| Research Article | ||
Open Vet. J.. 2024; 14(8): 1952-1959 Open Veterinary Journal, (2024), Vol. 14(8): 1952–1959 Research Article Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in EgyptAbdelmenem Ahmed Elmeligy1, Ali A. Ghania2,3 and Ahmed Fotouh4*1Pathology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 2Department of Food Science and Technology, Faculty of Agriculture, University of Tripoli, Tripoli, Libya 3Food and Drug Control Center, Tripoli, Libya 4Pathology and Clinical Pathology Department, MBA, Faculty of Veterinary Medicine, New Valley University, Kharga, Egypt *Corresponding Author: Ahmed Fotouh. Pathology and Clinical Pathology Department, MBA, Faculty of Veterinary Medicine, New Valley University, Kharga, Egypt. Email: ahmedfotouh [at] vet.nvu.edu.eg Submitted: 16/05/2024 Accepted: 19/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
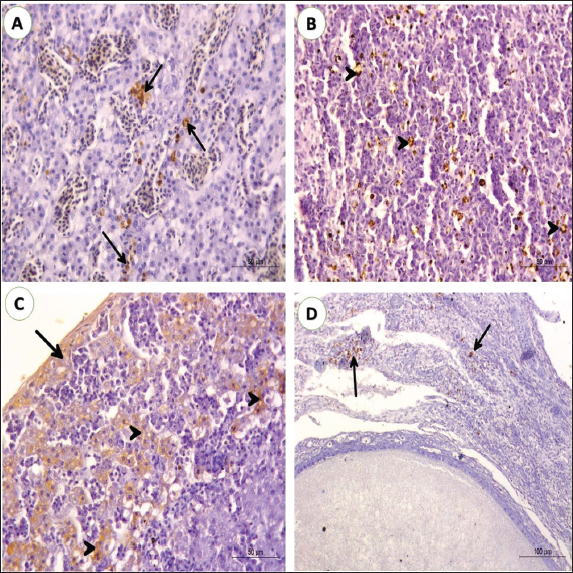
ABSTRACTBackground: Pigeon leukosis is primarily caused by avian leukosis virus subgroup A (ALV-A). It infects and transforms lymphoid cells, leading to the development of tumors in various lymphoid tissues and other organs especially the liver. Aim: This study was conducted to diagnose lymphoid leukosis in a naturally infected pigeon flock in Egypt. Methods: Tissue specimens from the liver, spleen, thymus, kidney, lung, proventriculus, gizzard, intestine, pancreas, heart, pectoral muscle, ovary, and testes were collected from infected birds for pathological and immunohistochemical examinations. Results: Clinical signs were generally nonspecific and comprised weakness, dehydration, and emaciation. Gross lesions were mostly in the liver and spleen, in the form of minute white nodules scattered on the liver surface. Microscopic examination of the liver, spleen, and kidneys showed masses of uniform sizes and the presence of differentiated lymphoid cells. These cells appeared as large mononuclear cells with poorly defined cell membranes. Immunohistochemical investigation exhibited that the ALV-A positive indicators were chiefly accessible in the liver, ovary, spleen, and kidney. Conclusion: Lymphoid leukosis in pigeons could be provisionally diagnosed by a pathological picture of characteristic tumors and confirmed by immunoreactivity of viral antigens in different tissues. Keywords: Pigeon, Lymphoid leukosis, Pathology, Immunohistochemistry. IntroductionAvian leukosis (ALV), is a viral disease that affects various species of birds, particularly chickens. It is caused by a retrovirus belonging to the family Retroviridae and the genus Alpharetrovirus. Avian leukosis viruses (ALVs) can infect chickens, turkeys, quails, pigeons, and pheasants (Payne and Venugopal, 2000; Zaib et al., 2022). There are different subgroups of ALVs, including A, B, C, D, and J. Subgroup J avian leukosis virus (ALV-J) was known to cause myeloid leukosis, a form of the disease that affects the bone marrow and can lead to tumor development within various organs (Fotouh et al., 2020). Subgroup A and B viruses are frequently accompanied by lymphoid leukosis, which affects the lymphoid tissues, including the spleen and bursa of Fabricius (Hu et al., 2017). Lymphoid leukosis can cause tumors, immunosuppression, and declined egg production in precious birds (Fandiño et al., 2023). In pigeons, the disease is rare and caused by subgroup A, which infects and transforms lymphoid cells, leading to the development of tumors in various tissues, especially the liver. The virus can be transmitted horizontally through direct contact with infected birds, as well as vertically from infected parents to their offspring through the egg (Zhang et al., 2019). The pathogenesis of lymphoid leukosis in pigeons is similar to that in chickens, once the ALV enters the pigeon’s body, it targets lymphocytes, particularly B cells, and replicates within them. The viral genome is combined into the host cell’s DNA, disrupting the normal regulation of cell growth and division. This results in the formation of tumors primarily in the lymphoid organs (Fotouh et al., 2024). ALV caused greater economic losses because the infected chickens usually show tumor development, low immunity, growing retardation, decrease egg production, and increased morbidity, and mortality (Li et al., 2013). The presence of tumors can lead to various clinical signs and symptoms in affected pigeons, including weight loss, lethargy, and decreased appetite. By affecting the immune system, the birds become more susceptible to secondary infections and increase overall mortality (Wang et al., 2017; Fotouh et al., 2024a). Lymphoid leukosis can lead to various pathological lesions in affected birds characterized by the development of tumors or neoplastic growths in the lymphoid organs. The affected lymphoid tissues, such as the spleen, thymus, and bursa of Fabricius, may exhibit infiltration of neoplastic cells. These cells can replace or disrupt the normal architecture of the lymphoid tissue, leading to the formation of tumor masses. These tumors can vary in size, ranging from small nodules to large masses. They may appear white to grayish and can be well-demarcated or diffuse. Tumor cells can infiltrate surrounding tissues and organs beyond the primary site. Metastasis, the spread of tumor cells to distant sites, can occur in lymphoid leukosis (Rahman et al., 2020). Common sites of metastasis include the liver, kidneys, lungs, and heart. In addition, the tumors may exhibit areas of hemorrhage and necrosis due to the disruption of blood vessels and compromised blood supply. These areas can appear dark red or black, indicating the presence of necrotic tissue (Zhang et al., 2019). This study aims to describe different pathological and immunohistochemical findings associated with lymphoid leukosis in pigeons. Materials and MethodsBirdsA breeder pigeon farm in Ismailia governorate, Egypt, suffered from mortalities and a severe drop in egg production (15%). The ages of these birds ranged from 3 to 5 years old. The capacity of the farm was about 3,000 birds. Both freshly dead and sacrificed birds were subjected to postmortem examination (P.M.) and recording of the gross lesions. Microscopic analysisTissue specimens were collected from the liver, spleen, thymus, kidney, lung, proventriculus, gizzard, intestine, pancreas, heart, pectoral muscle, ovary, and testes for microscopic investigation. These samples were fixed in neutral buffered formalin 10%. Then the tissue specimens were washed, dehydrated, cleared, and embedded in paraffin. Paraffin blocks were sectioned at 4 µm thickness and stained with hematoxylin and eosin (H&E) (Abo-Aziza et al., 2022; Elbarbary et al., 2023). Immunohistochemistry (IHC)To distinguish the existence of ALV-A antigen, Paraffin-embedded, sections were placed on positive glass slides at a thickness of 4 μm. The tissue slices were marked with a repetitive streptavidin biotin/horseradish peroxidase (HRP)-conjugated IHC procedure. Pre-treatment of sections with 3% H2O2 in methanol and raised with 5% bovine serum albumin in PBS for 10 min. The slides were incubated with the primary antibody (a rabbit anti-ALV-A surface protein) and raised with the secondary antibody (biotinylated goat anti-rabbit IgG, Santa Cruz, CA, USA). After several washes, the streptavidin/HRP was added. The site of antibody binding was imagined using DAB (3, 3-diaminobenzidine tetrahydrochloride) as chromogen which gave a dark brown precipitate (positive straining). Hematoxylin was used as the counterstain. Positive and negative control slides kindly taken from the pathology division, Animal Health Institute, Dokki, Giza, Egypt. The slides were examined microscopically under light microscopy (Fotouh et al., 2024b). Ethical approvalThis study protocol was achieved by ensuring ethics and rules for experimental animals and accepted by the New Valley Research Ethics Committee of the faculty of veterinary medicine, New Valley University below code (02/3/6–2024/18). ResultsClinical findings and mortalityClinical signs were generally nonspecific. They comprise weakness, inappetence, and emaciation. A severe drop in egg production reached to about 15%. In some birds, there was abdominal enlargement. Other birds may die without any apparent signs. The mortality rate was 1.6% within 2 months. Pathological findingsGross findings On gross examination of some adult dead pigeons, the liver appeared uniformly enlarged occupying most of the abdominal cavity with military nodules. These livers were mostly friable. The cut surface may have areas of necrosis. Several livers have hemorrhagic spots on the surface. The spleen and kidney were diffusely enlarged and mottled. The proventricular mucosa was thick and hemorrhagic in some cases. Nonfunctional ovaries and regressed ovarian follicles were visible changes in ovaries (Fig. 1). Microscopic and immunohistochemical findings Microscopic examination reveals that the thymus, hearts, lungs, muscles, proventriculus, gizzards, pancreas, and intestines were free from any neoplastic lymphoblastic infiltrations. Liver The microscopic examination of the miliary nodules showed numerous numbers of small-size foci of aggregated lymphoblastic cells. In some livers, massive lymphoblastic cell infiltration between the hepatic lobules was marked which replaced most of the hepatic parenchyma (Fig. 2A). The hepatocytes seemed as lonely islets in between the immense infiltration of lymphoblastic cells. The lymphoblastic cells are large mononuclear cells with ill-defined cell membranes. The nuclei looked vesicular which clumping and margination of chromatin with the presence of one or more noticeable nucleoli which is acidophilic. The hepatocytes around portal areas showed degenerative changes, atrophy, and disorganization (Fig. 2B). For further detection of the ALV-A antigen in different organs, immunohistochemistry was performed. In the liver, intense brown granules stain the lymphocytes around congested blood vessels as well as in Kupffer cells and macrophages indicating a positive reaction (Fig. 5A).
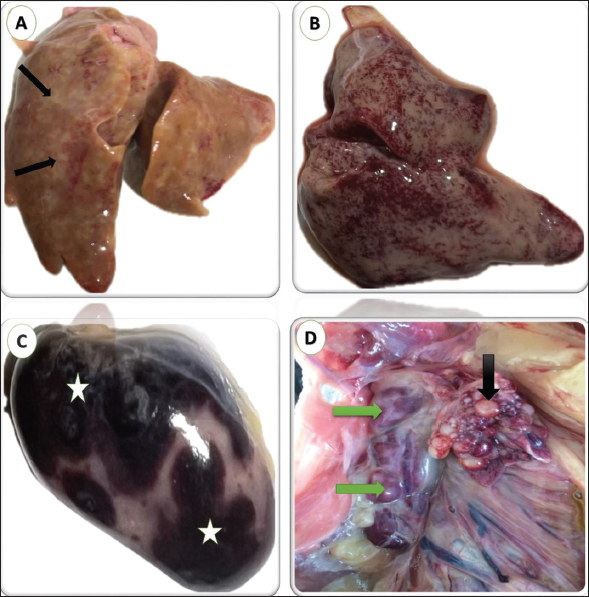
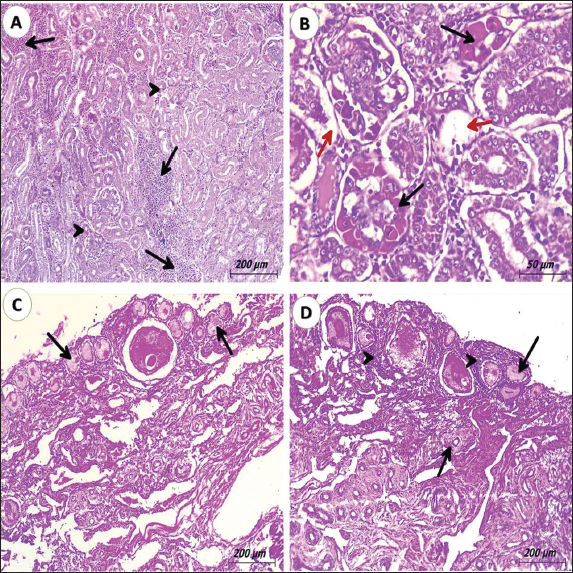
Fig. 1. gross lesions from pigeons (3 years old) naturally infected with lymphoid leukosis. A) Liver showing whitish nodules about 1–2 mm in diameter scattered all over the surface. B) Liver showing petechial hemorrhages all over the surface. C) Spleen showing severe enlargement with large hemorrhagic areas on the surface. D) Kidney shows enlargement and mottling (green arrow). Nonfunctional ovaries and regressed ovarian follicles (black arrow). Spleen Many spleens showed thickened and hyalinized capsules. The red pulp was completely replaced by diffusely infiltrating RBCs indicating massive hemorrhage that increased the size of the red pulp of the spleen and decreased the white/red pulp ratio (Fig. 3A). The white pulp is approximately decreased in size showing several lymphocytes comparison to the normal spleen. The proliferated lymphocytes showed mitotic activity and atypism. Massive propagation of large lymphoid cells all over splenic tissue particularly red pulp was seen in some tissues leading to ill demarcation between red and white pulp. (Fig. 3B). The brown granules by IHC indicate the positive reaction for the presence of specific viral particles in lymphocytes and macrophages distributed around lymphoid follicles and blood vessels. The brown granules were clear in the neoplastic cells at the white pulp (Figs. 5B). Kidneys Multifocal neoplastic aggregations of lymphoid cells were gathered around dilated blood vessels and between the deteriorated renal tubules (Fig. 4A). The renal tubules showed vacuolar degeneration, pressure atrophy, and precipitation of eosinophilic proteinaceous material in their lumens (hyaline casts) or sloughing of epithelial lining in some renal tubules together with pyknosis and karyorrhexis in some nuclei (Fig. 4B). Hypercellularity was observed in some glomeruli. Subcapsular and interstitial hemorrhages were prominent. By IHC staining, the brown granules indicate the positive response for the presence of definite viral particles in the lining epithelium of the renal tubules or macrophages at interstitial space (Fig. 5C).
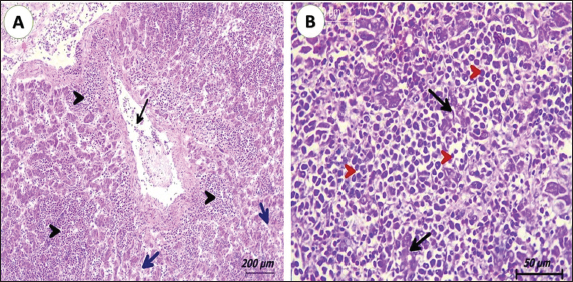
Fig. 2. Photomicrograph of liver from pigeons (3 years old) naturally infected with lymphoid leukosis. A) Liver showing multifocal aggregation of lymphoid cells (black arrowhead) around central veins (black arrow). Hepatocytes suffer from degeneration and pressure atrophy (blue arrow). (H&E, scale bar: 200 µm). B) Liver diffusely infiltrated by lymphoid cells, showing atypism and mitotic figures (red arrowhead). Islets of hepatocytes suffered from degeneration and atrophy (black arrow) (H&E, scale bar: 50 µm).
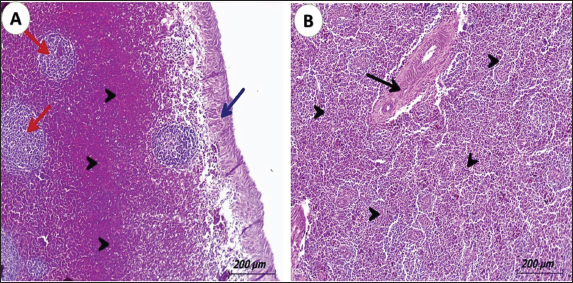
Fig. 3. Photomicrograph of spleen from pigeons (3 years old) naturally infected with lymphoid leukosis. A) Spleen showing hyalinized capsule (blue arrow). Severely hemorrhagic red pulp (black arrowhead) and depletion of white pulp (red arrow) (H&E, scale bar: 200 µm). B) Spleen displaying severe infiltrating of white pulp and red pulp with lymphoid cells (black arrowhead) and hyalinized splenic arterioles. (black arrow) (H&E, scale bar: 200 µm). OvaryThe ovarian stroma showing a mild infiltration with lymphoblast cells together with congestion of the vasculature was seen in some cases (Figs. 4D). The infiltrated tumor cells cause either necrosis or replacement of the ovarian stroma in other cases. In some birds, the infiltrating cells were in the interfollicular spaces and around ovarian follicles causing atrophied or undeveloped ovarian follicles (Figs. 4C). Examination of the ovary stained by IHC, the brown particles indicating the positive reaction for the presence of specific viral elements were detected in lymphocytes aggregated around ovarian follicle (Figs. 5D).
Fig. 4. Photomicrograph of kidney and ovary from pigeons (3 years old) naturally infected with lymphoid leukosis. A) kidney showing inter-tubular multifocal aggregation of lymphoid cells (black arrows). Many convoluted tubules suffer from degeneration and pressure atrophy (black arrowheads). (H&E, scale bar: 200 µm). B) Kidney revealing severe degeneration of convoluted tubules (red arrows). The glomerulus shows hyalinization (black arrows). (H&E, scale bar: 50 µm). C) Ovary display degeneration of ovarian stroma with atrophied ovarian follicles (black arrow) (H&E, scale bar: 200 µm). D) Ovary detecting mild lymphocytic infiltration around ovarian follicles (black arrowheads) and atrophied ovarian follicles. (black arrow). DiscussionThis study was carried out to illuminate the different pathological and immunohistochemical insights in pigeons due to ALV-A infection. Lymphoid leukosis is a neoplastic disease of chickens, turkeys, quails, pigeons, and pheasants (Payne and Venugopal, 2000; Payne and Nair, 2012) caused by subgroup A. The ALVs belong to the family Retroviridae. ALVs have been sub-classified into six subgroups, nominated A-E and J, which are created on their host variety and cross-neutralization forms (Zhang et al., 2010). In pigeons, the disease is rare and caused by subgroup A. It was revealed that pigeon cells were resilient to avian tumor viruses excluding ALV-D (Meyers et al., 1978). Since ALV-A was first isolated in the past century it has spread quickly all over the world causing economic losses in layer and breeder farms because the infected chickens usually exhibit tumors development (Miheso et al., 2017), depressed immunity, growth retardation, decrease egg production, and increased morbidity and mortality (Swayne et al., 2020). However, ALVs transmit to other several nations including Egypt. In Egypt, ALVs are transmitted to different types of chickens as native breeds and foreign breeds of layer chickens (Fotouh et al., 2024b). In addition to a higher number of culls, nonlaying birds and mortality in pigeons that accredited to either the direct outcome of the virus; in which death occurred due to tumor initiation by the virus (Průková et al., 2007), or due to the indirect effect of the virus through immunosuppression of the infected pigeons (Wang et al., 2012; Swayne et al., 2020). so, mixed bacterial infection especially E. Coli species (Abu El Hammed et al., 2022) and Salmonella species (Soufy et al., 2016) may occur; causing increased mortalities, a drop in egg production, and economic losses associated with reduced efficiency. Concerning post-mortem inspections, the liver, spleen, and kidneys were the most affected organs. The liver appeared uniformly enlarged occupying most of the abdominal cavity with diffuse tumor foci, which give a mottled or granular appearance in some cases. Other livers were mostly friable and the cut surface in tumor foci appears grayish to creamy white and may have areas of necrosis. The spleen and kidneys were diffusely hemorrhagic and enlarged. These findings were completely agreed with Zhang et al. (2019) and Rahman et al. (2020).
Fig. 5. Photomicrograph of liver, spleen, kidney, and ovary from pigeons (3 years old) naturally infected with lymphoid leukosis. A) Liver; the brown particles indicated the positive reaction for the presence of specific viral particles in lymphocytes aggregated in hepatic parenchyma (black arrows) (IHC stain, scale bar: 50 µm). B) Spleen; the brown particles indicated the positive response for the presence of exact viral elements in lymphocytes distributed in white and red pulps (black arrowheads). (IHC stain, scale bar: 50 µm). C) Kidney; high immunoreactivity in lining epithelium of convoluted tubules (black arrowheads) and lymphocytic infiltration under capsule (black arrows) (IHC stain, scale bar: 50 µm). D) Ovary; The brown particles indicated the positive response for the presence of definite viral particles in lymphocytes aggregated in ovarian stroma. (black arrows) (IHC stain, scale bar: 100 µm). Microscopic assessment of the liver determined the incidence of lymphoid leukosis. The liver lesions were accumulations of lymphoblastic cells. Sagarika et al. (2017) recorded the same findings in chickens. The antigen staining by IHC indicated the positive reaction for the presence of specific viral elements in the Kupffer cells and lymphocytes this provisions the preceding results in chickens which were established by noticing the virus from liver tissues using PCR. Those outcomes were consistent with Fotouh et al. (2020, 2024b), Sagarika et al. (2017), and Gharaibeh et al. (2001). The microscopic examination of the spleen sections revealed a lymphoid population in red and white pulps. The high affinity to splenic tissue may be attributed to species differences and tropism. Viral antigen was extreme in the lymphoid follicles of the spleen by IHC. These consequences were reinforced by Wang et al. (2013) and Fotouh et al. (2024b) who verified ALV-J-positive signals in the erythroblast cytoplasm, spleen, lung, and additional tissues particularly rich in blood. The microscopic evaluation of the kidney exposed multifocal aggregations of lymphoid cells. The tumor cells were infiltrated between the deteriorated renal tubules and round dilated blood vessels. The renal tubules showed pressure atrophy and hyaline casts or sloughing of epithelial lining in some renal tubules together with pyknosis of the nucleus. Some previous reports by Bande et al. (2016) and Chesters et al. (2002) designated those microscopic alterations, such as lymphoma caused by ALV-A chiefly arising in the livers, kidneys, and, spleens; in particular, several typical microscopic lesions might be seen in the liver (Payne, 1992; Begum et al., 2016). We concluded that ALV-A detected more in the liver and spleen and caused severe alterations. In some other cases, characteristic tumors produced by ALV-A arise in the livers and spleens of birds (Xu et al., 2016). This reflection may be correlated to the gathering of a large quantity of ALV-A in these organs. Ovaries mostly were undeveloped. Microscopically, mild infiltrates of neoplastic lymphoid cells were seen. These results agreed with Fenton et al. (2005). Viral antigens were detected around ovarian follicles and in interfollicular spaces, the same finding was reported by Fotouh et al. (2020) and Haridy et al. (2019) in chickens. This directly influences the production performance of pigeons and facilitates the vertical transmission of the virus, which is considered a new challenge that needs further studies. ConclusionThe current study was the first report in Egypt concerning the infection of pigeons by lymphoid leukosis. The finding confirmed the typical gross signs and the pathological picture of lymphoid leukosis which were characterized by enlarged organs having military nodules occupying most of the abdominal cavity also the ALV-A antigen detected in different organs. Further examination is required to determine the extent of the disease to other species as well as further inspection of pigeon flocks to demonstrate other leukosis subgroups. AcknowledgmentsThe authors are grateful to all colleagues from the Faculty of Veterinary Medicine, New Valley University, who made this study possible. Author’s contributionsAbdelmenem Ahmed Elmeligy: Supervision, and Visualization. Ali A. Ghania: Investigation, Methodology, Supervision, Writing—review and editing. Ahmed Fotouh: Methodology, Conceptualization, and Validation. All authors read and approved the final manuscript. Conflict of interestThe authors claim no conflicts of interest in publishing this research. FundingNone. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbo-Aziza, F.A.M., Zaki, A.A., Adel, R.M. and Fotouh, A. 2022. Amelioration of aflatoxin acute hepatitis rat model by bone marrow mesenchymal stem cells and their hepatogenic differentiation. Vet. World 15(5), 1347–1364. Abu El Hammed, W., Soufy, H., EL-Shemy, A., Fotouh, A., Nasr, M.N. and Dessouky, M.I. 2022. Prophylactic effect of oregano in chickens experimentally infected with avian pathogenic Escherichia coli O27 with special reference to hematology, serum biochemistry, and histopathology of vital organs. Egypt. J. Chem. 65(6), 269–282. Bande, F., Arshad, S.S. and Omar, A.R. 2016. Isolation and metagenomic identification of avian leukosis virus associated with mortality in broiler chicken. Adv. Virol. 2016, 9058403. Begum, M.D., Rahman, M.M., Akter, M.R., Haque, M.A., Rahman, M.K., Hossain, M.M. and Amin, M.N. 2016. Identification of avian leukosis virus from layer chicken by serological test and embryo inoculation technique. Asian-Aust. J. Biosci. Biotechnol. 1, 23–30. Chesters, P.M., Howes, K., Petherbridge, L., Evans, S., Payne, L.N. and Venugopal, K. 2002. The viral envelope is a major determinant for the induction of lymphoid and myeloid tumors by avian leukosis virus subgroups A and J, respectively. J. Gen. Virol. 83, 2553–2561. Elbarbary, N.K., Abdelmotilib, N.M., Gomaa, R.A., Elnoamany, F., Fotouh, A., Noseer, E.A. and Zaki, R.S. 2023. Impact of thawing techniques on the microstructure, microbiological analysis, and antioxidants activity of Lates niloticus and Mormyrus kannume fish fillets. Egypt. J. Aquat. Res. 49, 530–536. Fandiño, S., Gomez-Lucia, E., Benítez, L. and Doménech, A. 2023. Avian leukosis: will we be able to get rid of it? Animals 13(14), 2358. Fenton, S.P., Reddy, M.R. and Bagust, T.J. 2005. Single and concurrent avian leukosis virus infections with avian leukosis virus-J and avian leukosis virus-A in Australian meat-type chickens. Avian Pathol. 34(1), 48–54. Fotouh, A., Abdel-Maguid, D.S., Abdelhaseib, M., Zaki, R.S. and Darweish, M. 2024a. Pathological and pharmacovigilance monitoring as toxicological imputations of azithromycin and its residues in broilers. Vet. World 17(6), 1271–1280. Fotouh, A., Soufy, H., El-Begawey, M.B. and Nasr, S.M. 2020. Pathological, clinicopathological, and molecular investigations on chickens experimentally infected with avian leucosis virus type. J. Adv. Vet. Anim. Res. 8, 590–600. Fotouh, A., Shosha, E.A.EM., Zanaty, A.M. and Darwesh, M.M. 2024. Immunopathological investigation and genetic evolution of Avian leukosis virus Subgroup-J associated with myelocytomatosis in broiler flocks in Egypt. Virol. J. 21(1), 83. Gharaibeh, S., Brown, T., Stedman, N. and Pantin, M. 2001. Immunohistochemical localization of avian leukosis virus subgroup J in tissues from naturally infected chickens. Avian Dis. 45, 992–998. Haridy, M., Goryo, M., El-Neweshy, M. and Yanai, T. 2019. Visceral lymphomas due to co-infection of Marek’s disease virus- avian leucosis virus A-E in Japanese silkie fowl. Japanese J. Vet. Res. 67(1), 41–50. Hu, X., Zhu, W., Chen, S., Liu, Y., Sun, Z., Geng, T., Song, C., Gao, B., Wang, X., Qin, A. and Cui, H. 2017. Inhibition of ERK/MAPK suppresses avian leukosis virus subgroup A and B replication. Microb. Pathog. 102, 29–35. Li, D., Qin, L., Gao, H., Yang, B., Liu, W., Qi, X., Wang, Y., Zeng, X., Liu, S., Wang, X. and Gao, Y. 2013. Avian leukosis virus subgroup A and B infection in wild birds of Northeast China. Vet. Microbiol. 163(3-4), 257–263. Meyers, P., Ritts, G.D., Heise, J.M. and Qualtiere, L.F. 1978. Limited host range of avian tumor viruses on pigeon cells. Virology 90(1), 162–165. Miheso, K.O., Mbuthia, P.G., Njagi, L.W., Karanja, D.N., Gathumbi, P.K., Shah, D.N., Wanjohi, C.W. and Murithi, M.R. 2017. Seroprevalence of avian leucosis in chicken in nairobi and surrounding counties. Livest. Res. Rural. Dev. 29(3), 52. Payne, L.N. 1992. Biology of avian retroviruses. In: Levy JA, editor. The retroviridae. New York, NY: Plenum Press; vol. 1, pp: 299–404. Payne, L. and Venugopal, K. 2000. Neoplastic disease: Marek’s disease, avian leucosis and reticuloendotheliosis. Rev. Sci. Tech. 19, 544–564. Payne, L.N. and Nair, V. 2012. The long view: 40 years of avian leukosis research. Avian Pathol. 41, 11–19. Průková, D., Vernerová, Z., Pilcík, T., Stepanets, V., Indrová. M., Geryk, J., Plachý, J., Hejnar, J. and Svoboda, J. 2007. Differences in pathogenicity among strains of the same or different avian leukosis virus subgroups. Avian Pathol. 36(1), 15–27. Rahman, M.H., Kabir, R., Hossain, M., Hossain, I., Nooruzzaman, M., Begum, J.A., Ahmad, N. and Kabiraj, C. 2020. Pathological evaluation of lymphoid leukosis in a racing pigeon (Columba livia domestica). Indian J. Vet. Pathol. 44(1), 52–55. Sagarika, S., Das, D., Panda, S.K., Das, S., Jena, B. and Sahu, R.K. 2017. Avian leucosis in chickens: a clinico-pathological survey. J. Entomol. Zool. Stud. 5, 1697–1701. Soufy, H., Gab-Allah, M.S., Tantawy, A.A., Fotouh, A. and Nasr, S.M. 2016. Pathogenesis of experimental Salmonella Gallinarum infection (fowl typhoid) in Broiler chicks. Egyptian J. Vet. Sci. 47(2), 117–131. Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V. and Suarez, D.L. 2020. Diseases of poultry. 14th ed. Hoboken, NJ: Wiley; pp: 1488. Wang, J.Y., Cheng, M.H., She, R., Wu, Q.X., Shi, R.H. and Hu, F.J. 2017. Avian leucosis virus detection and liver pathology observation of slaughter broiler. Chin. Vet. Sci. 47, 114–120. Wang, G., Jiang, Y., Yu, L., Wang, Y., Zhao, X. and Cheng, Z. 2013. Avian leukosis virus subgroup J is associated with the outbreak of erythroblastosis in chickens in China. Virol. J. 10, 92. Wang, X., Zhao, P. and Cui, Z.Z. 2012. Identification of a new subgroup of avian leucosis virus isolated from Chinese indigenous chicken breeds. Virol. Sin. 28(6), 609–614. Xu, S., Yixin, W., Yang, L., Huaibiao, L., Zhi-zhong, C., Shuang, C. and Peng, Z. 2016. Sequence analysis and pathogenic study of a subgroup A avian leukosis virus isolated from Longshengfeng chickens. Chinese J. Prev. Vet. Med. 38(9), 705–710. Zaib, G., Hu, X. and Cui, H. 2022. Global maps of avian leukosis viruses: research trends and themes based on networking. Vet. Sci. 10(1), 16. Zhang, Q.C., Zhao, D.M., Guo, H.J. and Cui, Z.Z. 2010. Isolation and identification of a subgroup A avian leukosis virus from imported meat-type grandparent chickens. Virol. Sin. 25(2), 130–136. Zhang, Z., Hu, W., Li, B., Chen, R., Shen, W., Guo, H., Guo, H. and Li, H. 2019. Comparison of Viremia, cloacal virus shedding, antibody responses and pathological lesions in adult chickens, Quails, and pigeons infected with ALV-A. Sci. Rep. 9, 3027. | ||
| How to Cite this Article |
| Pubmed Style Elmeligy AA, Ghania AA, Fotouh A. Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. Open Vet. J.. 2024; 14(8): 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 Web Style Elmeligy AA, Ghania AA, Fotouh A. Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. https://www.openveterinaryjournal.com/?mno=201990 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.24 AMA (American Medical Association) Style Elmeligy AA, Ghania AA, Fotouh A. Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. Open Vet. J.. 2024; 14(8): 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 Vancouver/ICMJE Style Elmeligy AA, Ghania AA, Fotouh A. Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 Harvard Style Elmeligy, A. A., Ghania, . A. A. & Fotouh, . A. (2024) Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. Open Vet. J., 14 (8), 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 Turabian Style Elmeligy, Abdelmenem Ahmed, Ali A. Ghania, and Ahmed Fotouh. 2024. Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. Open Veterinary Journal, 14 (8), 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 Chicago Style Elmeligy, Abdelmenem Ahmed, Ali A. Ghania, and Ahmed Fotouh. "Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt." Open Veterinary Journal 14 (2024), 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 MLA (The Modern Language Association) Style Elmeligy, Abdelmenem Ahmed, Ali A. Ghania, and Ahmed Fotouh. "Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt." Open Veterinary Journal 14.8 (2024), 1952-1959. Print. doi:10.5455/OVJ.2024.v14.i8.24 APA (American Psychological Association) Style Elmeligy, A. A., Ghania, . A. A. & Fotouh, . A. (2024) Pathological and immunohistochemical studies of lymphoid leukosis in pigeons in Egypt. Open Veterinary Journal, 14 (8), 1952-1959. doi:10.5455/OVJ.2024.v14.i8.24 |