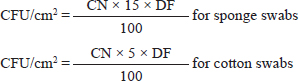| Research Article | ||
Open Vet. J.. 2024; 14(7): 1658-1667 Open Veterinary Journal, (2024), Vol. 14(7): 1658–1667 Research Article Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouseIsraa M. Jweer and Omar A. Al-Mahmood**Corresponding Author: Omar A. Al-Mahmood. Department of Veterinary Public Health, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: omar.a.almoula [at] uomosul.edu.iq Submitted: 17/05/2024 Accepted: 19/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
AbstractBackground: Meat contamination occurs in various ways, the most important of which are live animals before slaughter and the slaughter process (de-hiding and evisceration). For this, many substances were used that have an antimicrobial effect and can disinfect the surfaces of the carcass and extend its shelf life. Aim: This research aimed to study the efficiency of using some organic acids (lactic acid and beefxide) to reduce the microbial load (indicator microorganisms) on the surfaces of beef carcasses and some edible organs in the Mosul slaughterhouse. Methods: Two hundred sixty-four swabs (192 carcasses + 72 edible organ samples) were collected over the course of three months from the Mosul slaughterhouse in Nineveh Governorate between September 2023, and December 2023 (132 treated with organic acids and 132 not treated). The petrifilm method was used to detect indicator microorganisms in the samples. Results: Our results showed that the contamination rate in beef carcasses with generic Escherichia coli, coliforms, total coliform counts, and Enterobacteriaceae before treatment was 0.81, 1.22, 1.48, and 1.38 mean log colony forming unit (CFU/cm2), respectively. While the contamination rate in samples treated with organic acids for generic E. coli, coliforms, total coliform counts, and Enterobacteriaceae was −0.1, 0.31, 0.45, and 0.41 mean log CFU/cm2, respectively. Moreover, the level of contamination with indicator microorganisms in edible organs treated with organic acids was lower compared to untreated samples. Even though there was contamination with indicator microorganisms in the liver, heart, and kidney, there was no “significant” difference between them. Whereas there was no significant difference (p > 0.05) between lactic acid and beefxide solution in terms of reducing the rate of contamination of the indicator microorganisms in carcasses and the edible organs samples. Regarding the type of swabs used in the study, the results showed the effectiveness of sponge swabs, as the rate of microbial recovery (indicator microorganisms) was higher (p < 0.01) compared to cotton swabs. Conclusion: The study demonstrated the efficiency of using organic acids (lactic acid and beefxide solution) in reducing the microbial load to a level that does not cause diseases. Keywords: Indicator microorganisms, Organic acids, Beef, Slaughterhouse, Mosul. IntroductionMeat and its products are a large and important portion of the typical diet in many countries because they are linked to health, and cultural reasons. They are considered an essential source of animal protein necessary for the individual. From this standpoint, many countries are keen on the safety of food, including meat, which is considered of utmost importance to public health, especially when the environment of the slaughterhouses is highly contaminated (Soriyi et al., 2008). Meat contamination occurs in various ways, the most important of which are live animals before slaughter (infected animals), workers, and tools used in the slaughter process, and during the operations that take place after slaughter to prepare the carcasses (removing the skin and evisceration (Tiţa et al., 2020). Raw meat remains an essential source of many diseases caused by microbes that are transmitted to humans, and there is also difficulty in obtaining animal food free of germs despite decades of applying control procedures (Ruban and Fairoze, 2011). Foodborne illness and microbial spoilage of food occur due to failure or inability to control contaminants, such as foodborne pathogens, and organisms that spoil food at one or more stages of the food chain (Karanth et al., 2023). Indicator organisms were also used as signs that their presence in numbers exceeding the limits indicates the possibility of the emergence of pathogens, insufficient food processing hygiene, or poor quality of raw materials (Richiardi et al., 2023). The term “indicator organisms” has been used for nearly a century to evaluate the microbiological status of food production and food control systems, including assessing the quality or safety of raw or processed food products and verifying the effectiveness of microbial control measures (Schaffner and Smith, 2004). Due to the perishability of meat-based products, it is not easy to preserve them for a long time without using some appropriate processing and preservation techniques. Therefore, a wide range of new and advanced preservation, processing, and packaging techniques have been developed to maintain the quality of meat (Ubaid ur Rahman et al., 2018). Many chemicals are safe for food and humans and have an antimicrobial effect which were used because of their capability of disinfecting carcass surfaces for a long time and increasing the quality of meat and its shelf life (Saad et al., 2020). Treating the surfaces of the carcass with lactic acid has a positive result in inhibiting the growth of microorganisms. This is mainly due to the fact that organic acids tend to enhance the disruption of the driving force of the proton motive force created by microorganisms on the cell surface. This creates an unfavorable condition for the growth of microorganisms, as well as a lower pH of the surface to a level that is harmful to the microorganisms, especially bacteria, so that lactic acid can reduce or kill microorganisms on the surfaces of the carcasses (Van Ba et al., 2018). Among these chemicals and practices that use organic acids during the manufacturing process, which can greatly reduce the risk of infection with microbes, including Salmonella and Escherichia coli (Conner and Kotrola, 1995). Lactic acid, beefxide, and other organic acids were used in slaughterhouses in the United States of America, as part of the process of washing the carcass before cooling it, which led to improved quality and the safety of meat and meat products (USDA-FSIS, 2023). Wheeler and his colleagues stated that temperature and pH played a major role in the ability to inhibit microbes, and this was proven by the significant decrease in the numbers of E. coli when using lactic acid, beefxide, and peroxyacetic acid (Wheeler et al., 2014). Therefore, the study aimed to evaluate the efficiency of using lactic acid and beefxide to reduce the microbial load (indicator microorganisms) on beef carcasses in Mosul slaughterhouse. Materials and MethodsThe study included one slaughterhouse in Mosul city (Nineveh Governorate/Iraq), where 264 swabs of beef carcass and edible organs were collected from the Mosul slaughterhouse for the period extending from September 2023 to December 2023 through regular visits weekly (12 visits), over a period of three months. The total number of swabs for carcasses was 192 (96 swabs before treatment and 96 swabs after treatment), while the total number of swabs for edible organs was 72 (36 swabs before treatment and 36 swabs after treatment). Organic acids (2.5% lactic acid and 2.5% beefxide solution consisting of lactic acid and citric acid) were used for sample treatment. Two types of swabs were also used, 96 sterile sponge swabs (a special kit: Whirl-Pak® bag, gloves, and Butterfield’ Phosphate Buffer (World Bioproducts LLC, USA)) and 96 sterile cotton swabs (Bioline Diagnostics LLPs) were used. Swabs (100 cm2 per swab) were collected from each carcass (either the round or chuck area) or edible organs after wearing sterile gloves and preparing sponge and cotton swabs. A special sterile 10 x 10 cm (100 cm2) template (World Bioproducts LLC, USA) was used to mark the swabbing areas on the carcass, which were taken before treatment with organic acids. As for the method of taking the swab after treatment with organic acids, the carcass was sprayed with organic acid inside the slaughterhouse, then an hour later the swab was taken from the same carcass. The spraying method was directly on the carcass for 10 seconds and at a distance not exceeding 50 cm with low pressure using a manual sprayer. The swabbing process included 10 horizontal movements followed by 10 vertical movements for each site, after which the swab was placed in a sample bag (Whirl-Pak® bag, USA) for sponge swabs (USDA-FSIS, 2014), which contains 15 ml of phosphate buffer solution, or cotton swabs were placed in its special sterile tubes containing 5 ml of phosphate buffer solution with a unique identification code (Sample ID) for each sample. All samples were placed in a cooler container and transferred directly to the Veterinary Public Health Research Laboratory at the College of Veterinary Medicine/University of Mosul to perform the tests within a period not exceeding 4 hours. Serial dilution was performed on the cotton and sponge swabs samples after homogenizing the samples using the vortex mixer for 30 seconds. All treated and untreated samples were examined for indicators of microorganisms, which included (Generic E. coli, coliform, total coliforms, and Enterobacteriaceae) using 3M ™petrifilms (3M, USA). Then, took 1 ml of the dilutions and spread them using the spreader provided by the manufacturer on E. coli/coliform, and Enterobacteriaceae petrifilms and placed them in the incubator at 37°C for 24 hours. After 24 hours (according to the manufacturer’s instructions), the bacteria were counted, and the number was converted to colony forming unit (CFU/cm2) using the following equations: the detection limit for this method is −1.3 log.
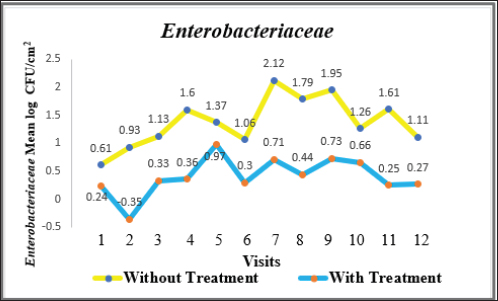
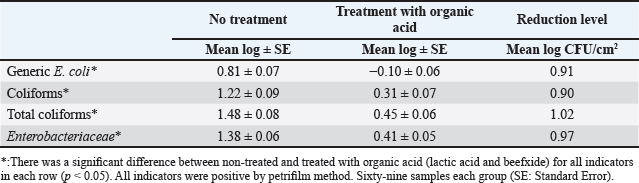
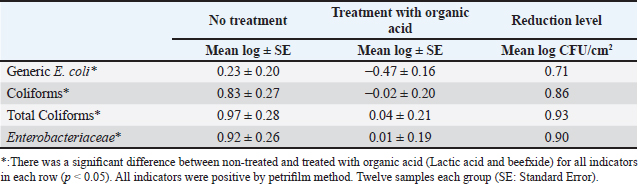
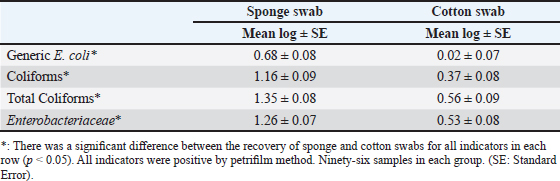
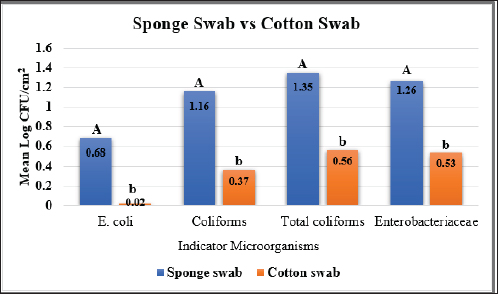
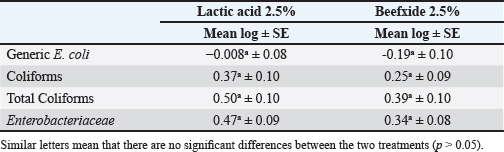
where CN stands for colony number on the petrifilm plate, and DF means the inverse of the dilution factor. The numbers 5 and 15 mean the amount of phosphate buffer solution placed in the sponge bag or cotton swab tube. The number 100 means the number of square centimeters (cm2) that were swabbed from the carcass or edible organs using sponge and cotton swabs. The number of colonies was then converted to a logarithmic number using an electronic calculator. Statistical analysisDescriptive and inferential statistical analysis was performed using JMP Pro16.1 software (2021 SAS Institute Inc., USA). The descriptive statistics calculated were the mean, the standard error, and the percentages. While the inferential statistics included determining the effect of independent variables [treatment with acids/non-treatment, type of sponge/cotton swab, edible organs (liver, kidney, and heart), visits] on generic E. coli, coliforms, total coliforms, and Enterobacteriaceae. The data were analyzed using analysis of variance (ANOVA). A one-way ANOVA was also used to test the difference between the means of two groups on a single dependent variable. While the Student's t-test and Duncan's test were used the multiple range test for comparison between groups taken from a normally distributed population. The results were significant at the probability level of (p < 0.05). Ethical approvalNot needed for this study. ResultsOur results showed that the contamination rate in beef carcasses with generic E. coli, coliforms, total coliform counts, and Enterobacteriaceae before treatment was 0.81, 1.22, 1.48, and 1.38 mean log CFU/cm2, respectively. While the contamination rate in samples treated with organic acids for generic E. coli, coliforms, total coliform counts, and Enterobacteriaceae was −0.1, 0.31, 0.45, and 0.41 Mean log CFU/cm2, respectively (Figs. 1–4; Table 1). In addition, the study demonstrated the efficiency of using organic acids in reducing (p < 0.05) the rate of contamination of all indicator microorganisms (used in the study) of carcass swab samples treated with organic acids compared to untreated samples during the study period. Moreover, the level of contamination with indicator microorganisms in edible organs treated with organic acids was lower compared to untreated samples (Tables 2–4). Even though there was contamination with indicator microorganisms in liver, heart, and kidney, there was no “significant” difference between them. Regarding the type of swabs used in the study, the results showed the effectiveness of sponge swabs, as the rate of microbial recovery (indicator microorganisms) was higher (p < 0.01) compared to cotton swabs (Table 5; Fig. 5). Lastly, there was no significant difference (p > 0.05) between lactic acid and beefxide solution in terms of reducing the rate of contamination of the indicator microorganisms in carcasses and edible organs samples, but both showed a significant reduction in all indicator microorganisms (Table 6). DiscussionMicroorganisms are present in all meat preparation and processing stations, which increases the importance of periodic monitoring of meat and processing facilities to ensure that carcasses and their meat are not contaminated with these organisms, especially the organisms that cause diseases (Matthews et al., 2019). The muscles are free of organisms before slaughtering (healthy animals), so handling and treating them in accordance with food safety practices keep the meat produced safe and hygienic after the slaughter process and has a longer shelf life (Santos et al., 2018), as some microorganisms cause spoilage of meat in addition to diseases. Microbial contamination of beef carcasses and other meat products may occur during slaughter and subsequent processing (Nakamura et al., 2023). Throughout the slaughter process, there may be microbial contamination introduced to the edible product from the skin, digestive tract, workers, and environment (Nakamura et al., 2023).
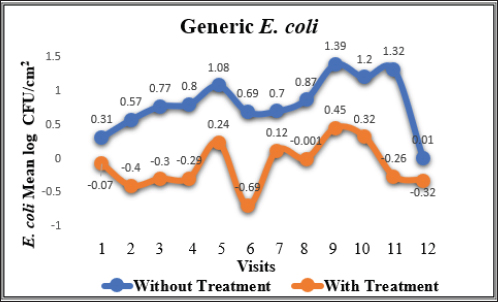
Fig. 1. Mean log of generic E. coli obtained from treated (organic acids) and non-treated beef carcasses throughout the study (12 visits). Eight samples per visit for each group. There was a significant difference between non-treated and treated with organic acid (p < 0.05) except visit 12.
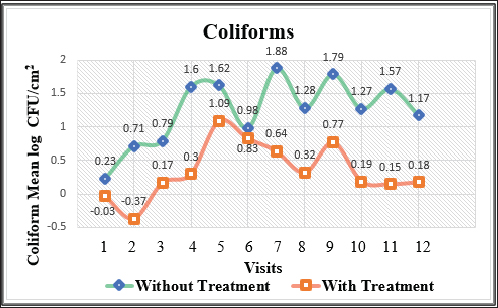
Fig. 2. Mean log of coliforms obtained from treated (organic acids) and non-treated beef carcasses throughout the study (12 visits). Eight samples per visit for each group. There was a significant difference between non-treated and treated with organic acid (p < 0.05) except visits 1, 5, and 6.
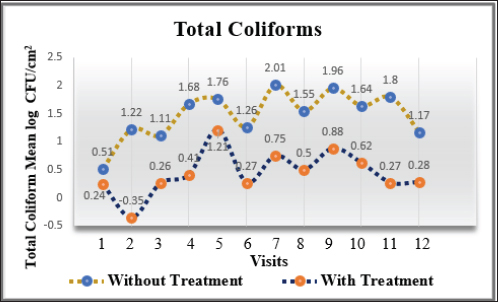
Fig. 3. Mean log of total coliforms obtained from treated (organic acids) and non-treated beef carcasses throughout the study (12 visits). Eight samples per visit for each group. There was a significant difference between non-treated and treated with organic acid (p < 0.05) except visits 1 and 5.
Fig. 4. Mean log of Enterobacteriaceae obtained from treated (organic acids) and non-treated beef carcasses throughout the study (12 visits). Eight samples per visit for each group. There was a significant difference between non-treated and treated with organic acid (p < 0.05) except visits 1, 5, 6, and 10. Table 1. Mean Log (CFU/cm2) of indicator organisms obtained from treated (organic acids) and non-treated beef carcasses.
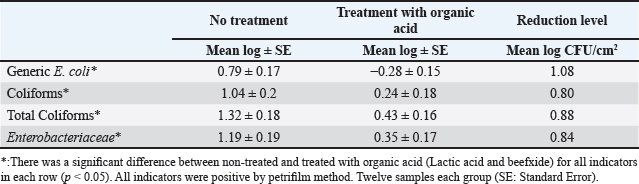
Table 2. Mean log (CFU/cm2) of indicator organisms obtained from treated (organic acids) and non-treated liver.
To our knowledge, the effect of organic acids on reducing the microbial load in beef carcasses or other animals in Iraq has not been studied. Therefore, we decided to study this important topic because it has a significant impact on public health in terms of positively reducing the microbial load and prolonging the shelf life of meat. So, our results showed that the indicator organisms (generic E. coli, coliforms, and Enterobacteriaceae) in samples not treated with organic acids were present on the carcass surfaces at a rate of 100%, but all numbers were less than the Maximum Acceptable Limits (E. coli < 2 log CFU/cm2) (USDA-FSIS, 2015), and Enterobacteriaceae (<2.5 log CFU/cm2) (EC, 2001). The reason for the low level of contamination may be due to the implementation of food safety practices in the Mosul slaughterhouse, where the slaughtering operations were supervised by a veterinary staff member who observed the slaughterhouse workers to carry out cleaning procedures for slaughtering tools or instruments (e.g., cleaning knives continuously), taking care not to cause any punctures in the bowels, and not to contaminate the carcass with the outer side of the skin. In addition to the fact that the slaughterhouse environment was relatively clean. Table 3. Mean log (CFU/cm2) of indicator organisms obtained from treated (organic acids) and non-treated heart.
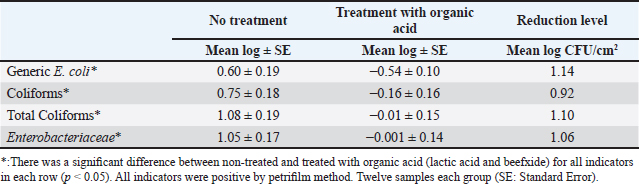
Table 4. Mean log (CFU/cm2) of indicator organisms obtained from treated (organic acids) and non-treated kidney.
Table 5. Mean Log (CFU/cm2) of indicator organisms recovered by sponge and cotton swabs.
To compare our findings with previous studies, our results revealed low indicator microorganisms compared to other local studies (Jawad and Abdul Alwahid, 2007; Hammadi, 2017; Al-Chalaby, 2020), as they showed an elevation in E. coli and coliforms. While our findings were similar to those studies conducted in different slaughterhouses around the world (Sofos et al., 1999; Wambui et al., 2018; Al-Mahmood et al., 2021), all studies showed low levels of indicator microorganisms in beef carcasses at the post-slaughter stage. In contrast, the results of researchers in Arab countries (Egypt and Saudi Arabia) (Omer et al., 2013; Khalafalla et al., 2016) showed higher contamination compared to our results. On the other hand, treatment with organic acids (lactic acid and beefxide solution) at a concentration of 2.5% proved effective in reducing the microbial load of indicator organisms in beef carcasses, as there was a significant decrease in all indicator organisms (E. coli, coliforms, total coliforms, and Enterobacteriaceae) in our study (overall mean), compared to samples that were not treated with organic acids. The reason why the indicators did not decrease significantly for some visits may be due to several reasons including: the fact that the first visit did not treat organic acids in a perfect manner, in addition to the confusion in the work, especially during the stage of spraying acids in the slaughterhouse; and the failure to control the necessary pressure when spraying in the other visits; and finally, it might be due to the contamination was relatively low, especially in the E. coli, which led to the fact that some visits did not have a “significant” difference, especially the last visit.
Fig. 5. Mean Log (CFU/cm2) of indicator microorganisms recovered by sponge and cotton swabs of beef carcasses throughout the study (12 visits). Different letters indicate that there is a significant difference between sponge and cotton swabs (p < 0.05) for each indicator. Table 6. The effect of using different organic acids (lactic acid 2.5% and beefxide 2.5%) on the mean log (CFU/cm2) of indicator organisms of beef carcasses.
Many previous studies have also shown the effectiveness of organic acids as antimicrobials in the processing of red meat (Hardin et al., 1995). Among those organic acids that are naturally present in meat is lactic acid, whose natural content in meat is 10 g/kg, which contributes to the tenderness of meat, in addition to its anti-bacterial effects and preservation of meat quality (Bolder, 1997). As for the beefxide solution, it also had a “significant effect in reducing the microbial load in carcass samples, and this was in agreement with” a study that demonstrated the reduction of E. coli on the surfaces of carcasses (Hendricks et al., 2014). Our results were similar to studies conducted by researchers in various countries of the world (Loretz et al., 2011; Wheeler et al., 2014; Gill et al., 2019; Casas, 2019) using organic acids, especially lactic acid, which led to a significant decrease in the number of indicator microorganism detected in beef carcasses using the petrifilm method. The effect of organic acids comes through penetrating the cell of microorganisms through diffusion, leading to a decrease in the pH inside the bacterial cell. Thus, interfering with metabolism, leading to a decrease in biological activity as a result of changes in the pH of the cell environments, and this was confirmed by researchers (Bearson et al., 1997; Alakomi et al., 2000). As for the studies that were completed in some countries near Iraq, their results also showed a significant decrease in total coliforms and Enterobacteriaceae in meat after treatment with organic acids, but the level of contamination was higher compared to our study (Elmali et al., 2012; Saad et al., 2020; Sallam et al., 2020). While there were no significant differences (p > 0.05) in an indicator microorganism between samples treated with lactic acid and samples treated with beefxide solution. The reason may be due to the use of a similar concentration of lactic acid and beefxide solution (2.5%). Moreover, the pH of the two acids is almost similar (Lactic acid: pH=2.5; Beefxide: pH=2.6), and this is consistent with other studies that did not show any significant differences between these two acids (Hendrick et al., 2014; Eastwood et al., 2018). Furthermore, the results confirmed that there was a “significant” reduction in the indicator microorganisms (generic E. coli, coliforms, total coliforms, and Enterobacteriaceae) in liver, heart, and kidney samples treated with organic acids, as the results of this study matched the results of Pokharel et al. (2016), who confirmed that there was a significant decrease in the indicator in heart and liver samples treated with lactic acid. Where the rate of decrease reached 0.41 and 1.06 log in their study, while the rate of reduction was close to the results of our study. The results of other researchers who used the petrifilm method to detect indicators of microorganisms after treatment with lactic acid also showed agreement with our results obtained from heart and liver samples, with a significant decrease in E. coli, coliforms, and Enterobacteriaceae (Arita, 2020). Whereas a study conducted in Egypt showed a high level of contamination in samples of the liver, kidney, and heart with E. coli and Enterobacteriaceae compared to our findings, where no treatment was used to reduce the level of contamination in their study (Abd-El-Malek and El-Khateib, 2018). Regarding microbial recovery from carcass surfaces, it depends on the sampling method used in the study (Martinez et al., 2010). It was also mentioned in previous research that excision can recover the largest number of bacteria from the surface of the carcass (Sharpe et al., 1996). However, several studies have found that the number of bacteria recovered by swabbing increases when rough materials are used to scrape bacteria from the carcass (Gallina et al., 2015). The results of our study showed the efficiency of sponge swabs in recovering microorganisms from beef carcasses compared to cotton swabs. The study proved that there was a “significant” difference (p < 0.01) between the indicator microorganisms of samples taken using the sponge method and cotton swabs. The findings of this study were similar to many studies conducted worldwide (Dorsa et al., 1996; Gill and Jones, 2000; Capita et al., 2004; Pearce and Bolton, 2005; Pepperell et al., 2005), as they all indicated the number of bacteria removed from carcasses using cotton swabs was less than it was using sponge swabs. It is important to note that the comparison between microbial recovery results for different samples is substantially affected by the fact that sampling sites cannot be 100% identical in terms of contamination (Milios et al., 2014). ConclusionThe rate of bacterial contamination in beef carcasses and edible organs (liver, heart, and kidney) was relatively acceptable, as it did not exceed the maximum permissible limit internationally. The study demonstrated the efficiency of using organic acids (lactic acid and beefxide solution) in reducing the microbial load to a level that does not cause diseases. However, there was no significant difference (p > 0.05) between lactic acid and beefxide solution in terms of reducing the rate of contamination of the indicator microorganisms in carcasses and edible organs samples, but both showed a significant reduction in all indicator microorganisms. The study confirmed the superiority of using sponge swabs due to their ability to recover microbial organisms from the carcass compared to cotton swabs. This study encourages the use of organic acids, such as lactic acid or beefxide solution in Iraqi slaughterhouses to reduce the microbial load, prolong the shelf life of meat, and delay its spoilage. Further research is recommended to study other acids, such as acetic acid, ascorbic acid, and propionic acid, and their effect on microbial load. AcknowledgmentsThe authors thank the workers at the cooperating slaughterhouses for allowing them to collect samples. Authors’ contributionsIsraa Mahdi and Omar Al-Mahmood: Conceptualization. Omar Al-Mahmood: Study design. Israa Mahdi collected data and sampling. Omar Al-Mahmood: Statistical analysis. Israa Mahdi and Omar Al-Mahmood: Writing. Conflict of interestThe researchers declare that there is no conflict of interest. FundingThis research received no specific grant from any funding agency. Data availabilityAll relevant data are provided in the manuscript. ReferencesAbd-El-Malek, A. and El-Khateib, T. 2018. Microbiological evaluation of some edible bovine by-products. Int. J. Current Micro. Appl. Sci. 7(1), 3449–3458. Alakomi, H.L., Skyttä, E., Saarela, M., Mattila-Sandholm, T., Latva-Kala, K. and Helander, I.M. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Enviro. Micro. 66(5), 2001–5005. Al-Chalaby, A.Y. 2020. Detection of Escherichia coli from imported and local beef meat in Mosul city. J. Pure Appl. Microbiol. 14(1), 383–388. Al-Mahmood, O.A., Bridges, W.C., Jiang, X. and Fraser, A.M. 2021. A longitudinal study: microbiological evaluation of two halal beef slaughterhouses in the United States. Food Cont. 125, 107945. Arita, E.J. 2020. In plant assessment of lactic acid intervention in variety meats through the beef processing line. MSc Thesis. Escuela Agrícola Panamericana, Zamorano, Honduras. Bearson, S., Bearson, B. and Foster, J.W. 1997. Acid stress responses in Enterobacteria. FEMS Microbiol. Lett. 147(2), 173–180. Bolder, N.M. 1997. Decontamination of meat and poultry carcasses. Trend. Food Sci. Tech. 8, 221–227. Capita, R., Prieto, M. and Alonso-Calleja, C. 2004. Review: sampling methods for microbiological analysis of red meat and poultry carcasses. J. Food Prot. 67, 1303–1308. Casas, D.E. 2019. The efficacy of lactic acid immersion as an antimicrobial intervention in beef sub-primal fabrication. Meat Mus. Bio. 3(2), 145–150. Conner, D.E. and J.S. Kotrola. 1995. Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl. Enviro. Micro. 61, 382–385. Dorsa, W.J., Cutter, C.N. and Siragusa, G.R. 1996. Evaluation of six sampling methods for recovery of bacteria from beef carcass surfaces. Lett. Appl. Micro. 22, 39–41. Eastwood, L.C., Arnold, A., Miller, R., Gehring, K. and Savell, J. 2018. Impact of multiple antimicrobial interventions on ground beef quality. Meat Mus. Bio. 2, 46–56. Elmali, M., Yaman, H., Tekinsen, K.K., Öner, S. and Çekin, E. 2012. Inhibitory effects of different decontamination agents on the levels of Listeria monocytogenes in the experimentally inoculated raw beef samples in the laboratory conditions. J. Fac. Vet. Med. 18, 763–768. European Commission (EC). 2001. Commission decision of 8 June 2001 (2001/471/EC). Official Journal of the European Communities - Legislation 165, 48–53. Gallina, S., Bianchi, D., Ru, G., Maurella, C., Barzanti, P., Baioni, E., Virgilio, S., Mioni, R., Lanni, L., Migliazzo, A. and Losio, M.N. 2015. Microbiological recovery from bovine, swine, equine, and ovine carcasses: comparison of excision, sponge and swab sampling methods. Food Cont. 50, 919–924. Gill, A., Tamber, S. and Yang, X. 2019. Relative response of populations of Escherichia coli and Salmonella enterica to exposure to thermal, alkaline, and acidic treatments. Int. J. Food Microbiol. 293, 94–101. Gill, C.O., and Jones, T. 2000. Microbial sampling of carcasses by excision or swabbing. J. Food Prot. 2, 167–173. Hammadi, R. 2017. Detection of E. coli, as a contaminant of minced meat in certain locations of Baqubah city-Diyala Province-Iraq. Kufa J. Vet. Med. Sci. 8(1), 72–78. Hardin, M.D., Acuff, G., Lucia, L., Oman, J. and Savell, J. 1995. Comparison of methods for decontamination from beef carcass surfaces. J. Food Prot. 58, 368–374. Hendricks, K.M., Arnold, A.N., Taylor, T., Gehring, K. and Savell, J. 2014. In Plant validation of two antimicrobial agents applied during production of further processed beef products. Int. Cong. Meat Sci. Tech. 60, 186–189. Jawad, A.H., and Abdul Alwahid, A.T. 2007. Bacterial quality of beef carcasses and sanitary condition of butcher’s shops in Basrah city. Bas. J. Vet. Res. 6(2), 1–10. JMP®, Pro 16.1. 2021. Cary, NC: SAS Institute Inc., 1989–2021. Karanth, S., Feng, S., Patra, D. and Pradhan, A.K. 2023. Linking microbial contamination to food spoilage and food waste: the role of smart packaging, spoilage risk assessments, and date labeling. Front. Micro. 14, 1198124. Khalafalla, F., Ali, F., Hassan, A. and El-Feky, K. 2016. Monitoring the bacterial contamination during different stages of beef carcass preparation at Beni-Suef abattoir, Egypt. Ben. Vet. Med. J. 30(1), 51–58. Loretz, M., Stephan, R. and Zweifel, C. 2011. Antibacterial activity of decontamination treatments for cattle hides and beef carcasses. Meat Sci. 88, 256–260. Martinez, B., Celda, M.F., Anastasio, B. and Lopez-Mendoza, C.C. 2010. Microbiological sampling of carcasses by excision or swabbing with three types of sponge or gauze. J. Food Prot. 73(1), 81–87. Matthews, K.R., Kniel, K.E. and Montville, T.J. 2019. Food microbiology: an introduction, Hoboken, NJ: John Wiley and Sons. Milios, K., Drosinos, E. and Zoiopoulos, P. 2014. Food safety management system validation and verification in meat industry: carcass sampling methods for microbiological hygiene criteria e a review. Food Cont. 43, 74–81. Nakamura, A., Takahashi, H., Koike, F., Kuda, T. and Kobayashi, M. 2023. Transition of microbial contamination on the surface of carcass during the cattle slaughter process. Food Micro. 112, 104245. Omer, E., Al-Ghamd, M., Alsubaie, A. and Fadlelmula, A. 2013. The effect of seasonal variation on the hygienic standard of beef carcasses in Al Baha region, Kingdom of Saudi Arabia. J. Med. Meda. Sci. 4(6), 230–236. Pearce, R.A. and Bolton, D.J. 2005. Excision vs sponge swabbing: a comparison of methods for the microbiological sampling of beef, pork, and lamb carcasses. J. Appl. Micro. 98, 896–900. Pepperell, R., Reid, C.A., Nicolau, S., Hutchison, M., Walters, L.D., Johnston, A.M. and Buncic, S. 2005. Experimental comparison of excision and swabbing microbiological sampling methods for carcasses. J. Food Prot. 68, 2163–2168. Pokharel, S., Miller, M.F., Parks, A.R. and Brashears, M.M. 2016. In-plant validation study to determine the efficacy of lactic acid as an antimicrobial intervention on beef heads and variety meats. Meat Sci. 112, 167. Richiardi, L., Pignata, C., Fea, E., Bonetta, S. and Carraro, E. 2023. Are indicator microorganisms predictive of pathogens in water? Water. 15(16), 2964. Ruban, S.W. and Fairoze, N. 2011. Effect of processing conditions on microbiological quality of market poultry meats in Bangalore, India. J. Ani. Vet. Adv. 10(2), 188–191. Saad, S., Hassanin, F., Salem, A., Saleh, E. and Abdellatif, Z. 2020. Efficiency of some organic acids as decontaminants in sheep carcasses. Ben. Vet. Medi. J. 38(2), 116–119. Sallam, K.I., Abd-Elghany, S., Hussein, M., Imre, K., Morar, A., Morshdy, A. and Sayed-Ahmed, M. 2020. Microbial decontamination of beef carcass surfaces by lactic acid, acetic acid, and trisodium phosphate sprays. Bio. Med. Res. Int. 2020, 1–11. Santos, E.C., Castro, V.S., Cunha-Neto, A., Santos, L.F., Vallim, D.C., Lisbôa, R.D., Carvalho, R.C., Junior, C.A. and Figueiredo, E.E. 2018. Escherichia coli O26 and O113: H21 on carcasses and beef from a slaughterhouse located in Mato Grosso, Brazil. Foodborne Pathog. Dis. 15, 653–659. Schaffner, D.W. and Smith, S. 2004. Microbiological analysis/Indicator organisms. Bio. Enviro. Sci. 22, 773–780. Sharpe, A.N., Isigidi Bin Kingombe, C., Watery, P., Parrington, L.J., Dudas, I. and Diotte, M.P. 1996. Efficient non-destructive sampler for carcasses and other surfaces. J. Food Prot. 59, 757–783. Sofos, J., Kochevar, S., Bellinger, G., Buege, D., Hancock, D., Ingham, S., Morgan, J.B., Reagan, J.O. and Smith, A.C. 1999. Sources and extent of microbiological contamination of beef carcasses in seven United States slaughtering plants. J. Food Prot. 62(2), 140–145. Soriyi, I., Agbogli, H.K. and Dongdem, J.T. 2008. A Pilot microbial assessment of beef sold in the Ashaiman Market, A suburb of Accra, Ghana. Africa. J. Food, Agri. Nut. Develop. 8(1), 91–103. Tiţa, O., Constantinescu, A.M. and Tiţa, M.A. 2020. Sources of pollutants and environmental factors protection in the meat processing industry. MATEC Web of Conferences. 305, 00069. Ubaid ur Rahman, A.S., Ishaq, A., Aadil, R.M., Zahoor, T. and Ahmad, M.H. 2018. Advanced meat preservation methods: a mini review. J. Food Safe. 38(4), e12467. United States Department Agriculture/Food Safety Inspection Services (USDA/FSIS). 2023. Safe and suitable ingredients used in the production of meat, poultry, and egg products—revision 58 FSIS Directive 7120.1 Amendment 6. United States Department of Agriculture (USDA)/Food Safety and Inspection Service (FSIS). 2015. Sampling requirements to demonstrate process control in slaughter operations. United States Department of Agriculture/Food Safety and Inspection Service (USDA-FSIS). 2014. National beef and veal carcass microbiological baseline data collection program actual study. Van Ba, H., Seo, H.W., Pil-Nam, S., Kim, Y.S., Park, B.Y., Moon, S.S., Kang, S.J., Choi, Y.M., and Kim, J.H. 2018. The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Sci. 137, 16–23. Wambui, J., Lamuka, P., Karuri, E., Matofari, J., and Njage, P. 2018. Microbial contamination level profiles attributed to contamination of beef carcasses, personnel, and equipment: case of small and medium enterprise slaughterhouses. J. Food Prot. 81(4), 684–691. Wheeler, T.L., Kalchayanand, N., and Bosilevac, J.M. 2014. Pre- and post-harvest interventions to reduce pathogen contamination in the U.S. beef industry. Meat Sci. 98, 372–382. | ||
| How to Cite this Article |
| Pubmed Style Jweer IM, Al-mahmood OA. Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. Open Vet. J.. 2024; 14(7): 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 Web Style Jweer IM, Al-mahmood OA. Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. https://www.openveterinaryjournal.com/?mno=201863 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i7.15 AMA (American Medical Association) Style Jweer IM, Al-mahmood OA. Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. Open Vet. J.. 2024; 14(7): 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 Vancouver/ICMJE Style Jweer IM, Al-mahmood OA. Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. Open Vet. J.. (2024), [cited January 25, 2026]; 14(7): 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 Harvard Style Jweer, I. M. & Al-mahmood, . O. A. (2024) Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. Open Vet. J., 14 (7), 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 Turabian Style Jweer, Israa M., and Omar Ahmed Al-mahmood. 2024. Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. Open Veterinary Journal, 14 (7), 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 Chicago Style Jweer, Israa M., and Omar Ahmed Al-mahmood. "Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse." Open Veterinary Journal 14 (2024), 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 MLA (The Modern Language Association) Style Jweer, Israa M., and Omar Ahmed Al-mahmood. "Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse." Open Veterinary Journal 14.7 (2024), 1658-1667. Print. doi:10.5455/OVJ.2024.v14.i7.15 APA (American Psychological Association) Style Jweer, I. M. & Al-mahmood, . O. A. (2024) Efficiency of using lactic acid and beefxide to reduce indicator microorganisms on beef in Mosul slaughterhouse. Open Veterinary Journal, 14 (7), 1658-1667. doi:10.5455/OVJ.2024.v14.i7.15 |