| Research Article | ||
Open Vet. J.. 2024; 14(7): 1668-1676 Open Veterinary Journal, (2024), Vol. 14(7): 1668–1676 Research Article Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat modelNajlaa H. Almohmadi1*, Mona Zahran2, Sameh M. El-Nabtity2 and Hazem M. Shaheen31Clinical Nutrition Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia 2Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt *Corresponding Author: Najlaa H. Almohmadi. Clinical Nutrition Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia. Email: Nhmohmadi [at] uqu.edu.sa Submitted: 01/06/2024 Accepted: 28/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
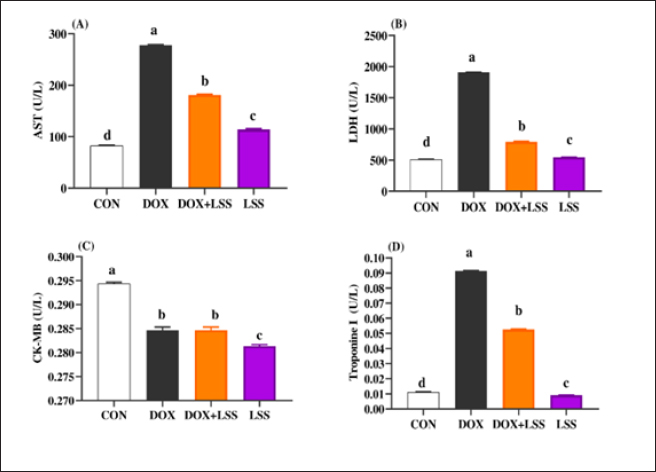
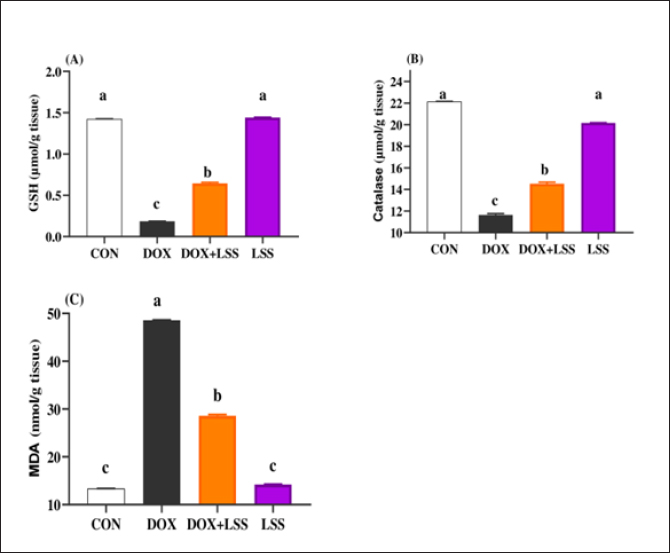
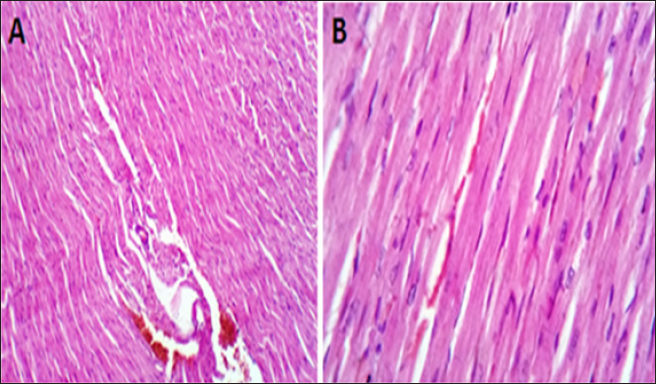
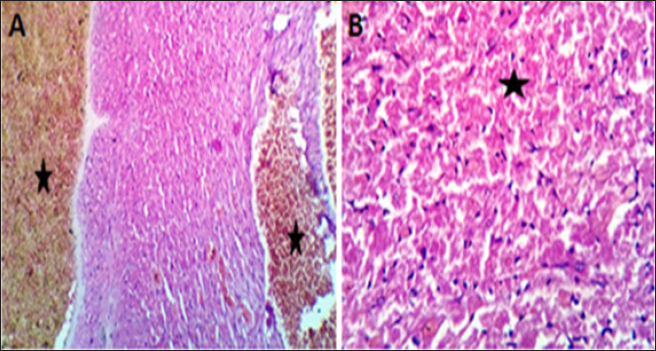
AbstractBackground: Doxorubicin (DOX) is a chemotherapeutic drug applied clinically for the remedy of cancer, but its possibly life-threatening cardiotoxicity effects remain a concern. Aim: After that, this study evaluates the cardioprotective impacts of Lagenaria siceraria (LSS) oil on DOX induced cardiomyopathy in rats. Methods: Wistar male rats (n=28, weighting 190–210 g) were arbitrarily allocated into four equal groups. Group 1 control group (CTR) received normal saline orally (1 ml/kg); group 2 (DOX) received DOX (10 mg/kg); group 3 (DOLS) received DOX + 3 g of Lagenaria siceraria seeds oil/kg; group 4 (LSSO) received LSSO (3 g/kg) daily for 18 days. The serum samples were collected to determine the creatine kinase-MB (CK-MB) isoenzyme, lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and Troponin I activity. At the same time, the catalase, malondialdehyde (MDA), and reduced glutathione (GSH) were assessed in heart tissues. Additionally, histopathological investigations for the heart tissue were performed. Results: Results revealed no significant change in CK-MB levels between the DOLS group compared to the CTR group (p > 0.05). DOX group confirmed a substantial increase in AST, LDH, and Troponin1 serum levels compared to DOLS and LLSO groups (p < 0.05). The study demonstrated the antioxidant activity of LSS oil against DOX-induced toxicity. The DOX group significantly reduced GSH and catalase levels, with an increase in MDA levels compared to DOLS and LLSO groups. Histopathological analysis showed protective properties of LSS oil against myocardial damage caused by DOX. Conclusion: This study highlights the favorable impacts of LSS oil in mitigating DOX-triggered cardiotoxicity in a rat model. Keywords: Antioxidant activity, Lagenaria siceraria, Doxorubicin, Cardiotoxicity, Rat. IntroductionDoxorubicin (DOX) is an anthracycline antibiotic widely utilized in clinical practice for treating several types of cancers (Gergely et al., 2015). Regrettably, this agent can induce acute, chronic cardiotoxic effects, including tachycardia, arrhythmia, even refractory late-onset cardiomyopathy, or transient depression of left ventricular function (Wen et al., 2018). Due to these side effects, the clinical use of DOX is limited. The prevalence of heart failure could elevate by up to 48% as the DOX dosage level increased to 700 mg/m2 (Li and Hill, 2014). Several studies pointed out that DOX can lead to harmful biological events in the myocardium, including lipid peroxidation (LP), oxidative stress, mitochondrial dysfunction, DNA breaks, apoptosis, or autophagy (Lipshultz et al., 2008). However, the cardiotoxic effects of DOX have been observed in both animal and clinical trials (Singal et al., 2000). Thus, there is an urgent need to search for functioning and advantageous compounds that minimize cardiotoxic outcomes due to DOX. Yaqtin [Lagenaria siceraria (LSS) (Molina), Family Cucurbitaceae] covers the views of Islamic scholars and folk medicinal use. LSS is an herbal plant usually used as a cardiotonic, cardioprotective, and general tonic and appears as a purgative, diuretic, and antimalarial effect (Shah and Seth, 2010; Habte et al., 2023). The fruit is palatable and considered a source of vitamin C, vitamin B-complex, saponins, β-carotene, pectin, and essential fixed oil and possesses the highest choline level—a lipotropic factor (Singh et al., 2010). The antioxidant potential of LSSO is attributed to its rich composition of bioactive compounds, including carotenoids, tocopherols, and phenolic compounds (Saeed et al., 2022). These antioxidants work together to chelate metal ions, scavenge free radicals, and prevent LP (Saeed et al., 2022). Furthermore, LSSO shows promise as a natural source of antioxidants with potential applications in the food, pharmaceutical, and cosmetic industries (Habte et al., 2023). Mali and Bodhankar (2010) found that pretreatment with LS fruit powder (500 mg/kg) for 51 days protects the hearts of rats from isoprenaline-induced cardiotoxicity. Moreover, the hepatoprotective effect of LSSO was also documented (Parveen et al., 2020). According to the biological actions of LSSO, we hypothesized that LSSO would mitigate the cardiovascular injury induced by DOX. Therefore, the present study aimed to explore the efficacy and pathways of L. Siceraria seed oil (LSSO) for treating DOX -triggered cardiotoxicity in rats. Materials and MethodsChemicals and the plant materialLSSO used in the present study was pale yellow and clear oil. It was obtained from the Faculty of Agriculture, Zagazig University. DOX, with its trade name Adriamycin, is in a 5 ml vial with a concentration (2 mg/ml) from a registered trademark of Pharmacia and Upjohn Company. The detection commercial kits for creatine kinase (CK), lactate dehydrogenase (LDH), Troponin I (TnI), aspartate transaminase (AST), catalase, malondialdehyde (MDA), and reduced glutathione (GSH) were obtained from Bio-diagnostics CO., (Cairo, Egypt) and used as per the manufacturers’ instructions. Animals and experimental proceduresTwenty-eight-10-week-old healthy adult Wistar rats weighing 190 ± 20 g were acquired from the Veterinary Medicine Faculty, Zagazig University farm. Animals were caged under well-managed atmospheric conditions (temperature: 25°C ± 2°C, relative humidity: 50%–70%) with 12 hours of dark/light cycle and had free access to water and feeds. The animal experiment was carried out following the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, and the local authorities of Zagazig University confirmed the rules of conduct. Later, 14 days after physiological adaptation, the rats were arbitrarily allocated into four investigational groups, each consisting of seven rats. Group 1: The control group (CTR) served as the CTR and received normal saline orally at a dosage of 1 ml/kg body weight daily for 18 days. Group 2 (DOX) received 10 mg/kg body weight of DOX intraperitoneally (Fard et al., 2008). Group 3 (DOLS) was injected with DOX intraperitoneally and then orally administered LSSO seeds oil (3g/kg b.w, DOLS) (Fard et al., 2008). Group 4 LSSO received orally at 3 g/kg b.w for 18 days. DOX was injected frequently at intervals. The test substance LS was performed once. The rats were monitored for any abnormal behavior and clinical signs. Blood and tissue samplingOn day 19, the rats were anesthetized by diethyl ether, and the blood from the jugular vein was collected into anticoagulant medication-free test tubes. The serum was separated (after centrifugations at 3,000 rpm for 15 minutes) and harvested and kept under –20°C until further determination of AST, LDH, CK-MB, Troponin 1, and antioxidant biochemical parameters. Subsequently, the heart tissues were excised immediately and washed with physiological saline (NaCl 0.9%), blotted on filter paper, and kept in 4% paraformaldehyde for histological study. Serum biochemical analysisThe serum AST was detected as previously reported (Tietz, 1986). The LDH assay was stated by (Wacker et al., 1956) and later qualified by (Gay et al., 1968). The determination of serum CK-MB described by (Tietz, 1986) and serum Troponin 1 was determined according to (Etievent et al., 1995). Oxidant /antioxidant hemostasis in heart tissue homogenatesThe heart tissues were mixed in ice-cold 0.15M KCL (10%, W/V) using a Biomasher (Nippi, Inc., Tokyo, Japan). Afterward, the homogenized heart tissues were centrifugated at 10,000 × g for 15 minutes at 4°C and the clear fluid above the sediment was collected for the assessment of LP indicator by MDA determination (Ohkawa et al., 1979) and antioxidant enzyme biomarkers. The GSH was measured by 5, 5-dithiobis (2-nitrobenzoat) at 412 nm and catalase activities were measured corresponding to the techniques by Nishikimi et al., (1972). Histopathological analysisThe heart tissues were excised to assess the histopathological analysis as a response to the study protocol. From each group, three rats were sacrificed and anesthetized as previously described. The tissues were washed with PBS (NaCl 0.9%), blotted on filter paper, and kept in 4% paraformaldehyde. After 3 days, the samples were exposed to a gradual percentage of ethanol alcohol, according to (Suvarna and Layton, 2013). Then, hematoxylin and eosin-stained sections were investigated for circulatory disturbances, degeneration, necrosis, and other pathological lesions. Statistical analysisData were displayed as mean ± SEM. The data were statistically examined by one-way ANOVA, followed by Tukey’s post-hoc test. Means were measured statistically significant at p < 0.05. Ethical approvalThe Ethics Committee (IACUC) approved the study of animal studies at the Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt, and care was taken to minimize the number of animals used. ResultsSerum biochemical analysisDOX-treated rats displayed substantially (p < 0.05) elevated serum amounts of AST (Fig. 1A), LDH (Fig. 1B), and Troponin1 (Fig. 1D) compared to the CTR group. Meanwhile, the LSSO group showed a noticeable decrease in serum amounts of AST, LDH, and Troponin1. Compared to the DOX group (p < 0.05). The LSSO group explained a substantial (p < 0.05) reduction in serum amounts of CK-MB, while there were no clear variations among the DOX and DOLS rats groups regarding the heart CK-MB (Fig. 1C). Antioxidative enzymesThe results showed the impact of treatments on antioxidant activities and the beneficial impact of LSSO (Fig. 2). Notably, DOX injection triggered a substantial (p < 0.05) decrease in the values of catalase (10.93 ± 0.709) (Fig. 2B) and GSH (0.1125 ± 0.007) (Fig. 2A) with an increase in MDA level (49.375 ± 2.57) (Fig. 2C). In contrast, the LSSO-treated group showed a substantial (p < 0.05) raise in catalase and GSH levels, and a significant (p < 0.05) decline in MDA amounts. The combination of DOX with Lagenaria seed oil resulted in intermediate levels of catalase, MDA, and GSH. Co-treatment with LSS oil and DOX improved this profile, but it was still lower than the control levels (13.83, 26.125, and 0.64). Histopathological analysisThe examined sections from the heart of the CTR group showed normal cardiomyocytes, intermuscular and coronary blood vessels with preserved endocardial and valvular endothelium, and Purkinje fibers (Fig. 3A and B). Coveralls, the heart sections from the DOX-treated group showed extensively congested coronary and intermuscular blood with a large to a moderate number of cardiac muscle fibers (60–65) showing hyaline degeneration, dissociation, and/or perinuclear vacuolation (Fig.4A and B). In the DOX + oil-treated group, the heart sections showed valvular stagnation and dissection, with moderately congested coronary and intermuscular blood vessels. Some cardiac muscle fibers showed hyaline degeneration, swelling, and perinuclear vacuolation (35%–40%, Fig. 5A and B). The heart sections from oil-treated rats also showed normal cardiomyocytes, intermuscular, and coronary blood vessels with preserved endocardial and valvular endothelium and Purkinje fibers (Fig. 6A and B).
Fig. 1. (A-D) Impacts of doxorubicin (DOX) (10 mg/kg.bw.), LSSO (3 g/kg.b w) and their combination on the serum aspartate aminotransferase (AST) activity (Fig. 1A), LDH (Fig. 1B), creatine kinase-MB isoenzyme (CK-MB, Fig. 1C) and Troponine I (Fig. 1D) of rats. The CON group, where rats received normal saline orally (1 ml/kg b.w). a, b,c, and d mean in the same row with different superscripts are significantly (p < 0.05) different.
Fig. 2. (A-C) Impacts of doxorubicin (DOX) (10 mg/kg.bw.), LSSO (3 g/kg.b.w), and their combination on the rats heart tissues of reduced GSH (Fig. 2A), catalase (Fig. 2B), and MDA (Fig. 2C). The CON group, where rats were received normal saline orally (1 ml/kg bw). a, b,c, and d mean in the same row with different superscripts are significantly (p < 0.05) different. DiscussionPhytochemicals contain a vast array of active compounds that have been utilized for centuries to treat various diseases (Abd El-Hack et al., 2016, 2019, 2020a,b, 2023a,b). At the same time, cancer represents the major disease in the world, and many chemical compounds have been used to remedy this disease. However, these molecules could present side effects on other organs in the body. Among them, DOX is one of the most popular drugs for treating cancer. However, it can cause cardiotoxicity in animal models and clinical experiments (Singal and Iliskovic, 1998). Therefore, it is important to identify effective treatments for DOX-induced cardiotoxicity (Zhao et al., 2018). LSS, a plant commonly used in India for various medicinal purposes, has antioxidant, free radical scavenging, cardioprotective, and hepatoprotective properties. Recent studies (Saeed et al., 2022) have demonstrated the cardioprotective effects of LSSO against DOX-induced cardiotoxicity, supported by previous research showing the cardioprotective potential of L. siceraria fruit extract (LSFJ) against DOX-encouraged cardiotoxicity in animals (Fard et al., 2008). AST, also known as serum SGOT (glutamic oxaloacetic transaminase), is an essential enzyme in amino acid metabolism, primarily found in the heart, liver, skeletal muscle, brain, kidney, and red blood cells. Increased serum AST levels were observed in rats subjected to DOX related to control rats. At the same time, treatment with powder (LSS) and DOX reduced AST levels compared to the DOX group (Fard et al., 2008). Consistent with our data, the study of (Singh et al., 2023) shows that LSS has strong antioxidant properties and can protect against DNA damage and liver damage.
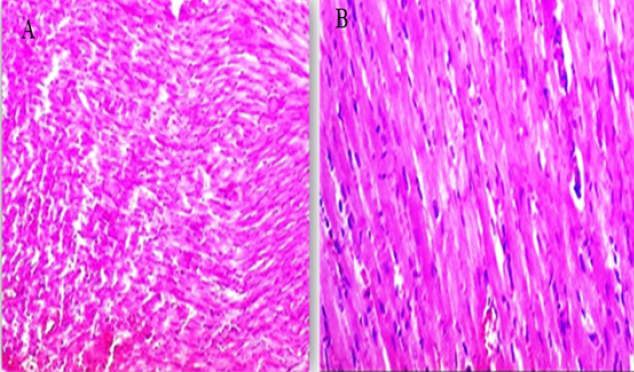
Fig. 3. (A, B) Histological photograph (H & E) of heart tissue of rats CTR who received normal saline orally (1 ml/kg b w) for 18 days. Heart tissues of rats under magnification of 10X (A) and 40X (B). Histological analysis shows normal cardiomyocytes intermuscular and coronary blood vessels.
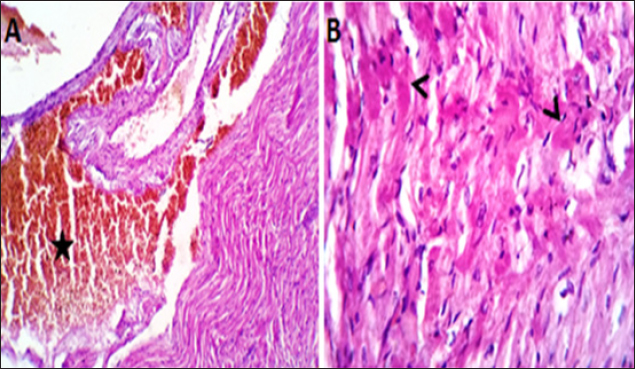
Fig. 4. (A, B) Histological photograph (H & E) of heart tissue of rats (Doxorubicin group) received DOX (10 mg/kg b.w) for 18 days. Heart tissues (A) show severe congestion of coronary and intermuscular blood vessels (stars, X10). (B). A moderate number of cardiac muscle fibers show hyaline degeneration and dissociation (star, X40).
Fig. 5. (A, B) Histological photograph (H & E) of heart tissue of rats (DOLS) received DOX (10 mg/kg b.w) + 3 g of LSSO/kg of b.w for 18 days. Heart tissues (A) show valvular stagnation of the blood and dissection of the coronary and intermuscular blood vessels were moderately congested (stars, X10). (B). Some of the cardiac muscle fibers (30%–35%) showed hyaline degeneration, swelling and perinuclear vacuolation (star, X40).
Fig. 6. (A-B) Histological photograph (H & E) of heart tissue of rats received 3 g of LSSO/kg of b.w for 18 days. Heart tissues of rats that received LSS showed normal cardiomyocytes, intermuscular (Fig. 6A, X10), and coronary blood vessels with preserved endocardial and valvular endothelium and Purkinje fibers (Fig. 6B, X40). LDH is an enzyme implicated in transforming pyruvate to lactate, with LDH-1 primarily found in the heart muscle and LDH-2 in the blood serum. Elevated LDH-1 levels relative to LDH-2 indicate myocardial necrosis or infarction. LDH levels can also be elevated in conditions involving tissue breakdown or hemolysis, such as cancer, meningitis, and encephalitis. LSS juice has shown potential anti-inflammatory properties and can inhibit digestive enzymes such as glucosidase and amylase (Das et al., 2024). While this study highlights the anti-inflammatory effects of LSS, this potential effect has not been explored in our study and requires further investigation. Our results were comparable with those of (Fard et al., 2008), who reported that the toxication by DOX resulted in the highest level of LDH in the DOX group compared to other groups. Intoxicated rats treated with LSS extracts in the DOX + oil group showed a reduction in LDH concentration, suggesting its membrane-stabilizing activity compared to the lowest concentration of heart LDH found in the CTR. In the present study, acute exposure to DOX could cause cardiotoxicity, as evidenced by a considerable elevation in serum AST or LDH levels compared to the control, LSS + DOX, and LSS oil-treated groups. These findings were consistent with earlier experiments (Saad et al., 2001). Moreover, no noteworthy distinctions were found between the experimental groups regarding heart CK-MB levels. High levels of CK-MB indicate myocardial injury. In other papers, it has been found that DOX-induced higher levels of CK-MB in rat models (Naderi et al., 2023). Troponin I (TnI), is a marker of myocardial damage, and it is a protein that appears absolutely in myocardial cells with a high diagnostic and prognostic value (Antman et al., 2000). Serum levels of cardiac troponins are markers of cardiomyocyte degeneration or high ventricular end-diastolic pressure. They should be further assessed in adults and children treated with regular- and high-dose chemotherapy (Cardinale et al., 2004). In the recent work, TnI was significantly elevated after intraperitoneal injection with DOX compared to the CTR, while a substantial decline in TnI was respected in rats administered LSSO + DOX compared to the DOX-treated group. The level of TnI in the LSSO-treated rats was substantially inferior to that in the control rats. Our conclusions are consistent with those obtained by Singh et al. (2012). Early evidence shows that the underlying pathway of DOX-caused cardiotoxicity is caused by oxidative strain inside myocardial cells (Lin and Yin, 2013). During growth, the intrinsic antioxidant mechanisms develop intracellularly to reduce ROS levels and enhance cell survival. Necessary antioxidant enzymes, such as GSH, which can manage the reduction of hydrogen peroxide or other peroxides, and SOD, which can reduce O2− to hydrogen peroxide (Sugden and Clerk, 2006), play a crucial role in this process. In this study, the data suggests that LSS oil reduced oxidative damage within myocardial cells caused by DOX by decreasing ROS levels and upregulating catalase and GSH levels. Additionally, MDA, an end-product of lipid hydroperoxide degradation and a ROS biomarker, was significantly lower in the LSS oil group compared to the control rats, DOX group, and DOX + LSS oil-treated groups. These findings indicate that LSS oil effectively alleviated DOX-induced myocardial oxidative destruction. These findings are also consistent with earlier studies that have demonstrated the association of oxidative damage with LP in DOX-triggered cardiomyopathy (Patil and Balaraman, 2005). The histopathological examination further supported biochemical results. The histopathological analysis of myocardial tissue in control rats showed intact myocardial cell membranes. In contrast, DOX-treated rats exhibited congestion of coronary and intermuscular blood vessels, hyaline degeneration, and inflammatory cell infiltration, indicating the contribution of oxidative stress and inflammatory processes in myocardial injury (Upaganlawar and Balaraman, 2011). Administration of LSS oil in DOX-injected rats (LSS + DOX) caused moderate histopathological changes. ConclusionThe present study has demonstrated that Yaqtin oil LSSO has cardioprotective potential against DOX-induced cardiotoxicity. It helps maintain the integrity of heart tissues by reducing oxidative stress. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsM.Z. and S.M.E. performed the experiments; N.H.A., S.M.E., and H.M.S. designed the experiments; N.H.A., M.Z., S.M.E., and H.M.S. analyzed data and wrote the initial draft; all authors revised the manuscript. Data availabilityAll data are provided in the published article. ReferencesAbd El-Hack, M. E., Abdelnour, S. A., Taha, A. E., Khafaga, A. F., Arif, M., Ayasan, T., Abdel-Daim, M. M. 2020a. Herbs as thermoregulatory agents in poultry: an overview. Sci. Total Environ. 703, 134399. Abd El-Hack, M. E., Abdelnour, S., Alagawany, M., Abdo, M., Sakr, M. A., Khafaga, A. F., Gebriel, M. G. 2019. Microalgae in modern cancer therapy: current knowledge. Biomed. Pharmacother. 111, 42–50. Abd El-Hack, M. E., Alagawany, M., Farag, M. R., Tiwari, R., Karthik, K., Dhama, K. 2016. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharmacol. 12(3), 232–248. Abd El-Hack, M. E., de Oliveira, M. C., Attia, Y. A., Kamal, M., Almohmadi, N. H., Youssef, I. M., Taha, A. E. 2023a. The efficacy of polyphenols as an antioxidant agent: an updated review. Inter. J. Biol. Macromol. 250, 126525. Abd El-Hack, M. E., Elnesr, S. S., Alagawany, M., Gado, A., Noreldin, A. E., & Gabr, A. A. 2020b. Impact of green tea (Camellia sinensis) and epigallocatechin gallate on poultry. World’s Poult. Sci. J. 76(1), 49–63. Abd El-Hack, M. E., Kamal, M., Alazragi, R. S., Alreemi, R. M., Qadhi, A., Ghafouri, K., Abdelnour, S. A. 2023b. Impacts of chitosan and its nanoformulations on the metabolic syndromes: a review. Brazil. J. Bio. 83, e276530. Antman, E., Bassand, J.P. and Klein, W. 2000. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction: the Joint European Society of Cardiology/American College of Cardiology Committee. J. Am. Coll. Cardiol. 36, 959–969. Cardinale, D., Sandri, M.T., Colombo, A., Colombo, N., Boeri, M., Lamantia, G., Civelli, M., Peccatori, F., Martinelli, G., Fiorentini, C., Cipolla, C.M. 2004. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 109(22), 2749–2754. Das, M.J., Banerjee, D., Banerjee, A., Muchahary, S., Sinha, A., Gogoi, D., Chattopadhyay, P., Dasgupta, S., Deka, S.C. 2024. Safety and antidiabetic activity of Lagenaria siceraria (Molina) Standl juice in streptozotocin-induced diabetic rats. Ethnopharmacol. 319, 117111. Etievent, J.P., Chocron, S., Toubin, G., Taberlet, C., Alwan, K., Clement, F., Cordier, A., Schipman, N., Kantelip, J.P. 1995. Use of cardiac troponin I as a marker of perioperative myocardial ischemia. Ann. Thorac. Surg. 59, 1192–1194. Fard, M.H., Bodhankar, S. and Dikshit, M. 2008. Cardioprotective activity of fruit of Lagenaria siceraria (Molina) standley on doxorubicin-induced cardiotoxicity in rats. Int. J. Pharmacol. 4, 466–471. Gay, R.J., McComb, R.B. and Bowers, G.N. 1968. Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin. Chem. 14, 740–753. Gergely, S., Hegedűs, C., Lakatos, P., Kovács, K., Gáspár, R., Csont, T. and Virág, L. 2015. High throughput screening identifies a novel compound protecting cardiomyocytes from doxorubicin-induced damage. Oxidative Med. Cell. Longev. 2015, 178513. Habte, G., Tamiru, S. and Eyasu, K. 2023. In vivo antimalarial activity of the 80% methanolic crude fruit extract of Lagenaria siceraria (Molina) Standl. against Plasmodium berghei-infected mice. Heliyon 9(4), e15453. Li, D.L. and Hill, J.A. 2014. Cardiomyocyte autophagy and cancer chemotherapy. J. Mol. Cell Cardiol. 71, 54–61. Lin, M.c and Yin, M.c. 2013. Preventive effects of ellagic acid against doxorubicin-induced cardio-toxicity in mice. Cardiovasc. Toxicol. 13, 185–193. Lipshultz, S.E., Alvarez, J.A. and Scully, R.E. 2008. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart 94, 525–533. Mali, V.R. and Bodhankar, S.L. 2010. Cardioprotective effect of Lagenaria siceraria (LS) fruit powder in isoprenalin-induced cardiotoxicity in rats. Eur. J. Integr. Med. 2, 143–149. Naderi, Y., Khosraviani, S., Nasiri, S., Hajiaghaei, F., Aali, E., Jamialahmadi, T., Banach, M. and Sahebkar, A. 2023. Cardioprotective effects of minocycline against doxorubicin-induced cardiotoxicity. Biomed Pharmacother 158, 114055. Nishikimi, M., Rao, N.A. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. Parveen, S., Mishra, A. and Kumar, R. 2020. Hepatoprotective activity of Lagenaria siceraria fruit peel extract against anti-tubercular drugs induced hepatotoxicity in Rats. Biochem. J. 20(1), 183. Patil, L. and Balaraman, R. 2005. Protective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Orient. Pharm. Exp. Med. 5, 137–143. Saad, S.Y., Najjar, T.A. and Al-Rikabi, A.C. 2001. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol. Res. 43, 211–218. Saeed, M., Khan, M.S. and Amir, K. 2022. Lagenaria siceraria fruit: a review of its phytochemistry, pharmacology, and promising traditional uses. Front. Nutr. 9, 927361. Shah, B.N. and Seth, A.K. 2010. Pharmacognostic studies of the Lagenaria siceraria (Molina) Standley. Int. J. Pharmtech. Res. 2, 121–124. Singal, P. and Iliskovic, N. 1998. Adriamycin cardiomyopathy. N. Engl. J. Med. 339, 900–905. Singal, P.K., Khaper, N., Farahmand, F. and Belló-Klein, A. 2000. Oxidative stress in congestive heart failure. Curr. Cardiol. Rep. 2, 206–211. Singh, M.K., Mohd, F., Ayaz, A., Ankur, S. and Jyoti, Y. 2012. Protective effect of Lagenaria siceraria against doxorubicin-induced cardiotoxicity in Wistar rats. Int. J. Drug Dev. Res. 4, 298–295. Singh, S., Gautam, A. and Sharma, A. 2010. Centella asiatica (L.): a plant with immense medicinal potential but threatened. Int. J. Pharm. Sci. Rev. Res. 4, 003. Singh, U., Singh, P. and Singh, P.K. 2023. Evaluation of antioxidant potential, DNA damage and hepatoprotective properties of Lagenaria siceraria plant against acetaminophen-induced hepatotoxicity. Funct. Foods Health Dis. 13, 117–134. Sugden, P.H. and Clerk, A. 2006. Oxidative stress and growth-regulating intracellular signalling pathways in cardiac myocytes. Antioxid. Redox Signal. 8, 2111–2124. Suvarna, S. and Layton, C. 2013. Bancroft’s Theory and Practice of Histological Techniques. London, UK: Churchill Livingstone Elsevier. Tietz, N. 1986. Textbook of clinical chemistry, Philadelphia, PA: WB Saunders, p: 1279. Upaganlawar, A. and Balaraman, R. 2011. Cardioprotective effects of Lagenaria siceraria fruit Juice on Isoproterenol-induced myocardial infarction in Wistar Rats: a biochemical and histoarchitecture study. J. Young Pharm. 3, 297–303. Wacker, W.E., Ulmer, D.D. and Vallee, B.L. 1956. Metalloenzymes and myocardial infarction: Malic and lactic dehydrogenase activities and zinc concentrations in serum. N. Engl. J. Med. 255, 449–456. Wen, S.Y., Tsai, C.Y. and Pai, P.Y. 2018. Diallyl trisulfide suppresses doxorubicin-induced cardiomyocyte apoptosis by inhibiting MAPK/NF-κB signalling through attenuation of ROS generation. Environ. Toxicol. 33, 93–103. Zhao, L., Tao, X. and Qi, Y. 2018. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox. Biol. 16, 189–198. | ||
| How to Cite this Article |
| Pubmed Style Almohmadi NH, Zahran M, El-nabtity SM, Shaheen HM. Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. Open Vet. J.. 2024; 14(7): 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 Web Style Almohmadi NH, Zahran M, El-nabtity SM, Shaheen HM. Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. https://www.openveterinaryjournal.com/?mno=204053 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i7.16 AMA (American Medical Association) Style Almohmadi NH, Zahran M, El-nabtity SM, Shaheen HM. Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. Open Vet. J.. 2024; 14(7): 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 Vancouver/ICMJE Style Almohmadi NH, Zahran M, El-nabtity SM, Shaheen HM. Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. Open Vet. J.. (2024), [cited January 25, 2026]; 14(7): 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 Harvard Style Almohmadi, N. H., Zahran, . M., El-nabtity, . S. M. & Shaheen, . H. M. (2024) Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. Open Vet. J., 14 (7), 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 Turabian Style Almohmadi, Najlaa H., Mona Zahran, Sameh M. El-nabtity, and Hazem M. Shaheen. 2024. Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. Open Veterinary Journal, 14 (7), 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 Chicago Style Almohmadi, Najlaa H., Mona Zahran, Sameh M. El-nabtity, and Hazem M. Shaheen. "Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model." Open Veterinary Journal 14 (2024), 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 MLA (The Modern Language Association) Style Almohmadi, Najlaa H., Mona Zahran, Sameh M. El-nabtity, and Hazem M. Shaheen. "Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model." Open Veterinary Journal 14.7 (2024), 1668-1676. Print. doi:10.5455/OVJ.2024.v14.i7.16 APA (American Psychological Association) Style Almohmadi, N. H., Zahran, . M., El-nabtity, . S. M. & Shaheen, . H. M. (2024) Prophylactic impacts of Lagenaria siceraria against cardiomyopathy induced by doxorubicin in a rat model. Open Veterinary Journal, 14 (7), 1668-1676. doi:10.5455/OVJ.2024.v14.i7.16 |