| Research Article | ||
Open Vet. J.. 2024; 14(8): 1999-2006 Open Veterinary Journal, (2024), Vol. 14(8): 1999–2006 Research Article The adverse side effects of prenatal and postnatal rats’ exposure to silver nanoparticles Induced toxicitySultan Al-Haid1, Mahmoud Elalfy1,2*, Eman Alsyaed2 Mamdouh Abouelmagd2, Ahmad Al-Jazzar3, Fahad A. Al-Hizab3, Wageh Sobhy Darwish4, AbdelRahman Hereba5,6 and Mona Elhadidy7,81Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia 2Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 3Department of Pathology, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia 4Food Hygiene, Safety, and Technology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 5Department of Microbiology, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia 6Department of Biomedical Physics, Medical Research Institute, Alexandria University, Alexandria, Egypt 7Department of Medical Physiology, Faculty of Medicine, Mansoura University, Mansoura, Egypt 8Medical Physiology, Faculty of Medicine, Albaha University, Saudi Arabia *Corresponding Author: Mahmoud Elalfy. Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, P.O. Box 400, Al-Hofuf 31982, Al-Ahsa, Saudi Arabia. Email: malhefnawy [at] kfu.edu.sa Submitted: 26/05/2024 Accepted: 07/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
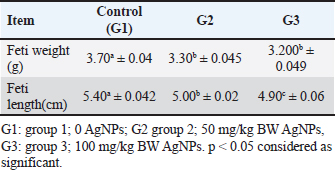
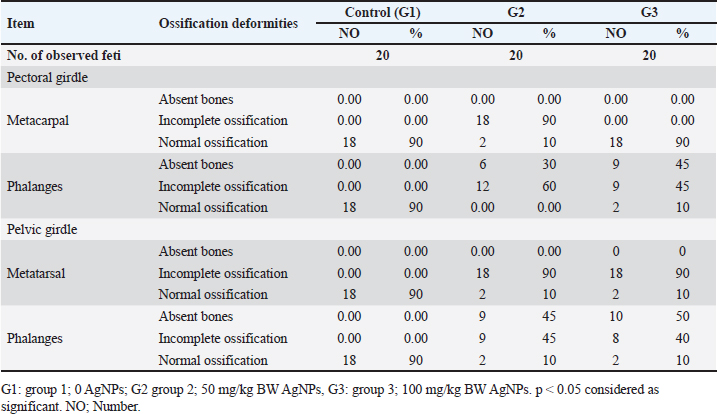
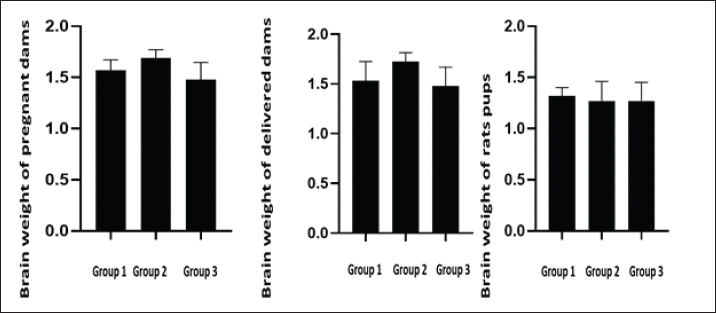
ABSTRACTBackground: Silver nanotechnology is widely applied in industry and medicine, with an increased likelihood of environmental and food contamination. Aim: This study aimed to explore the adverse effects of orally administering silver nanoparticles (AgNPs) to pregnant or lactating female rats on adults and the development of their offspring. Methods: Forty female albino rats were used to assess the immediate impacts of AgNPs in two separate experiments. The experimental group received 1 ml of AgNPs, dissolved in deionized water, at doses of 0, 50, and 100 mg/kg of body weight from the 6th to the 15th day of gestation in pregnant albino rats. After a 20-day gestation period, euthanasia was performed on the female rats, followed by a gross examination post-dissection. Results: The feti were preserved in ethyl alcohol and Poin’s solution for the identification of skeletal and visceral malformations. It was noticed that feti of dams that received AgNPs showed teratogenicities such as delayed ossification and deletion of bones or ribs. Notably, dams showed necrosis and satellitosis with evidence of behavioral alteration. While rats’ pups showed only brain edema and no behavioral changes. Conclusion: AgNPs at a dose of 50 or 100 mg/kg induced teratogenic effect in terms of delayed ossification, abnormal limb formation, and brain edema in rat pups, however, induced necrosis and satellitosis in dam rats. Hence, greater emphasis should be placed on preventing exposure to Ag-NPs, especially among pregnant females. Keywords: Neural effects, Prenatal, Postnatal toxicity, AgNPs, Teratogenicity. IntroductionAdministering silver nanoparticles (AgNPs) has many applications in the fabrication and design of medical and bio sensing devices (Alarcon et al., 2015). AgNPs demonstrate effective antimicrobial properties against a range of bacteria and viruses, including the human immunodeficiency virus, hepatitis B virus, and herpes simplex virus (Ciriminna et al., 2020). Moreover, AgNPs have garnered substantial interest owing to their unique optical, chemical, electrical, and catalytic characteristics that can be customized based on surface attributes, size, and shape. As a result, these nanoparticles (NPs) have found applications in diverse industries, serving as antimicrobial agents in the health sector, as well as in sensors, electronic components, and catalysis (Deshmukh et al., 2019). Disinfectants incorporating AgNPs have gained significant attention due to their practical applications in daily life. Consequently, AgNPs have been employed across various industries, including food packaging, textiles, animal husbandry, biomedical applications, and the creation of silver-based air and water filters. Remarkably, Ag NPs have demonstrated efficacy as disinfectants, particularly in recent outbreaks such as COVID-19 (Behbudi, 2021). AgNPs have drawn considerable interest and have been applied for their antibacterial, antifungal, anticancer, and antiangiogenic properties. There may be a health risk to humans because of the increased use of AgNPs. It is still unclear, nevertheless, how AgNPs might affect animal models (Zhang et al., 2015). Early exposure to the AgNPs may cause abnormal placental development and epigenetic changes in the embryo, which could compromise fetal and postnatal health. These findings enhance our knowledge of the evolution of AgNPs in biomedical applications and can be applied to studies concerning the safe use of AgNPs in a variety of biomedical applications (Zhang et al., 2015). Pregnant women, in particular, should take additional precautions to avoid exposure to Ag-NPs. A range of neurobehavioral disorders may arise in the offspring of pregnant animals exposed to Ag-NPs. (Ghaderi et al., 2015). The objective of this study was to investigate the neurobehavioral and developmental effects that AgNPs might induce in female rats, feti, and rat pups. Materials and MethodsExperimental animalsFemale Sprague Dawley rats, with weights falling within the range of 180–200 g. All rats were in good health and were accommodated in plastic cages (60 × 90 × 90 cm) with bedding comprised of wood shavings. Six male rats were utilized for mating purposes. Before the experiment, the animals underwent a 2-week acclimatization period during which they had unlimited access to water and were provided with a balanced diet that contained (percentage per 100 g) carbohydrate (48.8%), protein (21%), fat (3%), calcium (0.8%), phosphorus (0.4%), moisture (13%), ash (8%), and total energy (2,306 kal/100 g). ChemicalsAgNPs were purchased from Sigma-Aldrich (Saint Louis, MO, USA) (Nano powder, <100 nm particle size, contains Polyvinylpyrrolidone (PVP) as dispersant, 99.5% trace metals basis). Experimental designExperiment 1 A total of 18 female rats, weighing between 180 and 200 g, were allocated into three groups, each consisting of six rats. The pregnant female rats in the treated groups received oral administration of AgNPs, dissolved in deionized water, at doses of 0, 50, and 100 mg/kg body weight (equivalent to 1/100 and 1/50 of the LD50) from the 6th to the 15th days of gestation in pregnant female rats (Maneewattanapinyo et al., 2011). The administration continued until the 20th day of gestation. The protocol was approved form ethical committee in King Faisal University and followed all guidelines of animal management and welfare during treatment and authorization ((KFU-REC-2024-JUN-ETHICS18781). There was no PVP control group as PVP considered non toxic as reported in many articles that used coated AgNPs in teratogenicity studies. Experiment 2 Eighteen lactating female rats, ranging in weight from 180 to 200 g, were divided into three groups, each consisting of six rats, and were maintained until delivery. In this investigation, AgNPs (Sigma Aldrich, USA) were orally administered, and dissolved in deionized water, at dose levels of 0, 50, and 100 mg/kg body weight (equivalent to 1/50 and 1/100 of the LD50 previously recorded) starting from the first day of lactation until the 21st day. The control group received an intubation of deionized water. Dams were weighed before dosing to ensure consistent dosing throughout the experimental period. On the 21st day of lactation, all rats were euthanized using general anesthesia with an intraperitoneal injection of 40 mg/kg sodium thiopental (25 mg/l) (Marques et al., 2018). Brain tissue samples were collected and fixed in buffered formalin for subsequent histopathological examination. Gross and skeletal examinationThe gravid uterus was examined closely, the feti were separated, and the abdominal wall of the pregnant rat dams was opened and reflected over the thorax. The live-to-dead ratio and any obvious deformities were assessed on the separated feti. Additionally, the fetal body weight and crown rump were found. The feti of each dam was divided into two groups: the first group was preserved in an absolute solution of ethyl alcohol for skeletal malformation, and the second group was kept in Bouin’s solution for visceral and histopathological examination (Gad and Weil, 1982). The feti preparation processes included skinning, evisceration, tissue dissolution, and staining skeletal malformation (JG, 1965). Clinical signs and behavioural testThroughout the trial, the treated mothers and newborns were checked every day for any unusual signs, changes, or behaviours. Each rat was trained to mark its toe with ink during the behavioural assessment of its gait movement to its cage. All rats were then tested following the conclusion of their treatment and prior to sacrifice. Histopathological examinationSections of the brain from feti, pups, female pregnant dams, and delivered female rats were isolated and preserved in 10% neutral buffered formalin. The samples were prepared to produce tissue sections with a thickness of 5 microns, and these sections were then stained using hematoxylin and eosin. After staining, the sections were examined under a light microscope. Statistical analysisAnalysis of the data was conducted using a commercial statistical software program (SPSS for Windows, version 21; SPSS Inc., Chicago, IL, United States). The normality of the data distribution was assessed through the Shapiro–Wilk test. Based on the results of this test indicating normal distribution, one-way analysis of variance was employed to compare group means. Tukey’s test was applied to identify significant variations among mean values. All results were presented as the standard error of the mean, and statistical significance was defined at p < 0.05. Ethical approvalThe ethical protocol for the current experiment received approval from the Ethical Committee of King Faisal University, Saudi Arabia (KFU-REC-2024-JUN-ETHICS18781). ResultsA significant decrease in fetal body weight was evident in both groups receiving AgNPs compared to the control group. However, there was no statistically significant distinction in fetal weight between the groups treated with 50 and 100 mg/ kg BW of AgNPs as presented in Table 1 and Figure 1. Skeletal abnormalitiesThe skull of the group of mothers who received 1/100 LD50 AgNPs from the seventh to the fifteenth day of pregnancy revealed some anomalies in the feti’s skeleton, with the parietal, interpareital, occipital, and frontal bones showing incomplete ossification at percentages of 66.7%, 50%, 100%, and 33.3%, respectively. The axial bones displayed 100%, 83.3%, and 100% incomplete ossification of the coccygeal vertebrates, sternebrae, and xipho-sternebrae, respectively. The ribs displayed incomplete ossification, a wavy rib figure, and the absence of some ribs as displayed in Figure 1. In 90 % of the fetis analyzed, the pectoral girdle revealed incomplete ossification of the metacarpal bones, whereas 30% of the fetis displayed total phalangeal absence and 60 % displayed incomplete phalangeal ossification. In 90% of the fetish samples analyzed, the pelvic girdle revealed incomplete ossification of the metatarsal bones, while 45% displayed complete absence of phalanges and 45% displayed incomplete ossification of phalanges (Table 2). From the 6th to 15th day of pregnancy, the group that received LD50 AgNPs exhibited incomplete ossification of the parietal, intraparietal, occipital, and frontal bones, with percentages of 11.1%, 16.7%, 11.1%, and 33.3%, respectively (Fig. 1). Complete absence is also present in the occipital, parietal, and intraparietal bones, with corresponding percentages of 5.6%, 38.8%, and 38.8% (Fig. 1). 38.9% of the feti under examination had a complete absence of xipho-sternebrae, while the remaining axial bones displayed incomplete ossification of the xipho-sternebrae, sternebraes, and coccygeal vertebrates by 27.8%, 66.7%, and 100%, respectively. The data in Table 2 indicated that in 90% of the fetis analyzed, the pectoral girdle revealed incomplete ossification of the metacarpal bones, whereas 45% of the fetis displayed complete absence of phalanges and 45% displayed incomplete ossification of phalanges. In 90% of the fetish samples analyzed, the pelvic girdle revealed incomplete ossification of the metatarsal bones, whereas 8% displayed total absence of phalanges and 44.4% displayed incomplete ossification of phalanges. Gross and histology of brainThe data presented in Figures 2 and 3 revealed that notably, high doses of silver nanoparticles had reduced the weight of the brain non significantly in both dams in the prenatal or post-natal type of toxicity. While a low dose of silver nanoparticles increases brain weight non significantly when compared to the control group. Additionally, both doses of AgNPs had no effect of the brain weight of rat pups. Dams of female rats either in prenatal of post-natal short term of toxicity showed neuronal necrosis and satellitosis. While feti of dams received silver nanoparticles during gestation displayed proliferation of glial cells as displayed in Figure 4. Additionally, the brain of suckling pups (21-day old age) from the dam received 50 mg/kg or 100 mg/kg AgNPs from delivery until weaning, showing perivascular edema. Notably, as the presence of lesions of the brain, dams received AgNPs showed behavioral alterations, while rats’ pups had no changes in behavioral tests during the whole experiments. Table 1. Fetal body weight and fetal length from dams received different doses of silver nanoparticles orally from 7th day to 15th day of pregnancy (Mean ± SE).
DiscussionAround the world, nanotechnology is a major concern in a lot of fields. AgNPs were used in medicine and veterinary care for a variety of purposes, including antibacterial, disinfectant, anticancer, and household. In this work, we investigated the effects of AgNPs on the mother, the embryo’s toxicity, and the postnatal development in female albino rats at doses of 1/100 and 1/50 of LD50 (or 50, 100 mg/kg) from the 7th to the 15th day of gestation for the prenatal study, and from the 1st to the 21st day after delivery for the postnatal study. This research supported the findings of (Mahabady, 2012) who discovered that exposure to 0.4 and 0.8 mg/kg of AgNPs in the eighth and ninth days of rat gestation decreased the weight and length of embryos and did not result in skeletal malformation. The current study also demonstrated that AgNPs decrease the body weight and crown rump of the feti from dams received them during the pregnancy period. According to Prakash et al. (2018) oral administration of AgNPs to a pregnant woman may have caused placental translocation of the particles into the fetus’s organs, which may have resulted in a reduction of the feti body weight and crown rump. According to the current study, skeletal abnormalities in the ribs, skull, metacarpal and metatarsal bones, and phalanges were caused by AgNPs. In this context, Prakash et al. (2018). Multiple skeletal malformations as a teratogenic anomaly that was seen in foetuses from dam rats that were given repeated oral doses of larger size AgNPs colloidal solution. The increasing percentages were examined in correlation with the increase in dose. The control group showed a minimal 9% malformation rate, while the treated group exhibited higher percentages of 63% and 79% with doses of 14 mg/kg/day and 19 mg/kg/day, respectively. Among these skeletal abnormalities were instances of oligodactyly, syndactyly, and limb malformations.
Fig. 1. Skeletal malformation induced by oral administration of silver nanoparticles. (I): Feti from dam mice received 50 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) showed absence of carpal, tarsal, and phalanges of fore limb and hind limb and in complete ossification of metacarpal and metatarsal (arrow) (A) control. (II): Feti from dam mice received 50 mg/kg BW AgNPs from 6th to 15th of pregnancy (B) showed incomplete ossification of skull bones (inter parietal, parietal, occipital, and frontal) also in complete ossification of ribs and caudal vertebrae (arrow) control (A). (III): Feti from dam mice received 100 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) showed incomplete ossification of skull bones (inter parietal and parietal) and ribs (arrow) compared with control (A). (IV): Feti from dam mice received 100 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) showed incomplete ossification of occipital bone of the skull (arrow) compared with control (A). (V): Feti from dam rat received 100 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) compared with control (A) showed complete absence of skull bones (inter parietal and parietal) and incomplete ossification of caudal vertebrae (arrow). (VI): Feti from dam rats received 1/50 LD5 100 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) showed absence of carpal, tarsal, and phalanges of fore limb and hind limb and incomplete ossification of metacarpal and metatarsal (arrow) compared with control (A). (VII): Feti from dam rats received 100 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) showed absence of xiphosternbrae and incomplete ossification of sternbrae (arrow) compared with control (A). (VIII): Feti from dam rats received 100 mg/kg BW AgNPs from 6th to 15th day of pregnancy (B) showed incomplete ossification of ribs (arrow) compared with control (A). (IX): Feti from dam RATS received 50 mg/kg BW AgNPs (B) and feti from dam rats received 100 mg/kg BW AgNPs (C) from 6th to 15th of pregnancy showed incomplete ossification of skull bones and caudal vertebrates (arrow) compared with control (A). (X): Feti from dam RATS received 50 mg/kg BW AgNPs (B) and feti from dam rats received 100 mg/kg BW AgNPs (C) from 6th to 15th of pregnancy showed incomplete ossification of fore and hind limb bones (arrow) compared with control (A). Table 2. Pectoral and pelvic girdle malformation of feti from dams received different doses of silver nanoparticles from 6th to 15th day of pregnancy.
Fig. 2. Brain weight of dams and rats pups received silver nanoparticles during gestation or after delivery. (p < 0.05). Similarly, administering oral gavages at doses of 5, 10, 15, and 20 mg AgNPs/kg body weight could lead to toxic effects, including skeletal dysmorphology (Ghosh et al., 2012). AgNPs’ genotoxic effect, which caused apoptosis and DNA strand breaks, may be the source of their teratogenicity (Pani and Singh, 2017). AgNPs also caused imbalances in DNA damage, chromosome instability, inhibition of mitosis, and anti-reactive oxygen species (ROS) response, among other toxic effects (Xu et al., 2012).
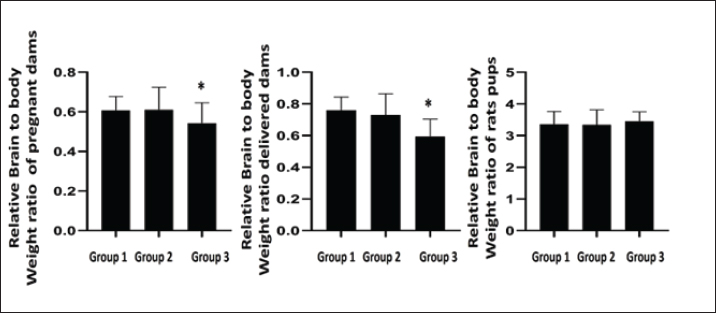
Fig. 3. The depicted figure represented relative brain to body weight ratio of pregnant dams, delivered rats and rat pups.
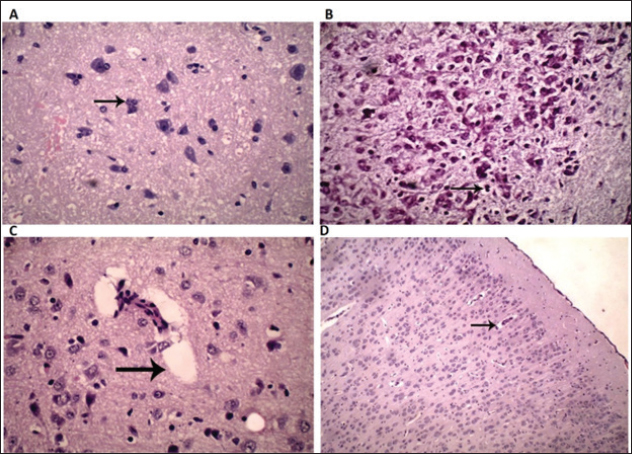
Fig. 4. Histopathological changes of brain of dams and rats pups received silver nanoparticles during gestation or after delivery. (A): Section from brain of parturient dam received 100 mg/kg BW AgNPs from 6th until 15th day of pregnancy, showing neuronal necrosis and satellitosis (arrow). (HE, 400x). (B): Section from brain of fetus from dam received 100 mg/kg BW AgNPs from 7th until 15th day of pregnancy, showing proliferation of glial cells (arrow). (HE, 400x). (C): Section from brain of suckling pups (21-day old age) from dam received 50 mg/kg AgNPs from delivery until weaning, showing perivascular edema (arrow). (HE, 400x). (D): Section from brain of suckling pups (21 day old age) from dam received 100 mg/kg BW AgNPs from delivery till weaning, showing perivascular edema (arrow) (HE,100x). The outcomes of the current study align with prior research, demonstrating that oral exposure to AgNPs led to an increase in silver concentration in the brains of adult rats. However, the amount observed was still notably lower than the silver concentration found in the brains of pups treated with AgNPs. This was because the small size of the AgNPs made them more likely than their ion form to cross biological barriers. The study also found that the AgNPs caused perivascular cuffing of lymphocytes, neuronal necrosis, and satellitosis in the brains of feti. Due to the blood–brain barrier’s (BBB) lack of functionality, it was able to cross the placental barrier, distribute to the fetus brain, and accumulate in other organs of the pups (Jiang et al., 2008; Abbott et al., 2010; Park et al., 2011; Philbrook et al., 2011; Lee et al., 2012; Melnik et al., 2013). Histological abnormalities in the cerebellum and brain tissue were induced by AgNPs. The BBB was not the sole pathway through which NPs could traverse; they could also compromise the integrity of the barrier. Moreover, NPs could instigate apoptosis by generating ROS that act as signaling molecules. Finally, NPs demonstrated the ability to alter the function of glutamate, the primary excitatory neurotransmitter in the brain, by enhancing the quantity of N-methyl-D-aspartate receptor receptors. This led to the degeneration of glutamatergic neurons and impaired cellular excitation, as identified in the study by Cortese-Krott et al. (2009). The toxicity observed was attributed to these effects of AgNPs. Contrarily, the oral administration of AgNPs to pregnant female rats, as detailed in the study by Rashno et al. (2014), did not induce any adverse effects on the pregnant dams or the fetal growth. The only observed impact was a temporary delay in the development of neurobehavioral reflexes in the offspring during the early postpartum period, with no reported issues later in the infants’ lives. Moreover, the present study corroborates a prior investigation that identified brain edema and the disruption of the blood-brain barrier resulting from nanoparticle exposure. The alterations in the brain were noted in regions where there was evidence of leakage of the blood-brain barrier protein tracer. AgNPs affect various components of the brain, encompassing both neuronal and non-neuronal regions, such as nerve, glial, and endothelial cells, as well as axons. The impact of NPs on endothelial cells resulted in perivascular edema, sponginess, and an increase in neutrophils approximately small blood vessels. AgNPs demonstrated a more pronounced influence on the physiological function of the brain (Sharma et al., 2010; Moradi-Sardareh et al., 2018). ConclusionOral administration of AgNPs to pregnant or lactating rats at a dose of 50 or 100 mg/kg induced toxic and teratogenic effects in terms of delayed ossification, abnormal limb formation, brain edema in rat pups, however induced necrosis and satellitosis in adult female rats. Hence, greater emphasis should be placed on preventing exposure to Ag-NPs, especially among pregnant females when used medical stuffs or cosmetics contain Ag-NPs. AcknowledgmentThis work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. GRANT- A142). Conflict of interestAll authors had no conflict of interest regarding any aspect of this publication. FundingDeanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. GRANT- A142). Authors’ contributionsAll authors contributed to the present study. Data AvailabilityData will be available upon reasonable request. ReferencesAbbott, N.J., Patabendige, A.A., Dolman, D.E., Yusof, S.R. and Begley, D.J. 2010. Structure and function of the blood–brain barrier. Neurobiol. Dis. 37(1), 13–25. Alarcon, E.I., Griffith, M. and Udekwu, K.I. 2015. Silver nanoparticle applications. Springer International Publishing, Switzerland, 10, 973-978. Behbudi, G.2021. Effect of silver nanoparticles disinfectant on covid-19. Adv. Nano. Res. 2(2), 63–67. Ciriminna, R., Albo, Y. and Pagliaro, M. 2020. New antivirals and antibacterials based on silver nanoparticles. Chem. Med. Chem. 15(17), 1619–1623. Cortese-Krott, M.M., Münchow, M., Pirev, E., Hessner, F., Bozkurt, A., Uciechowski, P., Pallua, N., Kröncke, K.D. and Suschek. C.V. 2009. Silver ions induce oxidative stress and intracellular zinc release in human skin fibroblasts. Free Radic. Biol. Med. 47, 1570–7. Deshmukh, S.P., Patil, S., Mullani, S. and Delekar, S. 2019. Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng. C 97, 954–965. Gad, S.C., and Weil, C. 1982. Principles and methods of toxicology. New York, NY: by Hayes AW, Raven Press, pp: 273–320. Ghaderi, S., Tabatabaei, S.R.F., Varzi, H.N. and Rashno, M. 2015. Induced adverse effects of prenatal exposure to silver nanoparticles on neurobehavioral development of offspring of mice. J. Toxicol. Sci. 40(2), 263–275. Ghosh, M., Manivannan, J., Sinha, S., Chakraborty, A., Mallick, S.K., Bandyopadhyay, M. and Mukherjee, A. 2012. In vitro and in vivo genotoxicity of silver nanoparticles. Mutat Res Genet. Toxicol. Environ. Mutagen. 749 (1-2), 60–69. JG, W. 1965. Embryonic considerations in teratology. Teratology: Principles and Techniques. Chicago, IL: University of Chicago Press, pp: 251–277. Jiang, W., Kim, B.Y., Rutka, J.T. and Chan, W.C. 2008. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3(3), 145–150. Lee, Y., Choi, J., Kim, P., Choi, K., Kim, S., Shon, W. and Park, K. 2012. A transfer of silver nanoparticles from pregnant rat to offspring. Toxicol. Res. 28, 139–141. Mahabady, M.K. 2012. The evaluation of teratogenicity of nanosilver on skeletal system and placenta of rat fetiin prenatal period. Afr. J. Pharm. Pharmacol. 6(6), 419–424. Maneewattanapinyo, P., Banlunara, W., Thammacharoen, C., Ekgasit, S. and Kaewamatawong, T. (2011). An evaluation of acute toxicity of colloidal silver nanoparticles. J. Vet. Med. Sci. 3(11), 1417–1423. Melnik, E., Demin, V., Demin, V., Gmoshinski, I., Tyshko, N. and Tutelyan, V. 2013. Transfer of silver nanoparticles through the placenta and breast milk during in vivo experiments on rats. Acta. Nat. 5(3), 107–115. Moradi-Sardareh, H., Basir, H.R.G., Hassan, Z.M., Davoudi, M., Amidi, F. and Paknejad, M. 2018. Toxicity of silver nanoparticles on different tissues of Balb/C mice. Life. Sci. 211, 81–90. Marques, T.R., Caetano, A.A., Henrique S. Cesar, P., Braga, M.A., Henrique A. Machado, G., de Sousa, R.V. and Corrêa, A.D. 2018. Antioxidant activity and hepatoprotective potential of lyophilized extract of acerola bagasse against CCl4-induced hepatotoxicity in Wistar rats. J. Food. Biochem. 42(6), e12670. Pani, J. and Singh, R. 2017. Skeletal dysmorphology in fetus following colloidal silver ingestion in pregnant Swiss albino mice. MOJ Anat. Physiol. 4(2), 251–256. Park, M.V., Neigh, A.M., Vermeulen, J.P., de la Fonteyne, L.J., Verharen, H.W., Briedé, J.J., van Loveren, H. and de Jong, W.H. 2011. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomater 32(36), 9810–9817. Philbrook, N.A., Winn, L.M., Afrooz, A.N., Saleh, N.B. and Walker, V.K. 2011. The effect of TiO2 and Ag nanoparticles on reproduction and development of Drosophila melanogaster and CD-1 mice. Toxicol. Appl. Pharmacol. 257(3), 429–436. Prakash, P., Royana, S. and Sankarsan, P. 2018. Multi-organ teratogenesis sequels of bigger size particles colloidal silver in primate vertebrates. J. Cytol. Histol. 9(2), 501. Rashno, M., Fatemi Tabatabaei, S.R., Khaksary Mahabady, M. and Ghaderi, S. 2014. Maternal exposure to silver nanoparticles in mice: effects on dams’ reproductive performance and pups’ neurobehavioral ontogeny. Anat. Sci. J. 11 (1), 41–52. Sharma, H.S., Hussain, S., Schlager, J., Ali, S.F. and Sharma, A. 2010. Influence of nanoparticles on blood–brain barrier permeability and brain edema formation in rats. In Brain edema XIV. Eds., Czernicki, Z., Baethmann, A., Ito, U., Katayama, Y., Kuroiwa, T. and Mendelow, AD. Vienna, Austria: Springer, pp: 359–364. Xu, L., Li, X., Takemura, T., Hanagata, N., Wu, G. and Chou, L.L. 2012. Genotoxicity and molecular response of silver nanoparticle (NP)-based hydrogel. J. Nanobiotechnology 10, 1–11. Zhang, X.F., Park, J.H., Choi, Y.J., Kang, M.H., Gurunathan, S. and Kim, J.H. 2015. Silver nanoparticles cause complications in pregnant mice. Int. J. Nanomed. 10, 7057–7071. | ||
| How to Cite this Article |
| Pubmed Style Al-haid S, Elalfy M, Alsyaed E, Abouelmagd M, Al-jazzar A, Al-hizab FA, Darwish WS, Hereba A, Elhadidy M. The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. Open Vet. J.. 2024; 14(8): 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 Web Style Al-haid S, Elalfy M, Alsyaed E, Abouelmagd M, Al-jazzar A, Al-hizab FA, Darwish WS, Hereba A, Elhadidy M. The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. https://www.openveterinaryjournal.com/?mno=203058 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.29 AMA (American Medical Association) Style Al-haid S, Elalfy M, Alsyaed E, Abouelmagd M, Al-jazzar A, Al-hizab FA, Darwish WS, Hereba A, Elhadidy M. The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. Open Vet. J.. 2024; 14(8): 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 Vancouver/ICMJE Style Al-haid S, Elalfy M, Alsyaed E, Abouelmagd M, Al-jazzar A, Al-hizab FA, Darwish WS, Hereba A, Elhadidy M. The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 Harvard Style Al-haid, S., Elalfy, . M., Alsyaed, . E., Abouelmagd, . M., Al-jazzar, . A., Al-hizab, . F. A., Darwish, . W. S., Hereba, . A. & Elhadidy, . M. (2024) The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. Open Vet. J., 14 (8), 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 Turabian Style Al-haid, Sultan, Mahmoud Elalfy, Eman Alsyaed, Mamdouh Abouelmagd, Ahmad Al-jazzar, Fahad A. Al-hizab, Wageh Sobhy Darwish, Abdelrahman Hereba, and Mona Elhadidy. 2024. The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. Open Veterinary Journal, 14 (8), 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 Chicago Style Al-haid, Sultan, Mahmoud Elalfy, Eman Alsyaed, Mamdouh Abouelmagd, Ahmad Al-jazzar, Fahad A. Al-hizab, Wageh Sobhy Darwish, Abdelrahman Hereba, and Mona Elhadidy. "The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity." Open Veterinary Journal 14 (2024), 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 MLA (The Modern Language Association) Style Al-haid, Sultan, Mahmoud Elalfy, Eman Alsyaed, Mamdouh Abouelmagd, Ahmad Al-jazzar, Fahad A. Al-hizab, Wageh Sobhy Darwish, Abdelrahman Hereba, and Mona Elhadidy. "The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity." Open Veterinary Journal 14.8 (2024), 1999-2006. Print. doi:10.5455/OVJ.2024.v14.i8.29 APA (American Psychological Association) Style Al-haid, S., Elalfy, . M., Alsyaed, . E., Abouelmagd, . M., Al-jazzar, . A., Al-hizab, . F. A., Darwish, . W. S., Hereba, . A. & Elhadidy, . M. (2024) The adverse side effects of prenatal and postnatal rats' exposure to silver nanoparticles Induced toxicity. Open Veterinary Journal, 14 (8), 1999-2006. doi:10.5455/OVJ.2024.v14.i8.29 |