| Research Article | ||
Open Vet. J.. 2024; 14(8): 2016-2028 Open Veterinary Journal, (2024), Vol. 14(8): 2016–2028 Research Article Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicityNajlaa H. Almohmadi1*, Ahmed K. Aldhalmi2, Mona Zahran3, Walaa E. Alhassani1, Shatha G. Felemban4, Sameh M. El-Nabtity3 and Hazem M. Shaheen51Clinical Nutrition Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia 2College of Pharmacy, Al-Mustaqbal University, Babylon, Iraq 3Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Medical Laboratory Sciences Department, Fakeeh College for Medical Sciences, Jeddah, Saudi Arabia 5Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt *Corresponding Author: Najlaa H. Almohmadi. Clinical Nutrition Department, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia. Email: Nhmohmadi [at] uqu.edu.sa Submitted: 02/06/2024 Accepted: 22/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: The liver is crucial for maintaining normal metabolism in the body. Various substances, such as toxic chemicals, drugs, and alcohol, can damage hepatocyte cells, leading to metabolic imbalances. Aim: The experiment aimed to determine the efficacy of Lagenaria siceraria seed oil (LSS) as a hepatoprotective agent against acute hepatotoxicity triggered by carbon tetrachloride (CCl4). Methods: A total of 20 rats were randomly separated into four groups. The control group: rats received 2 ml of distilled water orally, followed by 1.25 ml of olive oil intraperitoneally (i.p.) after 30 minutes. CCL4 group: rats were given a single intraperitoneal dose of 1.25 ml/kg b.w. of CCl4 in a 1:1 mixture with olive oil. Silymarin group: received 100 mg of silymarin per kg of b.w. diluted in 2 ml of distilled water orally, followed by CCl4 treatment after 30 minutes. LSS oil group: received LSS oil at 3g/kg b.w. orally, followed by CCl4 treatment after 30 minutes. Blood samples were collected to assess liver enzymes (AST, ALT, and ALP), proteins and bilirubin fractions, and redox status (catalase, reduced glutathione (GSH), and malondialdehyde (MDA)) were assessed in hepatic tissues. Changes in liver histopathological examination were also evaluated. Results: In CCl4-treated rats, there was a significant increase in serum liver marker enzyme activity (ALP, AST, and ALT) along with a significant elevation (p < 0.05) in total bilirubin, indirect bilirubin, and direct bilirubin compared to the control rats. However, all these parameters decreased in the CCl4+ Silymarin and CCl4+LSS groups compared to CCl4-treated rats. There was a significant decline in total protein level and serum albumin in all experimental groups compared to the control, while globulin levels significantly increased in all experimental groups. There was a significant (p < 0.05) reduction in the level of GSH and catalase, with an increase in MDA level in CCl4 rats compared to other rats. Histopathological investigation of the LSS-treated group showed a hepatoprotective effect against CCl4. Conclusion: The study revealed that LSS oil has antioxidant activity against CCl4-induced toxicity. Keywords: Antioxidant activity, Lagenaria siceraria seed oil, Carbon tetrachloride, Hepatotoxicity. IntroductionThe liver is crucial for maintaining normal metabolism in the body. Various substances, such as toxic chemicals, drugs, and alcohol, can damage hepatocyte cells, leading to metabolic imbalances (Zheng et al., 2022). Exposure to toxic chemicals can weaken the antioxidant system and lead to an overproduction of oxidative stress (OS). OS plays a role in promoting various health issues, including liver damage (Arroyave-Ospina et al., 2021). Additionally, the decline of antioxidant defenses in the cellular system may lead to liver dysfunction (Sadasivam et al., 2022). Carbon tetrachloride (CCl4) is a potent environmental hepatotoxin commonly applied to prompt liver impairment in experimental animal models to assess the effectiveness of hepatoprotective drugs (Zahoor et al., 2021; Wang et al., 2023). OS-triggered by CCl4 can damage unsaturated fatty acids in the membrane, leading to peroxidation reactions that affect membrane permeability and fluidity, causing OS. Moreover, CCl4 is a toxic compound that causes chemical hepatitis and liver damage in animals, commonly used in experiments to study liver damage and check the effectiveness of potential treatments. A single exposure to CCl4 can cause severe hepatic necrosis (Brautbar and Williams II, 2002; Manibusan et al., 2007). CCl4 induces cell destruction by forming covalent bonds with cellular components or by increasing lipid peroxidation, which breaks down unsaturated fatty acids and damages unsaturated phospholipids, leading to intracellular plasma membrane damage (Cheeseman et al., 1985). Liver toxicity from CCl4 is exacerbated by low tissue oxygen levels, which promote the formation of harmful radicals and metabolite binding. Although traditional liver medications are effective, their long-term side effects are worrisome (De Groot et al., 1988; Yasusuke and Yumi, 1990). Therefore, investigating natural substances as potential alternatives to synthetic hepatic protectors is crucial. Phytochemicals contain many active compounds used for centuries to treat various diseases (Abd El-Hack et al., 2016, 2019, 2020a,b, 2023). Lagenaria siceraria (Yaqtin, LSS) is a plant from the Cucurbitaceae family, commonly used in traditional medicine. The fruit of LSS is known for its antipyretic and diuretic properties. A water extract of LSS leaves mixed with sugar treats jaundice (Panchal et al., 2013). Lagenaria siceraria is a palatable fruit and a fitting source of vitamin B-complex, C, β-carotene, and pectin. It also contains superior choline levels, a lipotropic compound (Singh et al., 2010). The active compounds in LSS, such as saponins, phenolic, and alkaloids, have exhibited antioxidant, anti-inflammatory, and hepatoprotective effects (Rahman, 2003; Singh et al., 2010; Zahoor et al., 2021). The seeds of LSS fruits are used as a remedy for cough, fever, and earache. They are also considered a brain tonic and have anti-inflammatory properties (Saeed et al., 2022). These fruits contain saponins, previously studied for their hepatoprotective properties (Panchal et al., 2013). Natural phytochemicals have garnered significant interest in recent years due to their various beneficial bioactivities (Zahoor et al., 2021). Based on the antioxidant action of LLS reported in previous studies, we hypothesized that LLS could protect against liver damage induced by CCl4. In this experiment, we aimed to investigate the hepatoprotective properties of LSS on CCl4-induced hepatic tissue damage and explore the underlying mechanisms of its hepatoprotective action by examining blood health, OS, and antioxidant efficacy, using Silymarin as a standard hepatoprotective drug. Materials and MethodsChemicals and material sourcesLagenaria siceraria (LSS) pale yellow and clear oil was obtained from Zagazig University, College of Agriculture. CCl4 is a colorless, clear, volatile, and constant chlorinated hydrocarbon (Alexandria Chemical Company, Egypt). Doxorubicin, with the trade name ADRIAMYCIN, is a 5 ml vial with a concentration of 2 mg/ml and is the registered trademark of Pharmacia and Upjohn Company. The commercial kits for detecting liver enzymes, total proteins, albumin, bilirubin, direct bilirubin, glutathione (GSH), catalase, and MDA were obtained from bio-diagnostics Company (Cairo, Egypt). All chemicals used in the research were of analytical grade quality. Animals and experimental proceduresTwenty healthy Wistar rats weighing 190 ± 10 g were acquired from the Veterinary Medicine Faculty Farm, Zagazig University, and registered for this experiment. They were accommodated in cages with controlled humidity (50%–70%), temperature (25°C ± 2°C), and a 12-hour light/dark cycle, free to water and feed ad libitum. The rats were monitored for abnormal behavior and clinical signs throughout the experiments. After a one-week acclimatization period, the rats were randomly divided into four groups of five rats each, as follows:
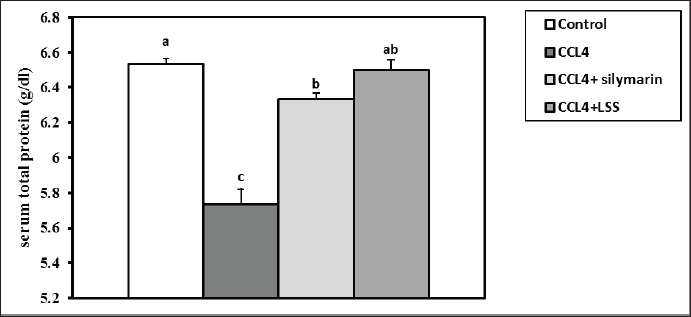
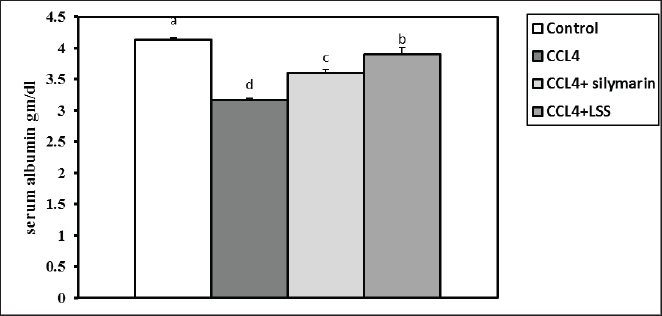
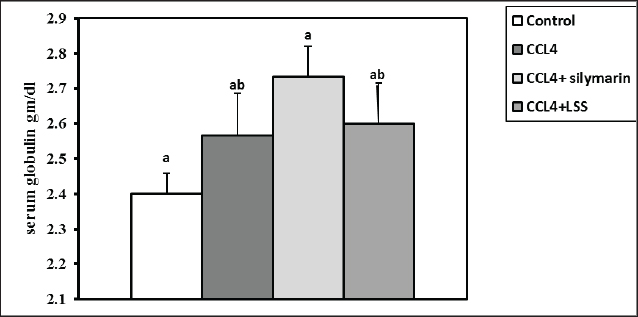
After 24 hours of CCl4 injection, all rats were sacrificed under anesthesia, and blood samples were collected from the retro-orbital venous plexus for assessing blood biochemistry. The hepatic tissues were then excised and kept in 10% of paraformaldehyde (PFA) for further histology screening. Blood and tissue samplingAfter thirty-six hours of the experiment, the animals were anesthetized with diethyl ether. Blood was collected from the retro-orbital venous plexus vein into sterilized test tubes for serum separation. The samples were centrifuged at 3,000 rpm for 15 minutes and stored at −20°C until biochemical examination. The rats were euthanized by cervical decapitation. The hepatic samples were excised and washed with physiological saline (NaCl 0.9%) and kept in 4% PFA for histopathological examination. Another part of the hepatic tissues was kept for further catalase, MDA, and GSH assays. Serum biochemical analysisThe total proteins and albumin were assessed using a colorimetric method with commercial kits provided by Bio-diagnostics Company (Cairo, Egypt). The globulins were calculated based on the total proteins and albumin differences. The A/G ratio was determined by dividing albumin by globulins. The serum ALT and AST corresponded to the assay described by Tietz (1986), and ALP was determined according to Kind and King (1954). The total bilirubin, direct bilirubin, and indirect bilirubin levels were measured following the guidelines by Abd El-Hack et al. (2019) and Ismail et al. (2019). GSH, MDA, and catalase determination in hepatic tissuesThe liver specimens were washed several times with PBS (0.1M, pH 7.4). Subsequently, 10% homogenates were prepared from hepatic tissues employing a disposable homogenizer tissue (Biomasher; Nippi, Inc., Tokyo, Japan) in cold potassium phosphate buffer (50 mM, pH 7.5) as described in the paper by Zhao et al. (2018). The homogenates were then centrifuged at 2000×g at 4°C for 15 minutes. The supernatants were collected for the estimation of malondialdehyde (MDA, a lipid peroxidation indicator) (Guarnieri et al., 1980; Renaudin, 2001) and antioxidant biomarkers such as catalase (Aebi, 1984) and GSH activities (Konrad et al., 1972), following the producer’s guidelines (Bio-diagnostics Company Cairo, Egypt). Histopathological analysisThe hepatic samples preserved in formalin were subjected to fixation and dehydration processes. The fixation involved immersing the tissue in 10% buffered formalin for three days with media change every 24 hours, followed by a 30-minute rinse in distilled water. Dehydration was carried out by sequential immersion in alcohol solutions (70%, 80%, 90%, and 100%) for 120, 90, 90, and 60 minutes, respectively. Subsequently, the tissues were cleared in xylene through a series of steps, including immersion in a 50% alcohol and 50% xylene mixture for an hour, followed by pure xylene for one and a half hours. The samples were then impregnated with molten paraffin wax, embedded, and sectioned. The paraffin sections (4–5 µm) were stained with hematoxylin and eosin (Suvarna and Layton, 2013) to evaluate fibrosis, steatosis, necrosis, or degenerative pathological lesions in the hepatic tissues. Statistical analysisNumbers are depicted as mean ± SEM and were analyzed using one-way analysis of variance, followed by Tukey’s post-hoc test. Results were considered statistically significant at p < 0.05. Ethical approvalThe Authors’ Institution Ethics Committee (IACUC) approved the study for animal studies at the Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Egypt. Care was taken to minimize the number of animals used. The animal experiments followed the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by Zagazig University authorities. ResultsSerum biochemical restored by LLS administrationCompared to the control rats, the total protein level showed a significant reduction (p < 0.05) in animals injected with CCl4 alone (14%) and CCl4+ Silymarin (2%). At the same time, there was no substantial change between the standard and CCl4+LSS oil groups (Fig. 1). The level of serum albumin significantly decreased (p < 0.05) in the CCl4, CCl4+ Silymarin, and CCl4+LSS groups (23.5%, 12.8%, and 5.5%), respectively (Fig. 2). Meanwhile, the globulin level notably boosted (p < 0.05) in the experimental groups (6.6%, 13.87%, and 8.33%), respectively (Fig. 3).
Fig. 1. Effect of Lagenaria siceraria seed oil (LSS), CCL4 and Silymarin on serum total protein concentration in male rat. a,b,c: columns labeled with different letters display significant variations (p<0.05).
Fig. 2. Effect of LSS, CCL4 and Silymarin on serum albumin gm/dl in male rat. a,b,c,d : columns labeled with different letters display significant variations (p<0.05).
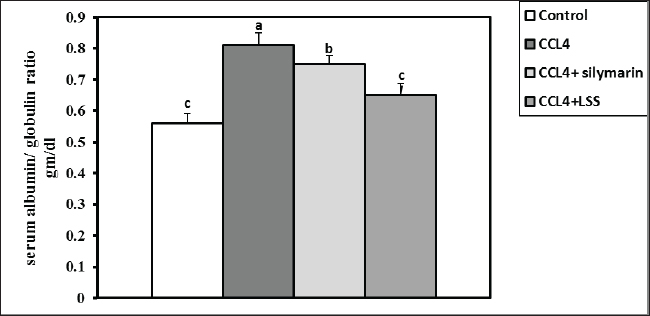
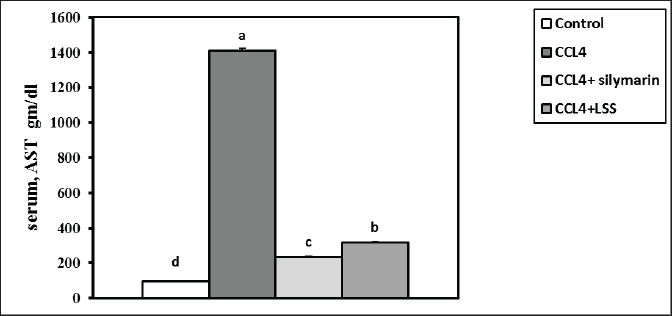
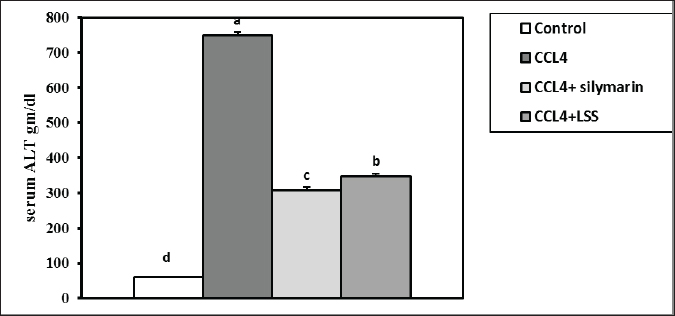
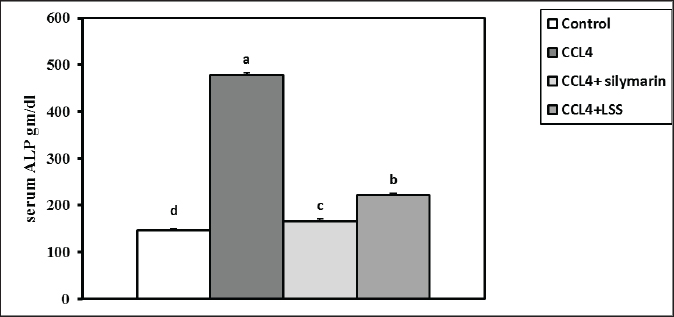
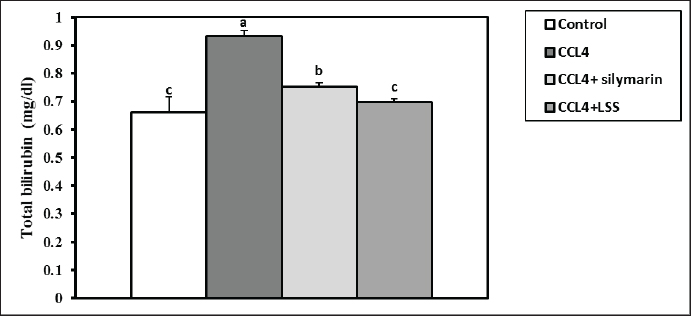
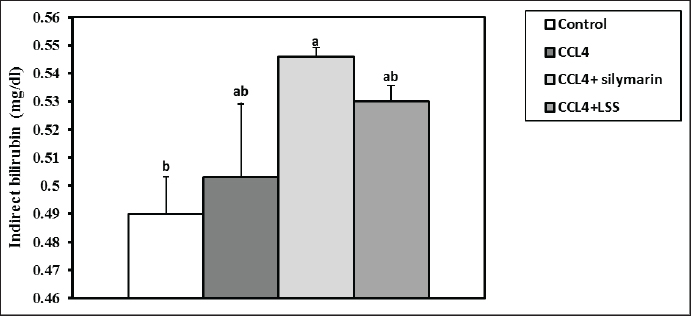
Fig. 3. Effect of LSS, CCL4 and Silymarin on serum globulin gm/dl in male rat. a,b: columns labeled with different letters display significant variations (p<0.05). The albumin/globulin ratio was substantially heightened in the CCl4 group, followed by the CCl4+Silymarin-treated animals. In contrast, there was no substantial diversity between the control and CCl4+LSS oil-treated rats (Fig. 4). The CCl4 group showed a highly significant increase (p < 0.05) in liver marker enzyme activity (ALP, AST, and ALT) compared to the control. At the same time, there was a moderate increase in CCl4+ Silymarin and CCl4+LSS oil-treated rats compared to the control (Figs. 5–7). Results in (Figs. 8–10) showed the effect of CCl4 administration with or without Silymarin or LSS on the serum level of indirect and direct bilirubin and total bilirubin, which confirmed a significant increase (p < 0.05) in total bilirubin, indirect bilirubin, and direct bilirubin in the CCl4 group compared to the control and other treated groups. There were no substantial changes between CCl4+ Silymarin and CCl4+LSS oil-treated rats in indirect and direct bilirubin. Additionally, the results showed no significant changes between the CCl4+LSS and control group in total bilirubin.
Fig. 4. Effect of LSS, CCL4 and Silymarin on serum albumin/globulin ratio gm/dl in male rat. a,b,c: columns labeled with different letters display significant variations (p<0.05).
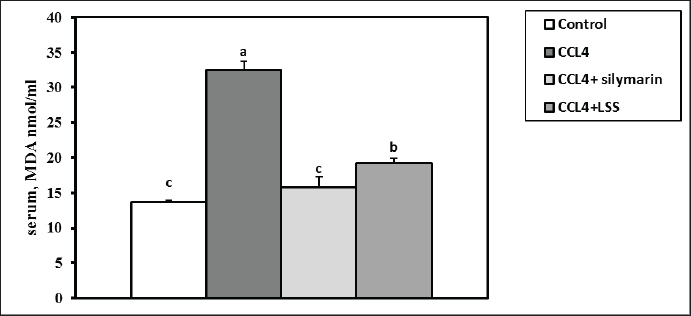
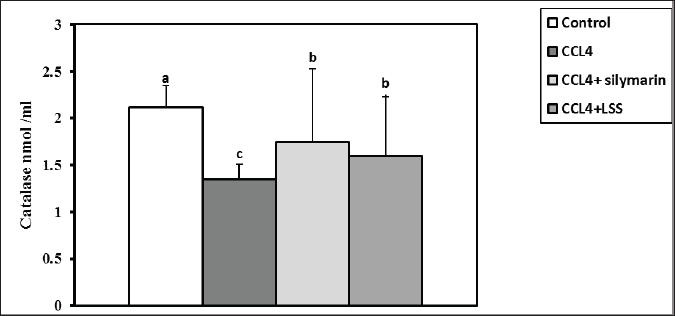
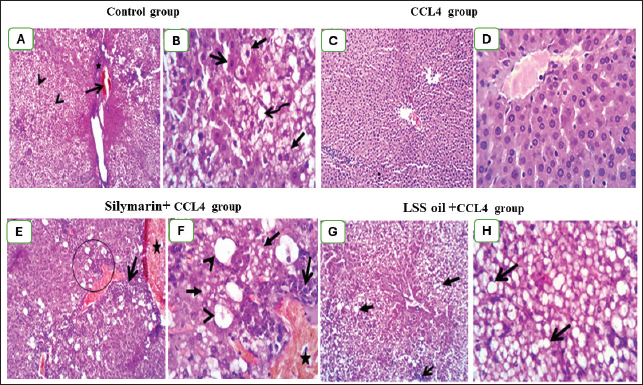
Fig. 5. Effect of LSS,CCL4 and Silymarin on Serum AST gm/dl in male rat. a,b,c,d : columns labeled with different letters display significant variations (p<0.05). LSS protected CCl4-induced liver damage via boosting antioxidant markers and reducing MDAMDA levels increased significantly by 137.7% (Fig. 11). The results demonstrate the effects of treatments on antioxidant activities and the positive influence of LSS. It is clear that CCL4 injection significantly decreased the values of catalase by 36% (Fig. 12). GSH by 68% (Fig. 13). There was no significant difference between the CCl4+Silymarin and CCL4+LSS groups in terms of catalase and GSH levels. Still, both groups demonstrated a substantial improvement related to CCL4-treated rats. Histopathological analysis after administration with LSSThe control group examined liver sections that showed normal hepatic parenchyma with preserved lobular arrangement, hepatic cords, sinusoids, and vascular tree (Fig. 14A and B). Sections from the livers of CCL4-treated rats (Fig. 14C and D) exhibited a large number of hepatocytes (70%–75%), particularly in a periportal arrangement with micro and macrosteatosis, along with ballooning changes in a variable number of cells with centrally or eccentrically situated pyknotic nuclei. The portal triads displayed moderately congested blood vessels, moderate infiltration of lymphocytes and macrophages, and mild biliary hyperplasia. Focal necrotic and apoptotic changes in some hepatocytes were also observed (Fig. 14C and D).
Fig. 6. Effect of LSS, CCL4 and Silymarin on Serum ALT gm/dl in male rat. a,b,c,d : columns labeled with different letters display significant variations (p<0.05).
Fig. 7. Effect of LSS, CCL4 and Silymarin on Serum ALP gm/dl in male rat. a,b,c,d : columns labeled with different letters display significant variations (p<0.05). On the other hand, sections from CCL4 + Silymarin-treated rats (Fig. 14E and F) revealed a large to moderate number of hepatocytes (50%–60%) with micro and macrosteatosis and the presence of a few cells with ballooning changes. The portal triads exhibited moderate to severe congestion of portal blood vessels and round cell infiltration. The Kupffer cells appeared hypertrophied, and a few apoptotic hepatocytes were also seen (Fig. 14E and F). Examined liver sections from CCL4 + LSS-treated rats (Fig. 14G and H) showed mild to moderate hydropic degeneration and fatty changes in hepatocytes (40%–45%), mostly in a centrilobular, peripherolobular, and periportal arrangement. The remaining hepatocytes displayed regenerative attempts with enlargement of the nuclei, which appeared hyperchromatic or double-nucleated (Fig. 14G and H).
Fig. 8. Effect of LSS, CCL4 and Silymarin on Serum total bilirubin gm/dl in male rat. a,b,c: columns labeled with different letters display significant variations (p<0.05).
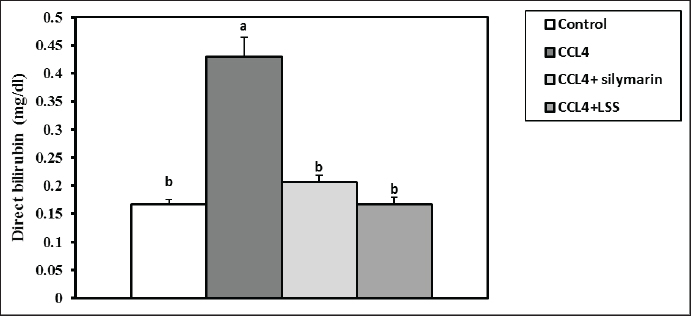
Fig. 9. Effect of LSS, CCL4 and Silymarin on serum direct bilirubin gm/dl in male rat. a,b: columns labeled with different letters display significant variations (p<0.05). DiscussionThe global burden of liver disease is significant, with over one million deaths annually. There is a need to discover effective off-the-shelf hepatoprotective agents. The current study shows that pre-treatment with LSS effectively reduces CCl4-induced liver damage in a rat model. The LSS can effectively restore liver enzymes and redox imbalance induced by CCl4. This study highlights the potential of LSS as a natural remedy for liver damage caused by toxic substances. Lagenaria siceraria has been used in ointments to treat various diseases worldwide. The plant has antioxidant, free radical scavenging, cardioprotective, and hepatoprotective properties (Kumar et al., 2012; Zahoor et al., 2021). LSS is conventionally employed in liver syndromes and diseases induced by different free radicals (Saha et al., 2011b). Hepatocellular damage increases serum enzyme indices circulated from the hepatocytes into the blood (Sreelatha et al., 2009). CCl4 induces hepatotoxicity by forming a covalent bond with membrane proteins and causing lipid peroxidation (Kanter et al., 2005; Wang et al., 2023).
Fig. 10. Effect of LSS, CCL4 and silymarin on serum indirect bilirubin gm/dl in male rat. a,b,c: columns labeled with different letters display significant variations (p<0.05).
Fig. 11. Effect of LSS, CCL4 and Silymarin on serum MDA nmol/ml in male rat. a,b,c: columns labeled with different letters display significant variations (p<0.05). Liver tissue contains enzymes used as indicators to verify biological liver injury. The enzymes AST, ALT, and ALP are essential for evaluating liver injury (Wang et al., 2023). The hepatoprotective impact of Lagenaria siceraria extracts was assessed by examining liver function biochemical parameters (Saha et al., 2011a). CCL4 has been shown to cause significant hepatic impairment, as indicated by a marked increase in serum ALP, AST, and ALT levels, which are indicators of cellular leakage and deficiency of functional reliability of hepatic cells (Althnaian et al., 2013). The existing report demonstrates the effect of CCL4 on liver-specific enzymes, showing a significant elevation in serum liver enzyme activities in CCL4-treated animals. Rats treated with CCL4, LSS, or Silymarin demonstrated a significant decrease in serum enzyme activities AST, ALT, and ALP. This research is consistent with earlier research by (Lakshmi et al., 2011), where the oral administration of the ethanolic extract of Lagenaria siceraria fruit (LSS) to various groups of rats decreased levels of serum ALT, AST, and ALP (Deshpande et al., 2008).
Fig. 12. Effect of LSS, CCL4 and silymarin on serum catalase nmol/ml in male rat. a,b,c : columns labeled with different letters display significant variations (p<0.05).
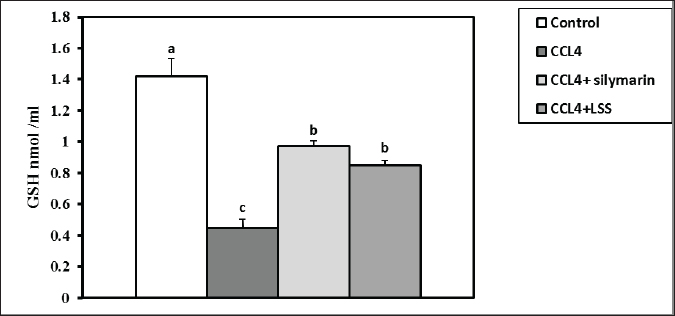
Fig. 13. Effect of LSS, CCL4 and Silymarin on Serum GSH nmol/ml in male rat. a,b,c: columns labeled with different letters display significant variations (p<0.05). Our results were consistent with (Shirwaikar and Sreenivasan, 1996), who reported that the hepatoprotective action of Lagenaria siceraria extract was evaluated by studying its impact on hepatotoxicity induced by the administration of CCl4, with Sylimarin used as a standard. Another biomarker for hepatotoxicity is the levels of total proteins and albumin, primarily produced by the liver. Hypoalbuminemia is commonly observed in advanced chronic liver diseases. In a recent study, acute administration of CCl4 resulted in significant hepatic damage, as evidenced by the decrease in serum albumin, total proteins, globulin levels, and A/G ratio. This decline was attributed to the disassociation of polyribosomes from the endoplasmic reticulum and mitochondrial dysfunction caused by CCl4 administration (Xian et al., 2024). Treatment with LSS in CCl4-induced liver injury showed an increase in serum albumin levels, total protein, globulin, and A/G ratio, confirming the hepatoprotective effect of LSS (Lakshmi et al., 2011). Hepatic damage was also observed with elevated serum total, direct, and indirect bilirubin levels. This increase is attributed to impaired bile excretion by the liver, resulting in elevated bile levels in the serum (Elkington et al., 1969).
Fig. 14. (A)–(H): Photographs of rat liver section in various treated and control groups. In the control group (14A and 14B), the liver sections of rats showed apparently normal hepatic parenchyma with preserved lobular arrangement, hepatic cords, sinusoids, and vascular tree. Liver section in rats treated with CCL4 treated group (14C) showing ballooning degeneration in a variable number of hepatocytes (arrow heads) beside congestion in portal blood vessels (open arrow) and moderate infiltration of round cells (lymphocytes, macrophages) in portal triads (star, 100X). Moreover, it shows ballooning degenerated cells (closed arrow), signet ring of adipocyte (curved arrow) and apoptotic cells with pyknotic nuclei (open arrow; 14D). The liver of rats in CCL4 + silymarin treated group (14E and 14F), showing vacuolation (closed arrow) mostly macrosteatosis and ballooing changed (arrow heads) with moderate congestion (star) and round cell infiltration in portal triads (open arrow). Lastly, sections from Liver of CCL4 + L.S.S oil treated group (14G, and 14H), showing (14G degenerative changes mostly fatty change in hepatocytes (closed arrows). Interstitial leukocytic infiltration is visible (open arrow), along with micro and macrosteatosis (14H). The first photo in each group was examined at 100X magnification, while the other one was examined at high magnification (400X). The administration of CCl4 to the animals showed a noticeable increase in total bilirubin (Bishayi et al., 2002). Our study reported that treatment with LSS in CCl4-induced liver damage led to a decline in serum total, direct, and indirect bilirubin levels, confirming the potential of LSS to alleviate liver biliary dysfunction. Rats treated with LSS showed a significant reduction in total serum bilirubin compared to those treated with CCl4 alone. The balance of serum total bilirubin and total protein levels through Lagenaria siceraria seeds treatment indicates improved liver function (Mani Senthilkumar et al., 2005). An extreme concentration of Reactive oxygen species (ROS) and other radicals leads to OS in the body, associated with various pathological conditions. Antioxidants can fight OS and ROS by scavenging free radicals or via their effective reductive capability (Halliwell, 2006). Under pathological conditions, an imbalance between OS and antioxidant defense can lead to oxidative damage in cellular membranes, resulting in lipid peroxidation, DNA degradation, and the formation of oligonucleosomal fragments. Elevated levels of lipid peroxides, such as MDA, can be highly cytotoxic (Halliwell, 2006; Raza et al., 2022). In the extant inquiry, the scavenging of both MDA and supporting the CAT and GSH synthesis indicate the potent antioxidant property of LSS. The significant antioxidant action of the extract, as evidenced by the increase in CAT and GSH levels, suggests its potential protective effect. In rats treated with CCl4, hepatotoxicity occurs due to an imbalance between ROS production and the antioxidant system, leading to cellular damage. This is indicated by the decreased levels of CAT and GSH and a significant reduction in lipid peroxidation in the treated groups, further enhancing its antioxidant activity in vivo (Halliwell, 2006; Raja et al., 2007). The protective effect of LSS on liver damage is also evident in histological examinations of hepatic tissue. Histopathological analyses have demonstrated that Lagenaria siceraria extract can protect hepatocytes from damage caused by hepatotoxic agents like CCl4 (Saeed et al., 2022). The protective effects of Lagenaria siceraria extract on the liver include reduced necrosis, inflammation, fibrosis in liver tissue, and the preservation of normal hepatocyte morphology. The extract’s hepatoprotective mechanisms involve scavenging free radicals, modulating antioxidant enzymes, inhibiting inflammatory mediators, and regulating apoptotic pathways in hepatocytes. Bioactive compounds in Lagenaria siceraria, such as triterpenoids, flavonoids, and saponins, are believed to contribute to its demonstrated hepatoprotective properties in histopathological studies. Further studies are needed to understand the molecular mechanisms of LSS to improve liver health and other organs. ConclusionThe study confirmed that LSS has a hepatoprotective effect against CCl4-induced hepatotoxicity in rats. This protective effect is attributed to the antioxidant capacity of LSS, which reduces MDA levels, promotes CAT and GSH activity, and maintains hepatic enzymes within normal ranges. Additionally, it helps maintain the integrity of liver cells and reduces OS, according to histological examination. AcknowledgmentThe authors thank their respective institutes for their support. FundingThis work received no external funds. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsM.Z. and S.M.E. performed the experiments; N.H.A., S.M.E., and H.M.S. designed the experiments; N.H.A., M.Z., S.M.E., A.K.A., W.E.A., S.G.F., and H.M.S. analyzed data and wrote the initial draft; A.K.A., W.E.A., and S.G.F. revised the manuscript. All authors have read and approved the submission of the final version of this manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAbd El-Hack, M.E., Abdelnour, S., Alagawany, M., Abdo, M., Sakr, M.A., Khafaga, A.F. and Gebriel, M.G. 2019. Microalgae in modern cancer therapy: current knowledge. Biomed. Pharmacother. 111, 42–50. Abd El-Hack, M.E., Alagawany, M. and Abdelnour, S. 2019. Responses of growing rabbits to supplementing diet with a mixture of black and red pepper oils as a natural growth promoter. J. Anim. Physiol. Anim. Nutr. 103(2), 509–517. Abd El-Hack, M.E., Alagawany, M., Farag, M.R., Tiwari, R., Karthik, K. and Dhama, K. 2016. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharmacol. 12(3), 232–248. Abd El-Hack, M.E., de Oliveira, M.C., Attia, Y.A., Kamal, M., Almohmadi, N.H., Youssef, I.M. and Taha, A.E. 2023. The efficacy of polyphenols as an antioxidant agent: an updated review. Inter. J. Biol. Macromol. 2023, 126525. Abd El-Hack, M.E., Abdelnour, S.A., Taha, A.E., Khafaga, A.F., Arif, M., Ayasan, T. and Abdel-Daim, M.M. 2020a. Herbs as thermoregulatory agents in poultry: an overview. Sci. Total Environ. 703, 134399. Abd El-Hack, M.E., Elnesr, S.S., Alagawany, M., Gado, A., Noreldin, A.E., and Gabr, A.A. 2020b. Impact of green tea (Camellia sinensis) and epigallocatechin gallate on poultry. World’s Poult. Sci. J. 76(1), 49–63. Abd El-Hack, M.E., Kamal, M., Alazragi, R.S., Alreemi, R.M., Qadhi, A., Ghafouri, K. and Abdelnour, S.A. 2023. Impacts of chitosan and its nanoformulations on the metabolic syndromes: a review. Brazilian J. Bio. 83, e276530. Aebi, H. 1984. Catalase in vitro, methods in enzymology, Amsterdam, The Netherlands: Elsevier, pp: 121–126. Althnaian, T., Albokhadaim, I. and El-Bahr, S.M. 2013. Biochemical and histopathological study in rats intoxicated with carbontetrachloride and treated with camel milk. SpringerPlus 2, 57. Arroyave-Ospina, J.C., Wu, Z., Geng, Y. and Moshage, H. 2021. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants 10, 174. Bishayi, B., Roychowdhury, S., Ghosh, S. and Sengupta, M. 2002. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl4 intoxicated mature albino rats. J. Toxicol. Sci. 27, 139–146. Brautbar, N. and Williams II, J. 2002. Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Inter. J. Hyg. Environ. Health 205, 479–491. Cheeseman, K.H., Albano, E.F., Tomasi, A. and Slater, T.F. 1985. Biochemical studies on the metabolic activation of halogenated alkanes. Environ. Health Perspect. 64, 85–101. De Groot, H., Littauer, A., Hugo-Wissemann, D., Wissemann, P. and Noll, T. 1988. Lipid peroxidation and cell viability in isolated hepatocytes in a redesigned oxystat system: evaluation of the hypothesis that lipid peroxidation, preferentially induced at low oxygen partial pressures, is decisive for CCl4 liver cell injury. Arch. Biochem. Biophys. 264, 591–599. Deshpande, J., Choudhari, A., Mishra, M., Meghre, V., Wadodkar, S. and Dorle, A. 2008. Beneficial effects of Lagenaria siceraria (Mol.) Standley fruit epicarp in animal models. Indian J. Exp. Biol. 46(4), 234–242. Elkington, S., Schreiber, W. and Conn, H., 1969. Hepatic injury caused by L-alpha-methyldopa. Circulation 40, 589–596. El-Maddawy, Z.K. and Gad, S.B. 2012. Hepato-renal protection of silymarin in comparison with vitamin E in rats. Glob. J. Pharmacol. 6(3), 236–244. Ismail, I.E., Abdelnour, S.A., Shehata, S.A., Abd El-Hack, M.E., El-Edel, M.A., Taha, A.E. and Tufarelli, V. 2019. Effect of dietary Boswellia serrata resin on growth performance, blood biochemistry, and cecal microbiota of growing rabbits. Frontiers Vet. Sci. 6, 471. Guarnieri, C., Flamigni, F. and Caldarera, C. 1980. Role of oxygen in the cellular damage induced by re-oxygenation of hypoxic heart. J. Mol. Cell. Cardiol. 12, 797–808. Halliwell, B. 2006. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 141, 312–322. Kanter, M., Coskun, O. and Budancamanak, M. 2005. Hepatoprotective effects of Nigella sativa L and Urtica dioica L on lipid peroxidation, antioxidant enzyme systems and liver enzymes in carbon tetrachloride-treated rats. World J. Gastroenterol. 11, 6684. Khan, A.A. and Alzohairy, M.A. 2011. Hepatoprotective effects of camel milk against CCl4-induced hepatotoxicity in Rats. Asian J. Biochem. 1, 171-180. Kind, P.R. and King, E.J. 1954. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Pathol. 7, 322–326. Konrad, P.N., Valentine, W. and Paglia, D. 1972. Enzymatic activities and glutathione content of erythrocytes in the newborn: comparison with red cells of older normal subjects and those with comparable reticulocytosis. Acta Haematol 48, 193–201. Kumar, A., Partap, S., Sharma, N.K. and Jha K. 2012. Phytochemical, ethnobotanical and pharmacological profile of Lagenaria siceraria:-a review. J. Pharmacogn. Phytochem. 1, 24–31. Lakshmi, B., Kumar, P., Neelima, N., Umarani, V. and Sudhakar, M. 2011. Hepatoprotective effects of L. siceraria. Res. J. Pharmaceut. Biol. Chem. Sci. 2, 130–137. Mani Senthilkumar, K.T., Rajkapoor, B. and Kavimani, S. 2005. Protective effect of enicostemma littorale. Against CCl4-induced hepatic damage in rats. Pharm. Biol. 43, 485–487. Manibusan, M.K., Odin, M. and Eastmond, D.A. 2007. Postulated carbon tetrachloride mode of action: a review. J. Environ. Sci. Health C. 25, 185–209. Panchal, C., Sawale, J.A., Poul, B. and Khandelwal, K. 2013. Hepatoprotective activity of Lagenaria siceraria (Molina) Standley fruits against paracetamol-induced hapatotoxicity in mice. Int. J. Pharm. Sci. Res. 4, 371. Rahman, A.H. 2003. Bottle gourd (Lagenaria siceraria) a vegetable for good health. Nat Prod Rad 2, 249–250. Raja, S., Ahamed, K.F., Kumar, V., Mukherjee, K., Bandyopadhyay, A. and Mukherjee, P.K. 2007. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J. Ethnopharmacol. 109, 41–47. Raza, S.H.A., Abdelnour, S.A., Alotaibi, M.A., AlGabbani, Q., Naiel, M.A., Shokrollahi, B., and Zan, L. 2022. MicroRNAs mediated environmental stress responses and toxicity signs in teleost fish species. Aquaculture, 546, 737310. Renaudin, J., 2001. Determination of nitrite and nitrate content in water. Analyt. Sci. 24(12), 1599–603 Sadasivam, N., Kim, Y.-J., Radhakrishnan, K. and Kim, D.-K., 2022. Oxidative stress, genomic integrity, and liver diseases. Molecules 27, 3159. Saeed, M., Khan, M.S., Amir, K., Bi, J.B., Asif, M., Madni, A., Kamboh, A.A., Manzoor, Z., Younas, U. and Chao, S. 2022. Lagenaria siceraria fruit: a review of its phytochemistry, pharmacology, and promising traditional uses. Front. Nutr. 9, 927361. Saha, P., Mazumder, U., Haldar, P., Gupta, M., Sen, S.K. and Islam, A. 2011a. Antioxidant and hepatoprotective activity of Lagenaria siceraria aerial parts. Pharmacog. J. 3, 67–74. Saha, P., Mazumder, U., Haldar, P., Islam, A. and Kumar, R.S. 2011b. Evaluation of acute and subchronic toxicity of Lagenaria siceraria aerial parts. Int. J. Pharm. Sci. Res. 2, 1507. Shendge, P.N. and Belemkar, S. 2021. Acute and 28-day oral toxicity studies of methanolic extract of Lagenaria siceraria (Cucurbitaceae) fruit in rats. Drug Chem. Toxicol. 44(5), 493–501. Shirwaikar, A. and Sreenivasan, K. 1996. Chemical investigation and antihepatotoxic activity of the fruits of Lagenaria siceraria. Indian J. Pharm. Sci. 58, 197. Singh, S., Gautam, A., Sharma, A. and Batra, A. 2010. Centella asiatica (L.): a plant with immense medicinal potential but threatened. Int. J. Pharm. Sci. Res 4, 3. Sreelatha, S., Padma, P. and Umadevi, M. 2009. Protective effects of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. Toxicol. 47, 702–708. Suvarna, S. and Layton, C. 2013. Bancroft’s theory and practice of histological techniques. churchill livingstone. In Eds., Aughey E, Frye FL. London, UK: Manson Publishing, 21, 173–186. Tietz, N., 1986. Textbook of clinical chemistry. Philadelphia, PA: WB Saunders, pp: 1279. Wang, C., Liu, Y., Gong, L., Xue, X., Fu, K., Ma, C. and Li, Y. 2023. Phillygenin Ameliorates Carbon Tetrachloride-Induced Liver Fibrosis: Suppression of Inflammation and Wnt/β-Catenin Signaling Pathway. Inflammation 46, 1543–1560. Xian, S., Yang, Y., Nan, N., Fu, X., Shi, J., Wu, Q. and Zhou, S. 2024. Inhibition of mitochondrial ROS-mediated necroptosis by Dendrobium nobile Lindl. alkaloids in carbon tetrachloride induced acute liver injury. Ethnopharmacol. 330, 118253. Yasusuke, M. and Yumi, N. 1990. Effects of oxygen deficiency and calcium omission on carbon tetrachloride hepatotoxicity in isolated perfused livers from phenobarbital-pretreated rats. Biochem. Pharmacol. 40, 1865–1876. Zahoor, M., Ikram, M., Nazir, N., Naz, S., Batiha, G.E.-S., Kamran, A.W., Tomczyk, M. and Kabrah, A. 2021. A comprehensive review on the medicinal importance; biological and therapeutic efficacy of Lagenaria siceraria (Mol.)(bottle gourd) Standley fruit. Curr. Top. Med. Chem. 21, 1788–1803. Zhao, L., Tao, X., Qi, Y., Xu, L., Yin, L. and Peng, J. 2018. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 16, 189–198. Zheng, W.V., Li, Y., Cheng, X., Xu, Y., Zhou, T., Li, D., Xiong, Y., Wang, S. and Chen, Z., 2022. Uridine alleviates carbon tetrachloride-induced liver fibrosis by regulating the activity of liver-related cells. J. Cell Mol. Med. 26, 840–854. | ||
| How to Cite this Article |
| Pubmed Style Almohmadi NH, Aldhalmi AK, Zahran M, Alhassani WE, Felemban SG, El-nabtity SM, Shaheen HM. Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. Open Vet. J.. 2024; 14(8): 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 Web Style Almohmadi NH, Aldhalmi AK, Zahran M, Alhassani WE, Felemban SG, El-nabtity SM, Shaheen HM. Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. https://www.openveterinaryjournal.com/?mno=204090 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.31 AMA (American Medical Association) Style Almohmadi NH, Aldhalmi AK, Zahran M, Alhassani WE, Felemban SG, El-nabtity SM, Shaheen HM. Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. Open Vet. J.. 2024; 14(8): 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 Vancouver/ICMJE Style Almohmadi NH, Aldhalmi AK, Zahran M, Alhassani WE, Felemban SG, El-nabtity SM, Shaheen HM. Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 Harvard Style Almohmadi, N. H., Aldhalmi, . A. K., Zahran, . M., Alhassani, . W. E., Felemban, . S. G., El-nabtity, . S. M. & Shaheen, . H. M. (2024) Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. Open Vet. J., 14 (8), 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 Turabian Style Almohmadi, Najlaa H., Ahmed K. Aldhalmi, Mona Zahran, Walaa E. Alhassani, Shatha G. Felemban, Sameh M. El-nabtity, and Hazem M. Shaheen. 2024. Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. Open Veterinary Journal, 14 (8), 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 Chicago Style Almohmadi, Najlaa H., Ahmed K. Aldhalmi, Mona Zahran, Walaa E. Alhassani, Shatha G. Felemban, Sameh M. El-nabtity, and Hazem M. Shaheen. "Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity." Open Veterinary Journal 14 (2024), 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 MLA (The Modern Language Association) Style Almohmadi, Najlaa H., Ahmed K. Aldhalmi, Mona Zahran, Walaa E. Alhassani, Shatha G. Felemban, Sameh M. El-nabtity, and Hazem M. Shaheen. "Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity." Open Veterinary Journal 14.8 (2024), 2016-2028. Print. doi:10.5455/OVJ.2024.v14.i8.31 APA (American Psychological Association) Style Almohmadi, N. H., Aldhalmi, . A. K., Zahran, . M., Alhassani, . W. E., Felemban, . S. G., El-nabtity, . S. M. & Shaheen, . H. M. (2024) Hepatoprotective efficacy of Lagenaria siceraria seeds oil against experimentally carbon tetrachloride-induced toxicity. Open Veterinary Journal, 14 (8), 2016-2028. doi:10.5455/OVJ.2024.v14.i8.31 |