| Research Article | ||
Open Vet. J.. 2024; 14(8): 2007-2015 Open Veterinary Journal, (2024), Vol. 14(8): 2007–2015 Research Article Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected miceRisqa Novita1,2*, Agik Suprayogi3, Andria Agusta2, Arifin Budiman Nugraha4, Tomoyoshi Nozaki5, Kurnia Agustini2 and Huda Shalahudin Darusman1,61Primatology Study Program, Graduate School of IPB University, Bogor, Indonesia 2Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Cibinong Science Center, Cibinong, Indonesia 3Department of Anatomy, Physiology and Pharmacology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 4Division of Parasitology and Medical Entomology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 5Department of Biomedical Chemistry, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan 6Primate Research Center, IPB University, Bogor, Indonesia *Corresponding Author: Risqa Novita. Primatology Study Program, Graduate School of IPB University, Bogor, Indonesia. Email: my_risqa [at] apps.ipb.ac.id Submitted: 31/05/2024 Accepted: 25/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
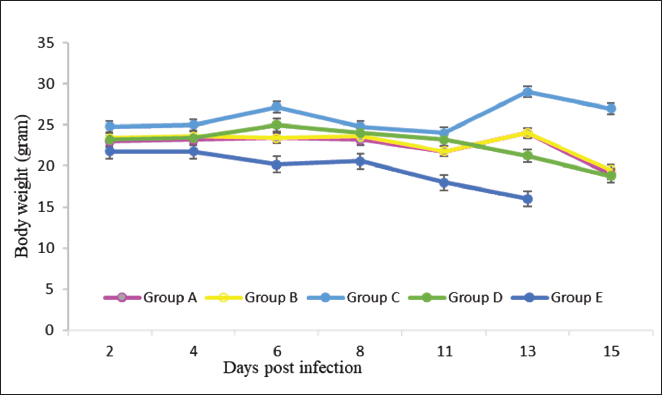
ABSTRACTBackground: Malaria remains a significant global health burden, with drug resistance posing a major challenge to its control. The emergence of resistance to antimalarial drugs represents a critical issue in malaria management, as it heightens the likelihood of morbidity and mortality associated with the disease. There is an urgent requirement for a novel candidate drug with a distinct mechanism of action. Aim: In light of the ongoing challenges in malaria management, particularly the emergence of drug resistance, this study aimed to investigate the efficacy of a novel combination therapy of borrelidin and fumagilin against Plasmodium berghei infection on Swiss Webster mice. The findings of this study could contribute to developing new and effective antimalarial treatments. Methods: This study employed a unique approach, using Swiss Webster mice aged 6–8 weeks and dividing them into five groups, each with five mice. The therapeutic efficacy of the combination treatment was evaluated through a comprehensive assessment of parasitemia levels, survival rates, and histological changes in the liver and spleen. This rigorous methodology ensures the reliability and validity of our findings. Results: The combination of borrelidin and fumagilin led to the lowest parasitemia at 5%, contrasting with the control group reaching 15%. Moreover, the combination group exhibited the highest inhibition rate of 69.6% on day nine post-infection. Histopathological alterations were limited to sinusoid dilation, hepatocyte ballooning, and the presence of hemozoin. Conclusion: These findings suggest that the combination of borrelidin and fumagilin holds promise as a potential antimalarial therapy. Keywords: Antibiotic, Drug resistance, Malaria, SDG’s. IntroductionMalaria continues to be a significant global health burden, especially in areas where Plasmodium spp. is prevalent (Talapko et al., 2019). According to data from WHO, malaria still occurs in Africa, Southeast Asia, the eastern Mediterranean, South America, and the West Pacific (WHO, 2023). Despite numerous efforts to control the disease, challenges like drug resistance and limited treatment options still exist, making it necessary to explore new therapeutic strategies. Indonesia and India are among the highest contributors to malaria cases in Asia. Indonesia, like other malaria-endemic countries, has cases of resistance to several antimalarial drugs, namely chloroquine and sulfadoxine-pyrimethamine (Rahmasari et al., 2022). The primary treatments for uncomplicated Plasmodium falciparum malaria involve the use of combination therapies based on Artemisinin-based combination therapy, which is in line with WHO’s recommendation (Siqueira-Neto et al., 2023). The potential for the parasite to build resistance against existing antimalarial medications underscores the urgency of creating new drugs with unique mechanisms of action (Belete, 2020). Exploring malaria drug discovery via natural products faces various challenges, potentially dampening enthusiasm for further research into these scaffold families. Future antimalarial treatments are anticipated to be formulated as combination therapies to mitigate resistance risks (Siqueira-Neto et al., 2023). Borrelidin and fumagilin are two antibiotics that have been explored for their potential as antimalarial drugs. Borrelidin is an 18-membered macrolide compound that has shown efficacy against drug-resistant strains of P. falciparum, the parasite responsible for malaria. Its potency is demonstrated by an IC50 value of 0.93 ng/ml (Sugawara et al., 2013). Borrelidin protects against lethal murine malaria at a low 0.25 mg·kg−1·day−1 dose. It has been found to protect mice from lethal infections and induce protective immune responses after treatment. Borrelidin’s antimalarial activity correlates with the accumulation of trophozoites in peripheral blood, and all infected mice treated with borrelidin survived and developed immunity, protecting them from reinfection on 75 and 340 days after the initial infection (Azcárate et al., 2013). Borrelidin, originally isolated from Streptomyces rochei, exhibits potent inhibitory activity against threonyl-tRNA synthetase (ThrRS), an essential enzyme in protein synthesis in Plasmodium spp. Inhibition of ThrRS disrupts the parasite’s ability to generate essential proteins, thus impeding its growth and proliferation within the host organism. These findings suggest that borrelidin can serve as a scaffold for antimalarial drug design (Novoa et al., 2014). Fumagilin, on the other hand, has yet to be extensively studied for its antimalarial potential. Fumagilin derived from Aspergillus fumigatus, targets Methionine Aminopeptidase 2 (MetAP2), an essential protein processing and maturation enzyme with an IC50 8 nM (Garrabrant et al., 2004). Fumagilin interferes with crucial cellular processes by inhibiting MetAP2 in Plasmodium, leading to parasite death. It is known to have potent antiparasitic activity with an IC50 of 4–17 ng/ml (Zhang et al., 2002). However, the efficacy of fumagilin in animal models has not yet been published, and it is one of the novelties of this study. World Health Organization recommended an antimalarial combination to enhance therapeutic efficacy by administering two or more blood schizontocidal drugs with independent modes of action improve the overall effectiveness of the treatment. This approach reduces the risk of treatment failure and the development of resistance to individual components of the combination. The rational combination of borrelidin and fumagilin presents several potential advantages over traditional monotherapy approaches. By targeting distinct molecular pathways essential for parasite survival, combination therapy may mitigate the emergence of drug resistance, a critical concern in malaria treatment. Moreover, synergistic interactions between borrelidin and fumagilin could target different stages of the parasite’s life cycle, enhance the drug’s efficacy and reduce the likelihood of resistance development, reduce treatment duration, and potentially lower the risk of adverse effects associated with higher drug doses (Chen et al., 2009; Ishiyama et al., 2011). Further research is needed to understand its potential as an antimalarial drug and to compare its efficacy with artemisinin. This study evaluated the effectiveness of combining borrelidin and fumagilin in treating malaria using a murine model of Plasmodium berghei infection. Materials and MethodsExperimental animalsAdult Swiss Webster female mice weighing 25–30 g and aged 6–8 weeks were used in the study. The mice were allowed to acclimate for a week, were exposed to a 12-hour cycle of light and darkness from 06:00 to 18:00, and had free access to the pellets and water ad libitum. The experiments were conducted at The Animal Laboratory of the Agency for Health Policies Development, Indonesian Ministry of Health, Bogor, from 20 October 2023 to 30 December 2023. Material collectionBorrelidin (B3061-1MG) was delivered from Sigma Aldrich, Fumagilin (F6771-5MG) was delivered from Sigma Aldrich, and P. berghei ANKA was obtained from the Agricultural Instruments Standardization Agency, Indonesian Ministry of Agriculture, Bogor. Inoculation of P. bergheiThe parasites were retrieved from the frozen stock at −80°C before commencing the experiment and intraperitoneally injected into the donor mice. Plasmodium berghei was then passed from the donor mouse to healthy mice thrice until the parasitemia reached approximately 50%. Parasitemia levels were monitored daily through Giemsa-stained blood smears. Subsequently, the donor mice were euthanized, and blood was collected via cardiac puncture. The blood was diluted with phosphate buffer saline, with each 0.5 ml containing 2 × 106 P. berghei-infected red blood cells (iRBCs). Each experimental group received 0.5 ml of iRBCs intraperitoneally (Cahyaningsih et al., 2022). In vivo antimalarial efficacy testThe mice were divided into five groups, each containing five mice. Group A received 0.25 mg/kg body weight (BW) of borrelidin intraperitoneally, while Group B was given 20 mg/kg of fumagilin orally. Group C was given a combination of 0.25 mg/kgBW of borrelidin intraperitoneally and 20 mg/kgBW of fumagilin orally. Group D received 20 mg/kg BW of artemisinin orally (Tu, 2017), and Group E was used as an infected-untreated group. Treatment commenced on day five post-infection (pi) when the average parasitemia had risen to 10% (Cahyaningsih et al., 2022). The therapeutic efficacy was sustained for 4 days, encompassing days 5 through 9 pi. Determination of weight changesAn analytical balance measured body weight on days 0, 4, 6, 8, and 11 (Ohaus SPX 2202). Changes in body weight were calculated and statistically to determine which group significantly differed from the positive control group. Monitoring of parasitemiaParasitemia was observed daily by examining thin blood smears stained with 10% Giemsa solution. Microscopic examination was conducted at a magnification of 1,000x (Eclipse 100i, Nikon, Japan). The percentage of parasitemia was determined by counting parasites per 1,000 RBCs. Histopathological examination of liver and spleenThe Plasmodium infection primarily impacts the kidney, liver, spleen, lungs, and brain, with the liver and kidney particularly susceptible to toxicity (Nigatu et al., 2017; Chin et al., 2019). The organs were harvested on day 15 following infection and subjected to microscopic examination to identify any histopathological alterations to assess the effect of a combination therapy involving borrelidin and fumagilin. The organs were gathered and stored in a 10% buffered formalin (neutral) solution until examination. Subsequently, the organs were processed, sectioned, and stained according to established protocols with certain adjustments. Data analysisThe data were analyzed using Windows SPSS version 23.0. The significance of the treatment effect was determined through the Kruskal-Wallis non-parametric test, with a significance level set at p < 0.05. Post-hoc pairwise comparisons were subsequently conducted to compare results among the groups. Ethical approvalThe Health Research Ethics Commission of the National Research and Innovation Agency (BRIN) has approved the experiments on mice (accession number: 101/KE.03/SK/09/2023). ResultsTreatment with borrelidin and fumagilin combination therapy (Group C) significantly reduced compared to the control group (Group E) (p < 0.05). Additionally, mice receiving combination therapy exhibited higher survival rates than those treated with single therapy or the control group (p < 0.05). Histopathological examination revealed reduced tissue damage and inflammatory cell infiltration in the liver and spleen of mice treated with combination therapy compared to single therapy or control groups. Body weightThe body weight of the animals was measured and recorded on days 0, 4, 6, 8, and 11 pi to assess the effect of borrelidin and fumagilin treatment on changes in body weight compared to the control group as shown in Figure 1. The control group consistently had the lowest body weight values during the study compared to the treatment groups. Statistical analysis confirmed this with p < 0.05, indicating a significant difference between the groups. Further analysis demonstrated significant body weight differences between the treatment and control groups.
Fig. 1. The Body weight of mice was monitored every 2 days. (Group A: 0.25 mg/kg BW borrelidin i.p; Group B: 20 mg/kg BW fumagillin p.o; Group C: 0.25 mg/kg BW borrelidin i.p and 20 mg/kg BW fumagillin p.o; Group D: 20 mg/kg BW artemisinin p.o; Group E: infected-untreated group).
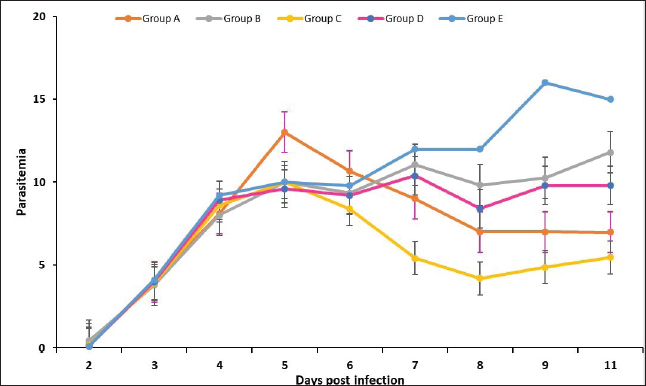
Fig. 2. Effect of drug treatment on the growth of Plasmodium berghei in mice. The parasitemia rate after treatment in the groups. The treatment regime was started from day 5 to 8 post-infection (Group A: 0.25 mg/kg BW borrelidin i.p; Group B: 20 mg/kg BW fumagillin p.o; Group C: 0.25 mg/kg BW borrelidin i.p and 20 mg/kg BW fumagillin p.o; Group D: 20 mg/kg BW artemisinin p.o; Group E: infected-untreated group).
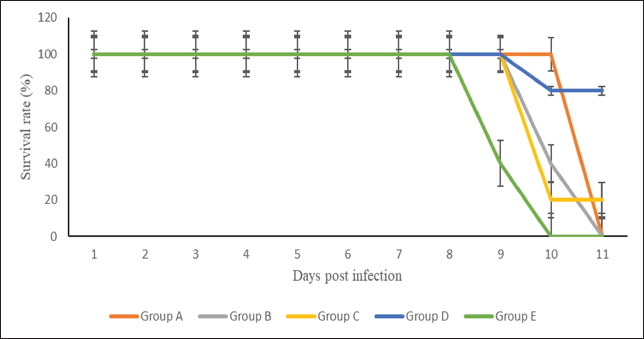
Fig. 3. Survival rate of the groups of mice (Group A: 0.25 mg/kg BW borrelidin i.p; Group B: 20 mg/kg BW fumagillin p.o; Group C: 0.25 mg/kg BW borrelidin i.p and 20 mg/kg BW fumagillin p.o; Group D: 20 mg/kg BW artemisinin p.o; Group E: infected-untreated group). In vivo antimalarial efficacy testThe effects of borrelidin, fumagilin, and their combination on the development of P. berghei were evaluated based on the percentage of parasitemia, inhibition, and survival rate in infected-treatment mice compared to infected-untreated mice. Our study indicated that mice treated with the combination of borrelidin and fumagilin, from day 6 pi (48 hours after the first treatment) until day 11 pi (6 days after the first treatment), showed a significant difference in parasitemia compared to the control (p < 0.05), which yielded similar results to group A and D, but differenced from group B. It demonstrated that the combination of borrelidin and fumagilin has an efficacy comparable to artemisinin. Meanwhile, group B was observed to have the highest parasitemia after group D throughout the study. As seen in Figure 2, all treatment groups experienced a decrease in parasitemia, possibly because of the treatment (Group A, B, C, D), compared to Group E, which continued to exhibit an increase in parasitemia. Group B consistently showed the second-highest parasitemia levels after group D throughout the study, while Group C had the lowest parasitemia percentage. Although group C had the lowest parasitemia, group D exhibited the highest survival rate by the end of the study (Fig. 3). However, the percentage inhibition of Group C was highest compared to other treatment groups from day 6 to 11 pi. However, the survival rate of all treatment groups was 100% from day 6 to 11 pi, except for the mice treated with artemisinin (Group D). The highest percentage of parasite suppression was found in group D from day 7 to 11 pi, with 55, 65, 69.6, and 63.6%, respectively (Fig. 3). Organ pathological changesIn the liver histopathological examination, slight changes were observed, including sinusoid dilation, hepatocyte ballooning, and abundant hemozoin, while P. berghei was not detected (Fig. 4). Meanwhile, the histopathological examination of the spleen only observed hemozoin and sinusoid dilation, as shown in Figure 5. In this study, hemorrhages occurring in the liver are characterized by sinusoidal dilatation, hemozoin, and ballooning hepatocytes. However, only minor alterations were detected. These findings can be attributed to the relatively mild hepar damage in group C, implying that the combination therapy given may have had a beneficial effect. DiscussionMalaria, caused by Plasmodium parasites, is a major global health problem, particularly in tropical and subtropical regions (Talapko et al., 2019). Unlike many viral infections, malaria offers limited immunity against subsequent reinfections, whether due to an incomplete immune response or the vast array of genetic variants (Siqueira-Neto et al., 2023). The emergence of drug-resistant strains of Plasmodium underscores the urgent need for novel therapeutic strategies. Combination therapies, which involve the simultaneous administration of two or more drugs with different mechanisms of action, have been advocated to combat drug resistance and improve treatment outcomes in malaria (Shibeshi et al., 2020; White, 2022). In preclinical studies, borrelidin and fumagilin are antibiotics that have shown promising antimalarial activity. Borrelidin inhibits protein synthesis in the parasite, while fumagilin targets angiogenesis, a process critical for parasite survival and proliferation (Frottin et al., 2016; Saint-Léger et al., 2016a).
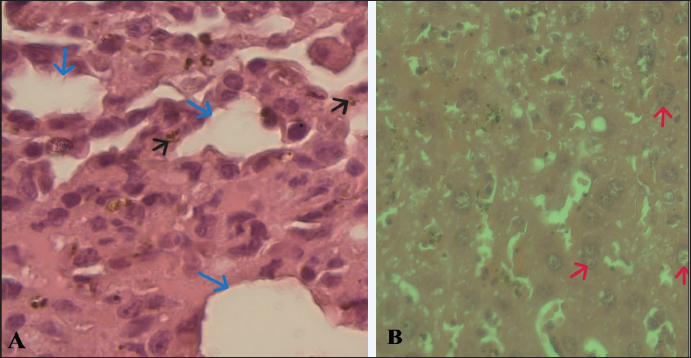
Fig. 4. A. Sinusoidal dilatation (blue arrow), hemozoin (black arrow). B. Ballooning hepatocytes (red arrow) were pathological signs of liver damage of Group C (magnification 100x). Bar 10 µm.
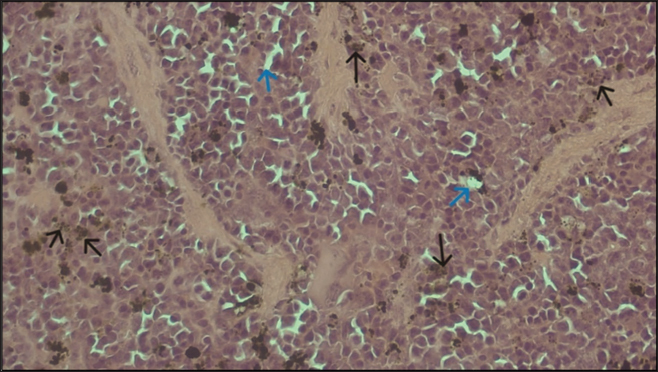
Fig. 5. Histopathologic of the spleen of Group C. Hemozoin and slight sinusoid dilation were visible at a magnification of 100x. Bar 10 µm. Compared to the other groups, the lowest percentage of parasitemia in group C implies a higher level of parasitemia inhibition within group C. This study showed that the rational combination of borrelidin and fumagilin has the lowest parasitemia, the highest survival rate, and mild hepar damage. As a result, the recovery rate for group C was superior to the other groups. Combining borrelidin and fumagilin has demonstrated significantly enhanced efficacy against Plasmodium berghei infection in mice. This synergistic effect can be attributed to several factors, primarily the distinct mechanisms of action and complementary modes of inhibiting the parasite’s proliferation and survival. Borrelidin operates by inhibiting ThrRS, an essential enzyme involved in protein synthesis in Plasmodium. Borrelidin disrupts the parasite’s ability to generate essential proteins by targeting this specific molecular pathway, impeding its growth and proliferation within the host organism. On the other hand, fumagilin exerts its antimalarial activity by inhibiting MetAP2, a crucial enzyme involved in protein processing and maturation. Consequently, the concurrent administration of borrelidin and fumagilin presents a multi-targeted approach, effectively blocking different stages of protein synthesis within the parasite’s lifecycle (Chen et al., 2006; Arico-Muendel et al., 2009; Bhikshapathi et al., 2010; Saint-Léger et al., 2016b). The combination therapy may lead to increased drug accumulation within Plasmodium-infected cells. Borrelidin and fumagilin may exhibit synergistic effects on cellular uptake mechanisms or intracellular trafficking pathways, facilitating enhanced drug concentrations at the site of action. This phenomenon can potentiate the individual efficacies of both compounds, resulting in a more pronounced antimalarial effect than monotherapy with either borrelidin or fumagilin alone. Furthermore, the combined regimen could mitigate the emergence of drug resistance in Plasmodium populations. As borrelidin and fumagilin target distinct molecular pathways, the likelihood of the parasite developing cross-resistance to both drugs is reduced. This aspect is crucial in combating the persistent challenge of antimalarial drug resistance, as the emergence of resistant strains often undermines the efficacy of single-agent therapies. Meanwhile, the histopathological examination of the spleen only observed hemozoin and sinusoid dilation, as shown in Figure 5, indicating reinfection prevention. In comparing these compounds, artemisinin stands out for its well-established role in malaria treatment, while borrelidin and fumagilin offer diverse pharmacological potentials beyond antimalarial applications. Artemisinin’s mechanism of action involves its activation by the heme released from hemoglobin digestion within the malaria parasite. This activation generates free radicals, particularly reactive oxygen species, which damage essential biomolecules within the parasite, ultimately leading to its death. Additionally, artemisinin can interfere with the parasite’s calcium homeostasis and mitochondrial function, further contributing to its antimalarial effects. Borrelidin exerts its pharmacological effects by inhibiting ThrRS, an essential enzyme involved in protein synthesis. By blocking this enzyme, borrelidin disrupts the production of proteins necessary for bacterial and cancer cell survival, ultimately leading to their death. Fumagilin’s mechanism of action involves inhibition of the enzyme methionine aminopeptidase, which is crucial for removing the initiator methionine from nascent polypeptides during protein synthesis. Fumagilin disrupts protein synthesis in microorganisms by inhibiting MetAP (Sin et al., 1997; O’Neill et al., 2010; Giessen & Marahiel, 2014). Fumarranol, an analogue of fumagilin, has been shown to inhibit malaria growth by interacting with Plasmodium falciparum Methionine Aminopeptidase 2. After treatment with fumarranol, Plasmodium yoelii showed a significant reduction in parasitemia levels in mice, leading to an extended mean survival time in a dose-dependent manner as long as 30 days at a dose of 120 mg/kg BW (Chen et al., 2009). The administration of fumagilin alone resulted in higher parasitemia than the combination group of borelidin and fumagillin in this study. This suggests that administering fumagilin by itself may not effectively suppress the growth rate of plasmodium. On the other hand, the borrelidin group could suppress plasmodium growth and, statistically, showed no significant difference compared to the combination group of borrelidin and fumagilin and the artemisinin group. This indicates that the efficacy of administering borelidin alone and combined with fumagillin is comparable to that of artemisinin. Malaria leads to body weight loss due to the immune system’s response to the parasite, which triggers the secretion of the hormone leptin and nitric oxide (Pulido-Mendez et al., 2002). This aligns with this study’s findings that mice’s body weight in the untreated control group was lower compared to the treatment group. The spleen and liver are organs susceptible to the effects of malaria infection. Hyperplastic Kupffer cells, portal tract inflammation, sinusoidal congestion, and hemozoin pigment deposition are important pathological features associated with higher levels of malaria. (Viriyavejakul et al., 2014). The histopathological results were similar to those observed in the spleen, where hemosiderin and cytoplasmic vacuolation were also detected. Infection with P. berghei leads to liver histopathological alterations, including sinusoidal dilation, presence of polymorphonuclear cells, hemosiderin deposition, degeneration, necrosis of hepatocytes, vacuolisation of epithelial cells, infiltration by mononuclear cells, and megalocytosis. However, in the histopathological examination of Group C, only sinusoidal dilation and ballooning hepatocytes were observed, indicating that the liver damage in Group C was not severe, possibly due to the administration of the combination of borrelidin and fumagilin. Similarly, slight sinusoidal dilation and hemosiderin deposition were observed in the spleen histopathological findings. Hemosiderin, a protein or amino acid derived from blood, is produced due to damage to red blood cells. The buildup of hemosiderin in liver tissue occurs due to the excessive destruction of red blood cells (hemolysis) triggered by parasite presence during the initial stages of infection, resulting in anemia in infected animals. The liver is the primary site of infection and replication of Plasmodium in the human body. In the liver, hepatocytes are the primary target cells for Plasmodium. The process of hepatocyte ballooning in murine malaria is a critical aspect of the disease pathogenesis. Hepatocyte ballooning is when the hepatocytes become swollen and lose their typical structure. The accumulation of parasitized erythrocytes causes this process, releases toxic products by the parasites, and activates releases toxic products by the parasites and activates the host’s immune response. The accumulation of parasitized erythrocytes in the liver sinusoids leads to the compression of hepatocytes, causing them to swell and lose their typical structure. The release of toxic products by the parasites, such as hemozoin and lactate dehydrogenase, further contributes to the ballooning process. The activation of the host’s immune response, including the release of cytokines and the activation of complement proteins, also plays a role in the ballooning process. Hepatocyte ballooning is a critical aspect of the disease pathogenesis in murine malaria. It is associated with the development of severe clinical symptoms, such as hepatomegaly, jaundice, and anemia. The ballooning process also contributes to the development of liver fibrosis and cirrhosis, which can lead to long-term liver damage. Furthermore, hepatocyte ballooning is a valuable marker for the diagnosis and prognosis of malaria (Mandell et al., 2010; Bertrram et al., 2012). The use of antibiotics as standalone treatments for malaria is not recommended due to their slower action compared to standard antimalarials (Bertram et al., 2012), this aligns with the findings of this study, which suggests that combining the antibiotics borrelidin and fumagilin yields comparable efficacy to that of artemisinin. However, this study has some limitations. This study did not measure the pharmacokinetics between borrelidin and fumagilin compared to artemisinin, and no statistical evaluation of cumulative versus synergistic effects of the two combined therapies was conducted. ConclusionOur study provides evidence for the effectiveness of combining borrelidin and fumagilin as a novel candidate for antimalarial therapy. The combination therapy demonstrated superior efficacy to monotherapy in reducing parasitemia levels and improving survival rates in Plasmodium berghei-infected Swiss Webster mice. Further research is warranted to elucidate the precise molecular mechanisms underlying this synergism and a pharmacokinetics study to measure the half-life of borrelidin and fumagilin in mice. AcknowledgmentThe authors sincerely thank Mawar Subangkit, PhD from the Laboratory of Veterinary Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, for his valuable insights on this manuscript, particularly in the statistical analysis section. The authors also acknowledge Dr Jontari Hutagalung, Asmidah Karmini, and Asiah from The Animal Laboratory of the Agency for Health Policies Development of the Indonesian Ministry of Health for their technical assistance and valuable contributions to this study. Conflict of interestThe authors declare no conflict of interest in this study. FundingThis work was (partially) supported by the Rumah Program Grant Year 2023 from the National Research and Innovation Agency of the Republic of Indonesia (contract No 16/III.9/HK/2023), Science and Technology Research Partnership for Sustainable Development (SATREPS) from the Japan Agency for Medical Research and Development (AMED), and Japan International Cooperation Agency (JICA). Author’s contributionAll authors developed the concept and design of the study. RN and ABN performed material performation, data curation, and analysis. AS, AA, KA, TN, and HSD supervised the research process. RN drafted the manuscript under the supervision of AS, AA, ABN, KA, TN, and HSD. All authors reviewed and approved the final version of the manuscript. Data availabilityAll data corroborating the results of this study are incorporated within the manuscript. ReferencesArico-Muendel, C., Centrella, P. A., Contonio, B. D., Morgan, B. A., O’Donovan, G., Paradise, C. L., Skinner, S. R., Sluboski, B., Svendsen, J. L., White, K. F., Debnath, A., Gut, J., Wilson, N., McKerrow, J. H., DeRisi, J. L., Rosenthal, P. J. and Chiang, P. K. 2009. Antiparasitic activities of novel, orally available fumagilin analogs. Bioorg. Med. Chem. Lett. 19(17), 5128–5131. Azcárate, I. G., Marín-García, P., Camacho, N., Pérez-Benavente, S., Puyet, A., Diez, A., Ribas De Pouplana, L. and Bautista, J. M. 2013. Insights into the preclinical treatment of blood-stage malaria by the antibiotic borrelidin. Br. J. Pharmacol. 169(3), 645–658. Belete, T. M. 2020. Recent progress in the development of new antimalarial drugs with novel targets. Drug Des. Devel. Ther. 14, 3875–3889. Bertrram, G. K., Susan, B. M. and Anthony, J.T. 2012. Basic and Clinical Pharmacology. 11th edition, pp: 1–1232 Bhikshapathi, D.V.R.N., Shravan K.Y., Madhusudan Rao, Y. and Kishan, V. 2010. Borrelidin: a prospective drug. Indian J. Biotechnol. 9(1), 18–23. Cahyaningsih, U., Sa’diah, S., Syafii, W., Sari, R. K., Maring, A. J. and Nugraha, A. B. 2022. Antimalarial efficacy of aqueous extract of strychnos ligustrina and its combination with dihydroartemisinin and piperaquine phosphate (DHP) against Plasmodium berghei Infection. Korean J. Parasitol. 60(5), 339–344. Chen, X., Chong, C. R., Shi, L., Yoshimoto, T., Sullivan, D. J. and Liu, J. O. 2006. Inhibitors of Plasmodium falciparum methionine aminopeptidase 1b possess antimalarial activity. PANAS. 103(39), 14548–14553. Chen, X., Xie, S., Bhat, S., Kumar, N., Shapiro, T. A. and Liu, J. O. 2009. Fumagilin and fumarranol interact with P. falciparum methionine aminopeptidase 2 and inhibit malaria parasite growth in vitro and in vivo. Chem. Biol. 16(2), 193–202. Chin, V. K., Asyran, A. M. Y., Zakaria, Z. A., Abdullah, W. O., Chong, P. P., Nordin, N., Ibraheem, Z. O., Majid, R. A. and Basir, R. 2019. TREM-1 modulation produces positive outcome on the histopathology and cytokines release profile of Plasmodium berghei-infected mice. J. Parasit. Dis. 43(1), 139–153. Frottin, F., Bienvenut, W. V., Bignon, J., Jacquet, E., Jacome, A. S. V., Van Dorsselaer, A., Cianferani, S., Carapito, C., Meinnel, T. and Giglione, C. 2016. MetAP1 and MetAP2 drive cell selectivity for a potent anticancer agent in synergy, by controlling glutathione redox state. Oncotarget, 7(39), 63306–63323. Garrabrant, T., Tuman, R. W., Ludovici, D., Tominovich, R., Simoneaux, R. L., Galemmo, R. A. and Johnson, D. L. 2004. Small molecule inhibitors of methionine aminopeptidase type 2 (MetAP-2). Angiogenesis. 7(2), 91–96. Giessen, T. W. and Marahiel, M. A. 2014. The tRNA-dependent biosynthesis of modified cyclic dipeptides. Int. J. Mol. Sci. 15(8), 14610–14631. Ishiyama, A., Iwatsuki, M., Namatame, M., Nishihara-Tsukashima, A., Sunazuka, T., Takahashi, Y., Ömura, S. and Otoguro, K. 2011. Borrelidin, a potent antimalarial: stage-specific inhibition profile of synchronized cultures of Plasmodium falciparum. J. Antibiot. 64(5), 381–384. Mandell, G.L., John, E.B. and Raphael D. 2010. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Lancet Infect. Dis. 10(5), 303–304. Nigatu, T. A., Afework, M., Urga, K., Ergete, W. and Makonnen, E. 2017. Toxicological investigation of acute and chronic treatment with Gnidia stenophylla Gilg root extract on some blood parameters and histopathology of spleen, liver and kidney in mice. BMC Res. Notes. 10(1), 1–13. Novoa, E. M., Camacho, N., Tor, A., Wilkinson, B., Moss, S., Marín-García, P., Azcárate, I. G., Bautista, J. M., Mirando, A. C., Francklyn, C. S., Varon, S., Royo, M., Cortés, A. and De Pouplana, L. R. 2014. Analogs of natural aminoacyl-tRNA synthetase inhibitors clear malaria in vivo. PANAS. 111(51), E5508–E5517. O’Neill, P. M., Barton, V. E. and Ward, S. A. 2010. The molecular mechanism of action of artemisinin—The debate continues. Molecules. 15(3), 1705–1721. Pulido-Mendez, M., De Sanctis, J. and Rodríguez-Acosta, A. 2002. Leptin and leptin receptors during malaria infection in mice. Folia Parasitol. 49(4), 249–251. Rahmasari, F. V., Asih, P. B. S., Dewayanti, F. K., Rotejanaprasert, C., Charunwatthana, P., Imwong, M. and Syafruddin, D. 2022. Drug resistance of Plasmodium falciparum and Plasmodium vivax isolates in Indonesia. Malar. J. 21(1), 1–32. Saint-Léger, A., Sinadinos, C. and Ribas de Pouplana, L. 2016a. The growing pipeline of natural aminoacyl-tRNA synthetase inhibitors for malaria treatment. Bioengineered 7(2), 60–64. Saint-Léger, A., Sinadinos, C. and Ribas de Pouplana, L. 2016b. The growing pipeline of natural aminoacyl-tRNA synthetase inhibitors for malaria treatment. Bioengineered. 7(2), 60–64. Shibeshi, M. A., Kifle, Z. D. and Atnafie, S. A. 2020. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect. Drug Resist. 13, 4047–4060. Sin, N., Meng, L., Wang, M. Q. W., Wen, J. J., Bornmann, W. G. and Crews, C. M. 1997. The anti-angiogenic agent fumagilin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. PANAS. 94(12), 6099–6103. Siqueira-Neto, J. L., Wicht, K. J., Chibale, K., Burrows, J. N., Fidock, D. A. and Winzeler, E. A. 2023. Antimalarial drug discovery: progress and approaches. Nat. Rev. Drug Discov. 22(10), 807–826. Sugawara, A., Tanaka, T., Hirose, T., Ishiyama, A., Iwatsuki, M., Takahashi, Y., Otoguro, K., Omura, S. and Sunazuka, T. 2013. Borrelidin analogues with antimalarial activity: design, synthesis and biological evaluation against Plasmodium falciparum parasites. Bioorg. Med. Chem. Lett. 23(8), 2302–2305. Talapko, J., Škrlec, I., Alebić, T., Jukić, M. and Včev, A. 2019. Malaria: the past and the present. Microorganisms. 7(6), 1–17. Tu, Y. 2017. Pharmacological studies on Artemisinin. From Artemisia Annua L. to Artemisinins: the discovery and development of artemisinins and antimalarial agents. Chapter 14. 1st edition. Cambridge, MA: Academic Press, pp: 1–426. Viriyavejakul, P., Khachonsaksumet, V. and Punsawad, C. 2014. Liver changes in severe Plasmodium falciparum malaria: histopathology, apoptosis and nuclear factor kappa B expression. Malar. J. 13(1), 1–9. White, N. J. 2022. The assessment of antimalarial drug efficacy in vivo. Trends Parasitol. 38(8), 660–672. World Health Organization. 2020. Zero malaria starts with me: history of malaria elimination in Indonesia helps to shape a malaria-free future. accessed on April 3rd, 2024. https://www.who.int/indonesia/news/feature-stories/detail/zero-malaria-starts-with-me-history-of-malaria-elimination-in-indonesia-helps-to-shape-a-malaria-free-future Zhang, P., Nicholson, D. E., Bujnicki, J. M., Su, X., Brendle, J. J., Ferdig, M., Kyle, D. E., Milhous, W. K. and Chiang, P. K. 2002. Angiogenesis inhibitors specific for methionine aminopeptidase 2 as drugs for malaria and leishmaniasis. J. Biomed. Sci. 9(1), 34–40. | ||
| How to Cite this Article |
| Pubmed Style Novita R, Suprayogi A, Agusta A, Nugraha AB, Nozaki T, Agustini K, Darusman HS. Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. Open Vet. J.. 2024; 14(8): 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 Web Style Novita R, Suprayogi A, Agusta A, Nugraha AB, Nozaki T, Agustini K, Darusman HS. Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. https://www.openveterinaryjournal.com/?mno=203896 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.30 AMA (American Medical Association) Style Novita R, Suprayogi A, Agusta A, Nugraha AB, Nozaki T, Agustini K, Darusman HS. Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. Open Vet. J.. 2024; 14(8): 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 Vancouver/ICMJE Style Novita R, Suprayogi A, Agusta A, Nugraha AB, Nozaki T, Agustini K, Darusman HS. Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 Harvard Style Novita, R., Suprayogi, . A., Agusta, . A., Nugraha, . A. B., Nozaki, . T., Agustini, . K. & Darusman, . H. S. (2024) Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. Open Vet. J., 14 (8), 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 Turabian Style Novita, Risqa, Agik Suprayogi, Andria Agusta, Arifin Budiman Nugraha, Tomoyoshi Nozaki, Kurnia Agustini, and Huda Shalahudin Darusman. 2024. Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. Open Veterinary Journal, 14 (8), 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 Chicago Style Novita, Risqa, Agik Suprayogi, Andria Agusta, Arifin Budiman Nugraha, Tomoyoshi Nozaki, Kurnia Agustini, and Huda Shalahudin Darusman. "Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice." Open Veterinary Journal 14 (2024), 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 MLA (The Modern Language Association) Style Novita, Risqa, Agik Suprayogi, Andria Agusta, Arifin Budiman Nugraha, Tomoyoshi Nozaki, Kurnia Agustini, and Huda Shalahudin Darusman. "Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice." Open Veterinary Journal 14.8 (2024), 2007-2015. Print. doi:10.5455/OVJ.2024.v14.i8.30 APA (American Psychological Association) Style Novita, R., Suprayogi, . A., Agusta, . A., Nugraha, . A. B., Nozaki, . T., Agustini, . K. & Darusman, . H. S. (2024) Antimalarial activity of borrelidin and fumagilin in Plasmodium berghei-infected mice. Open Veterinary Journal, 14 (8), 2007-2015. doi:10.5455/OVJ.2024.v14.i8.30 |