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase. Sci. Rep. 6, 31906. Csikós, E., Horváth, A., Ács, K., Papp, N., Balázs, V.L., Dolenc, M.S., Kenda, M., Kočevar G. lavač, N. Nagy, M., Protti, M., Mercolini, L., Horváth, G. and Farkas, Á. 2021. On behalf of the oemonom. Treatment of benign prostatic hyperplasia by natural drugs. Molecules. 26(23), 7141. Das, K. and Buchholz, N. 2019. Benign prostate hyperplasia and nutrition. Clin. Nutr. ESPEN. 33, 5–11. Demark-Wahnefried, W., Robertson, C.N., Walther, P.J., Polascik, T.J., Paulson, D.F. and Vollmer, R.T. 2004. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 63(5), 900–904. Devi, S. and Singh, R. 2017. Antioxidant and anti-hypercholesterolemic potential of Vitis vinifera leaves. Pharma. J. 9(6), 807–814 Egan, K.B. 2016. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol. Clin. North Am. 43(3), 289–297. Ghazi, A.M. and Al-Bayati, M.A. 2020. Anti-proliferative of the phytosome propolis, phytosome lycopene and synergistic effect on the benign prostatic hyperplasia cells in-vitro. Plant Arch. 20, 6579–6589. Hwangbo, H., Kwon, D.H., Choi, E.O., Kim, M.Y., Ahn, K.I., Ji, S.Y., Kim, J.S., Kim, K.I., Park, N.J., Kim, B.H. and Kim, G.Y. 2018. Corni Fructus attenuates testosterone-induced benign prostatic hyperplasia by suppressing 5α-reductase and androgen receptor expression in rats. Nutr. Res. Pract. 12(5), 378–386. Johnson, T.M. and Mirk, A. 2024. BPH and male lower urinary tract symptoms. In Geriatric medicine. Cham, Germany: Springer, pp: 979–997. Kortt, M.A. and Bootman, J.L. 1996. The economics of benign prostatic hyperplasia treatment: a literature review. Clin. Ther. 18(6), 1227–1241. La Vignera, S., Condorelli, R.A., Russo, G.I., Morgia, G. and Calogero, A.E. 2016. Endocrine control of benign prostatic hyperplasia. Andrology, 4(3), 404–411. Lee, G., Shin, J., Choi, H., Jo, A., Pan, S., Bae, D., Lee, Y. and Choi, C. 2017. Cynanchum wilfordii ameliorates testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase and androgen receptor activities in a rat model. Nutrients. 9(10), 1070. Madersbacher, S., Sampson, N. and Culig, Z. 2019. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: a mini-review. Gerontology 65(5), 458–464. Morgia, G., Cimino, S., Favilla, V., Russo, G.I., Squadrito, F., Mucciardi, G., Masieri, L., Minutoli, L., Grosso, G. and Castelli, T. 2013. Effects of Serenoa repens, selenium and lycopene (Profluss®) on chronic inflammation associated with benign prostatic hyperplasia: results of “FLOG”(Flogosis and Profluss in Prostatic and Genital Disease), a multicentre Italian study. Int. Braz. J. Urol. 39, 214–221. Mori, F., Oda, N., Sakuragi, M., Sakakibara, F., Kiniwa, M. and Miyoshi, K. 2009. New histopathological experimental model for benign prostatic hyperplasia: stromal hyperplasia in rats. J. Urol. 181(2), 890–898. Pal, P., Hales, K., Petrik, J. and Hales, D.B. 2019. Pro-apoptotic and anti-angiogenic actions of 2-methoxyestradiol and docosahexaenoic acid, the biologically derived active compounds from flaxseed diet, in preventing ovarian cancer. J. Ovarian Res. 12, 1–16. Ren, G.Y., Chen, C.Y., Chen, W.G., Huang, Y., Qin, L.Q. and Chen, L.H. 2016. The treatment effects of flaxseed-derived secoisolariciresinol diglycoside and its metabolite enterolactone on benign prostatic hyperplasia involve the G protein-coupled estrogen receptor 1. Appl. Physiol. Nutr. Metab. 41(12), 1303–1310. Said, M.M., Hassan, N.S., Schlicht, M.J. and Bosland, M.C. 2015. Flaxseed suppressed prostatic epithelial proliferation in a rat model of benign prostatic hyperplasia. J. Toxicol. Environ. Health 78(7), 453–465. Sarwar, S., Adil, M.A.M., Nyamath, P. and Ishaq, M. 2017. Biomarkers of prostatic cancer: an attempt to categorize patients into prostatic carcinoma, benign prostatic hyperplasia, or prostatitis based on serum prostate specific antigen, prostatic acid phosphatase, calcium, and phosphorus. Prostate Cancer, 2017, 5687212. Schweizer, M.T., Antonarakis, E.S., Bismar, T.A., Guedes, L.B., Cheng, H.H., Tretiakova, M.S., Vakar-Lopez, F., Klemfuss, N., Konnick, E.Q., Mostaghel, E.A. and Hsieh, A.C. 2019. Genomic characterization of prostatic ductal adenocarcinoma identifies a high prevalence of DNA repair gene mutations. JCO Precis Oncol. 3, 1–9. Shekhara, N.R., Anurag, A.P., Prakruthi, M. and Mahesh, M.S. 2020. Flax seeds (Linum usitatissimmum): nutritional composition and health benefits. J. Nutr. Metab. Health Sci. 3, 35–40. Wijerathne, C.U., Park, H.S., Jeong, H.Y., Song, J.W., Moon, O.S., Seo, Y.W., Won, Y.S., Son, H.Y., Lim, J.H., Yeon, S.H. and Kwun, H.J. 2017. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol. Pharm. Bull. 40(12), 2125–2133. | ||
| How to Cite this Article |
| Pubmed Style Abd-alhussien JK, Al-kurdy MJ, Hussein SA. In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. Open Vet. J.. 2024; 14(8): 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 Web Style Abd-alhussien JK, Al-kurdy MJ, Hussein SA. In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. https://www.openveterinaryjournal.com/?mno=200828 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.21 AMA (American Medical Association) Style Abd-alhussien JK, Al-kurdy MJ, Hussein SA. In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. Open Vet. J.. 2024; 14(8): 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 Vancouver/ICMJE Style Abd-alhussien JK, Al-kurdy MJ, Hussein SA. In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 Harvard Style Abd-alhussien, J. K., Al-kurdy, . M. J. & Hussein, . S. A. (2024) In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. Open Vet. J., 14 (8), 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 Turabian Style Abd-alhussien, Jamela K., Masar J. Al-kurdy, and Suha A. Hussein. 2024. In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. Open Veterinary Journal, 14 (8), 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 Chicago Style Abd-alhussien, Jamela K., Masar J. Al-kurdy, and Suha A. Hussein. "In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats." Open Veterinary Journal 14 (2024), 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 MLA (The Modern Language Association) Style Abd-alhussien, Jamela K., Masar J. Al-kurdy, and Suha A. Hussein. "In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats." Open Veterinary Journal 14.8 (2024), 1928-1935. Print. doi:10.5455/OVJ.2024.v14.i8.21 APA (American Psychological Association) Style Abd-alhussien, J. K., Al-kurdy, . M. J. & Hussein, . S. A. (2024) In vivo assessment of the anti-benign prostatic hyperplasia effect of hot ethanolic extract of flax seeds in male rats. Open Veterinary Journal, 14 (8), 1928-1935. doi:10.5455/OVJ.2024.v14.i8.21 |