| Research Article | ||
Open Vet. J.. 2024; 14(8): 2029-2039 Open Veterinary Journal, (2024), Vol. 14(8): 2029–2039 Research Article First molecular sequencing of Babesia gibsoni in ticks, IraqIsraa M. Essa*, Ghazi Y. Azzal and Nadia K. ThamerDepartment of Parasitology, College of Veterinary Medicine, University of Basrah, Basra, Iraq *Corresponding Author: Israa M. Essa. Department of Parasitology, College of Veterinary Medicine, University of Basrah, Basra, Iraq. Email: israa.essa [at] uobasrah.edu.iq Submitted: 05/06/2024 Accepted: 25/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
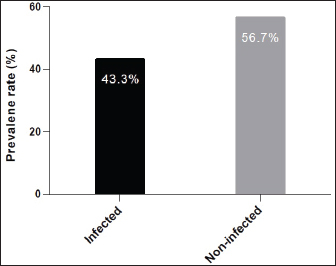
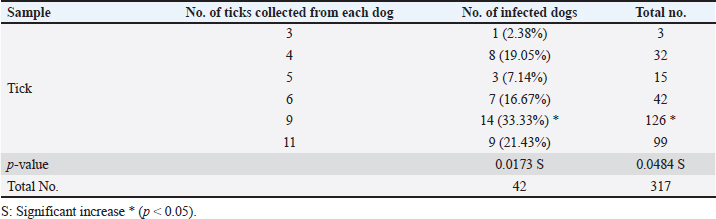
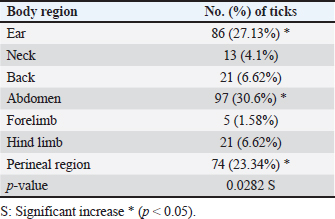
ABSTRACTBackground: Tick is one of the most important ectoparasites distributed worldwide and plays an obvious role in the transmission of different infections to humans and animals as dogs. Aim: This study conducted to molecular demonstration of Babesia gibsoni in ticks of stray dogs and phylogenetic analysis of study isolates to detect their identity to global isolates. Prevalence of ticks in dogs, identification of tick species, and their relationship to some risk factors were aimed, also. Methods: A total of 97 stray dogs were inspected grossly to detect and collect ticks that existed in different body parts. After collection, all ticks were examined morphologically to identify their species, and then molecularly by the polymerase chain reaction (PCR) assay to detect B. gibsoni in different species of ticks. Local B. gibsoni isolates were sequenced, documented in the National Center For biotechnology information (NCBI) database, analyzed phylogenetically, and compared with the global GenBank-NCBI isolates. Results: In the current study, ticks were detected in 43.3% of dogs, and were shown to be varied in number and distribution among different body parts of each dog. Concerning its distribution, ticks were observed significantly on the abdomen, ear, and perineal region. In relation to risk factors, ticks were increased significantly in dogs <6 months old in comparison to older dogs, males more than females; and in rural areas more than dogs of sub-urban and urban areas. Based on morphology, different tick species were seen including Hylaomma anatolicum (86.12%), R. sanguineus (11.99%), and Rhipicephalus turanicus (1.89%). Targeting the 18S rRNA gene, PCR assay reported 3.79% positive ticks to B. gibsoni that were seen in R. sanguineus (13.16%) and H. anatolicum (2.56%). Based on phylogenetic analysis data of five local B. gibsoni isolates, this study demonstrated their close relations to the global NCBI-BLAST B. gibsoni Iraqi isolate (ID: MN385424.1). Conclusion: This represents the first Iraqi study that demonstrated molecularly B. gibsoni in different species of ticks that infected stray dogs. Keywords: Canine babesiosis, Hylaomma anatolicum, Rhipicephalus sanguineus, Rhipicephalus turanicus, Phylogeny. IntroductionBabesia gibsoni is a tick-transmitted protozoan parasite, that belongs to Piroplasmida Order in the Aconoidasida Class under the Apicomplexa Phylum, which infects the domestic and wild canid species causing in a disease known as babesiosis (Baneth et al., 2019; Birkenheuer, 2021). Like other members, the life cycle of B. gibsoni requires two types of hosts; a tick and a canine host. Briefly, sporozoites enter the host’s circulation with tick saliva during blood sucking to attach and penetrate erythrocytes by endocytosis. Once inside, sporozoites transform into trophozoites that develop into merozoites throughout the binary fission process (Baneth, 2018; Conesa et al., 2020; Martínez-García et al., 2021). Post ingestion of erythrocytes that contain gametocytes, ticks become infected and may remain infective for many generations by the transovarial and transstadial transmission. This process occurs due to the development of gametocytes into female and male gametes in the gut of female ticks to produce later the motile zygotes that multiply to vermicules and invade numerous organs including ovaries. Usually, sporogony resides in the salivary glands of different developmental stages (larval, nymphal, and/or adult) in female ticks that get an infection (Chauvin et al., 2009; Friedhoff, 2018; Ravindran et al., 2023). Tick is an ectoparasite of widespread distribution, particularly in tropical and subtropical areas. Ticks have the ability to attach and feed the blood of different domestic and wild animals as well as humans to cause obvious health impacts, and to transmit several infectious viral, bacterial, and parasitic diseases (Mahlobo, 2018; Rajakaruna and Eremeeva, 2023). Scientifically, ticks are classified in the Parasitiformes order, Arachnida class, of the Chelicerata phylum (Proctor et al., 2015). Worldwide, different tick species belonging to Boophilus, Dermacentor, Haemaphysalis, Hyalomma, and Rhipicephalus genera can actively transmit several Babesia species (Aktas, 2014; Onyiche et al., 2021). Regarding B. gibsoni, Rhipicephalus sanguineus, Haemaphysalis bispinosa, Haemaphysalis longicornis, and Dermacentor variablis represent the most prevalent species in endemic areas (Karasová et al., 2022). For diagnosis of the infected ticks, traditional diagnostic methods such as microscopy may yield inconclusive results, especially in absence the history of infection (Lager, 2020; Kahlig et al., 2021). The DNA-based methods such as polymerase chain reaction (PCR) and phylogeny can be applied as a highly sensitive, specific, and accurate diagnostic assay because of their capability to provide additional valuable information for genetic markers (Umesha and Manukumar, 2018; Ghosh et al., 2019). Until recently, limited studies have been conducted in Iraq to detect B. gibsoni in dogs (Otranto et al., 2019; Badawi and Yousif, 2020a,b). Therefore, this study conducted to molecular demonstration of B. gibsoni in ticks of stray dogs and phylogenetic analysis of study isolates to detect their identity to global isolates. Prevalence of ticks in dogs, identification of tick species, and their relationship to some risk factors were aimed, also. Materials and MethodsSamplesTotally, 97 stray dogs were selected randomly from different rural, sub-urban, and urban areas in Basra province (Iraq) from November (2023) to April (2024). Initially, all study animals were subjected clinically to visual examination of the different parts of the body to detect and carefully manually collect of tick samples into labeled plastic tubes containing 70% Ethanol. MorphologyAll ticks were screened stereomicroscopically at the Iraqi Natural History Research Center and Museum (University of Baghdad, Baghdad, Iraq) to investigate their morphological characteristics following the taxonomic keys of related references (Estrada-Peña et al., 2004). Molecular assayAddPrep Genomic DNA Extraction Kit (AddBio, Korea) was served to extract the DNAs from all tick samples. Then, the obtained DNAs were tested spectrophotometrically by the Nnaodrop System (Thermo Scientific, UK) to estimate their concentration (ng/μl) and purity at an absorbance of 260/280. For DNA amplification, AddStart Taq Master Kit (AddBio, Korea) was utilized in addition to one set of primers (IMF: 5´-ACA ATT GGA GGG CAA GTC TG-3´) and (IMR: 5´-GGC AAA TGC TTT CGC AGT A-3´) was designed for the current study targeting the 18S rRNA gene based on the sequence data of the National Center For Biotechnology Information (NCBI)-GenBank B. gibsoni Turkish isolate (ID: KJ513206.1) and using the Primer3Plus Software. In the Thermal Cycler System (MJ-Mini BioRad, USA), the prepared MasterMix tubes at a final volume of 20 μl were subjected to the steps of PCR reaction as follows: 1 cycle for initial denaturation (95°C/5 minutes), 35 cycles for denaturation (95°C/5 minutes), annealing (58°C/30 seconds) and extension (72°C/1 minute), and 1 cycle for final extension (72°C/5 minutes). Electrophoresis of PCR products and Ladder marker (100–2,000 bp) in Agarose-Gel stained with 3 μl Ethidium Bromide was performed at 100 V and 80 Am for 90 minutes, and the resultants were visualized under the UV transilluminator (ATTA, Korea) to detect the positive samples at 375 bp. PhylogenyFor documentation of local B. gibsoni isolates in the NCBI, five positive DNA samples were sent for sequencing in the Macrogen Company (Korea) using the Sanger method. The received data of study B. gibsoni isolates were reported in the NCBI database and analyzed phylogenetically by the MEGA-6 Software to detect their identity to the global NCBI-GenBank isolates. Statistical analysisGraphPad Prism Software version 6.0.1 (GraphPad Inc, USA) was used to analyze the study data and indicate significant differences at p<0.05 using the t-test and odds ratio (Gharban, 2023). Ethical approvalThis study obtained a license from the Scientific Committee of the Department of Parasitology in the College of Veterinary Medicine (University of Basrah). ResultsThe prevalence rate of ticks among a total of 97 stray dogs was 43.3% (Fig. 1). Also, the overall ticks collected from the study animals were 317 samples that varied significantly (p<0.05) in their numbers and distributions throughout the different body parts of each dog. Significantly (p<0.0173), 14 (33.33%) of study dogs were showed a higher rate of tick infestation (having a totally of 126 ticks) when compared to 9 (21.43%), 7 (16.67%), and 8 (19.05%) stray dogs having a totally 99, 42 and 32 ticks, respectively. Whilst, 1 (2.38%) and 3 (7.14%) of study dogs were showed significantly (p<0.05) a lower rate of tick infestation; 3 and 15 ticks, respectively (Table 1). Concerning the presence of ticks on different body parts, significant increases (p<0.0282) were observed in the abdomen (30.6%), ear (27.13%), and perineal region (23.34%); and decreased significantly (p<0.05) in the forelimb (1.58%), neck (4.1%), hindlimb (6.62%), and back (6.62%), (Table 2).
Fig. 1. Prevalence rate of ticks among totally 97 stray dogs. Table 1. Number of ticks collected from the study dogs (total no: 42 dogs).
Table 2. Distribution of ticks on different body parts of study dogs (total no: 317 ticks).
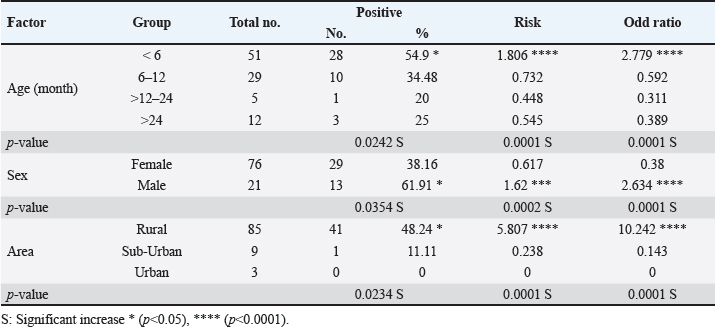
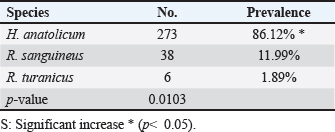
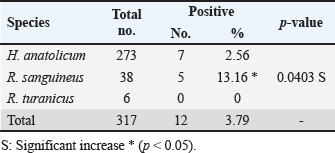
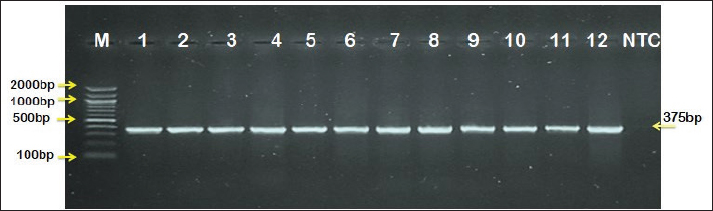
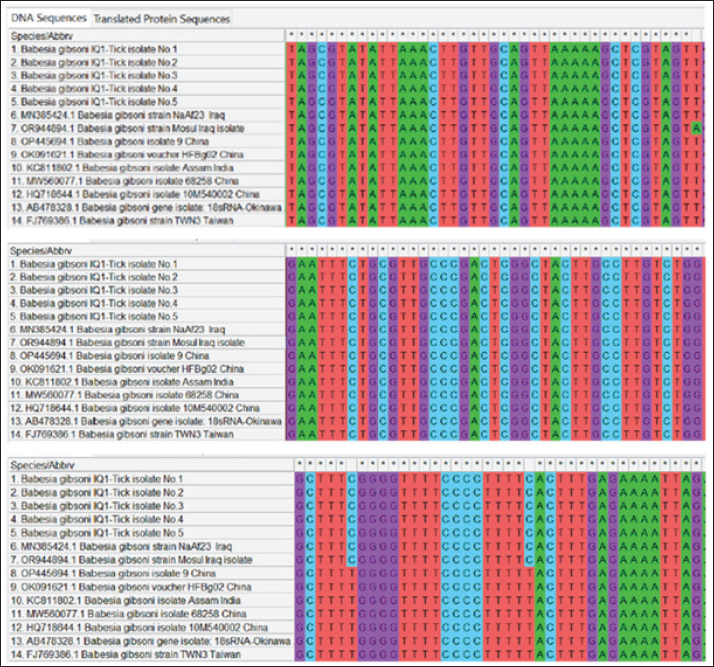
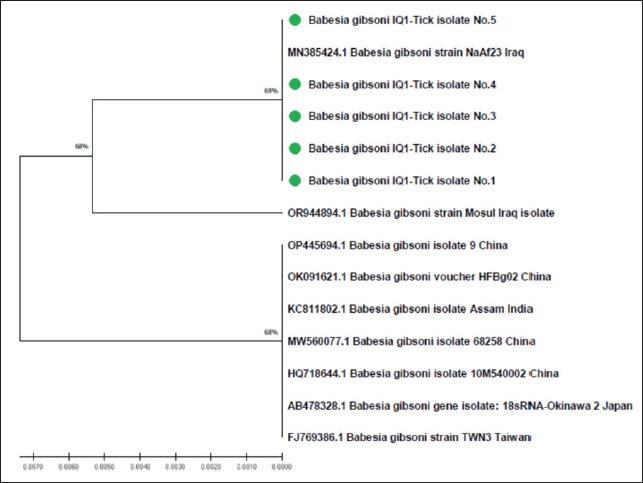
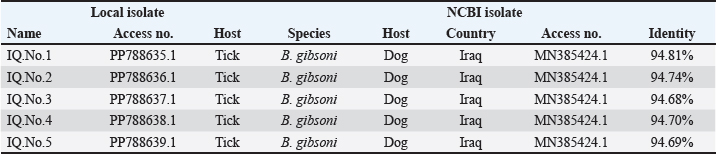
The prevalence rate of ticks was differed significantly (p<0.05) according to age, sex, and type of study areas (Table 3). Significantly, study dogs aged <6 months were showed a higher rate and risk of tick infestation (54.9% and 1.806, respectively) in comparison with the other age groups; 6-12 months (34.48% and 0.732, respectively), >12-24 months (20% and 0.448, respectively), and > 24 months (25% and 0.545, respectively). Significantly, male dogs showed an elevation in the prevalence rate of ticks (61.91%) and risk (1.62) than females (38.16% and 0.617, respectively), (p < 0.0354 and p < 0.0002, respectively). This study noticed that ticks were increased significantly (p < 0.0234) in dogs of rural areas (48.24%) more than those of sub-urban (11.11%) and urban (0%) areas. Subsequently, dogs from rural areas were seen at a significant (p < 0.0001) higher risk of infection (5.807) than those of sub-urban (0.238) and urban (0.238) areas. Based on its morphology, all tick samples were screened stereomicroscopically, and the findings revealed the presence of 3 different tick species including Hylaomma anatolicum (86.12%), R. sanguineus (11.99%), and Rhipicephalus turanicus (1.89%) (Table 4; Fig. 2). Molecularly, conventional PCR assay reported that the prevalence of B. gibsoni among study ticks was 3.79% (Fig. 3). Relation to tick species, a significant prevalence of B. gibsoni infections (p <0.0403) was seen in R. sanguineus (13.16%) than H. anatolicum (2.56%), and R. turanicus (0%) (Table 5). For phylogeny, five positive local B. gibsoni isolates were sequenced, named, and recorded in the NCBI database (Fig. 4). Targeting the 18S rRNA gene, multiple sequence alignment, phyologenetic tree analysis, and NCBI-BLAST homology demonstrated that the local study B. gibsoni isolates were closely related to the global NCBI-BLAST B. gibsoni Iraqi isolate (ID: MN385424.1) at a total similarity of 99.64%–99.66% and a total genetic changes/mutation of 0.007%–0.001% (Figs. 4–6; Table 6). DiscussionWorldwide, ticks represent the second only to mosquitoes in transmitting several infections in both humans and animals. This study showed that 43.3% of stray dogs were infected with ticks. In Iraq, the prevalence rate of canine ticks was 30.95%–40.47% in Mosul province (Arsalan et al., 2006), 16.7% in central provinces (Mohammad, 2015), 68.75% in Basra province (Hatem, 2020), and 10.3% in Erbil province (Aziz and AL-barwary, 2019). In other countries, the prevalence rate of canine ticks was 51.53% in USA (Raghavan et al., 2007), 22.92% in Great Britain (Smith et al., 2011), 46.39% in India (Sahu et al., 2013), 30% in UK (Abdullah et al., 2016), 67.9%–100% in Indonesia (Hadi et al., 2016), 45.7% in Italy (Maurelli et al., 2018), and 24.3% in Pakistan (Hussain et al., 2023). In general, many researchers have been concluded that higher rates of endoparasites and ectoparasites can be seen in stray than in pet dogs (Arsalan et al., 2006; Sahu et al., 2013), and this might because of the lack of systematic surveillance and preventive strategies in stray dogs. Léger et al. (2013) mentioned that alteration in abundance and spatial distribution of different tick species and their associated pathogens may occur due to globalization of human activities, habitat modification, and climate changes. Table 3. Association of infected dogs to age, sex, and type of areas.
Table 4. Classification of study ticks based on morphological characteristics.
Table 5. Distribution of B. gibsoni infections among different tick species.
Fig. 2. Classification of study ticks based on its morphology. (A) Hylaomma anatolicum, (B) R. sanguineus, and (C) R. turanicus.
Fig. 3. Agarose-gel electrophoresis of PCR products targeting 18S rRNA gene; in which, Lane (M): Ladder marker, Lanes (1-12): Positive samples to B. gibsoni infections at 375 bp, and Lane (NTC): Negative control (distilled water).
Fig. 4. Sequence data of local study B. gibsoni isolates in the GenBank-NCBI.
Fig. 5. Multiple sequence alignment analysis of local study and GenBank-NCBI B. gibsoni isolates in ticks targeting the 18S rRNA gene. The number of existing ticks on different body parts was shown in the current study as in agreement with that recorded by other studies that saw the presence of at least one tick on each infested dog (Smith et al., 2011; Maurelli et al., 2018). However, the low number of obtained ticks from study animals might be affected by the season in which the ticks were collected (autumn and winter). The presence of ticks on different body parts of study dogs might be because these are the most exposed sites to attachment ticks (Claerebout et al., 2013; Ramos et al., 2020). Hadi et al. (2016) mentioned that tick predilections in dogs distributed on the back (35%), head, ears, and neck (29%), legs and interdigital spaces (14.5%), as well as abdomen and tail (10.9%). Maurelli et al. (2018) reported that ticks were located insignificantly on different parts of the body including the head (37.4%), neck (28.8%), muzzle (15.5%), and back (15.3%). In the current study, significantly higher levels of tick infestation were identified in dogs of <6 months age old, males more than females, and dogs in rural than in other study areas. In multiple logistic regressions, Raghavan et al. (2007) mentioned that younger, male, and sexually intact dogs have an elevated risk of tick infection. Smith et al. (2011) revealed no significant association between infection and risk factors such as age, sex, and body size. Sahu et al. (2013) summarized that the prevalence rate of ticks was higher in dogs of <1 year of age (53.41%) than > 1 year (45.21%), and in males (53.97%) than females (38.31%). Hadi et al. (2016) recorded that purebred male dogs were more infested than the crossbred, local, and female dogs. In comparison to results to Maurelli et al. (2018), it found that ticks existed significantly in females (18.6%) more than males (6.6%). However, the effect of age on tick infestation might be due to the development of resistance and effective scratching activity in adult dogs than in younger age groups; while for sex variables, hormonal factors and activity might be playing some roles to predispose male dogs more to tick infestations.
Fig. 6. Phylogenetic tree analysis of local study and GenBank-NCBI B. gibsoni isolates in ticks targeting the 18S rRNA gene. Table 6. Homology Sequence identity for local and NCBI-BLAST B. gibsoni isolates.
In this study, H. anatolicum was the almost prevalent tick species than R. sanguineus and R. turanicus. In fact, various studies demonstrated that different species of ticks can parasitize dogs (Latrofa et al., 2017; Saleh et al., 2019, 2021). In Iraq, earlier studies in dogs have been detected two types of tick species in both Mosul province (Northern part) and Basra province (Southern part) including R. sanguieus and R. turanicus (Arsalan et al., 2006; Awad et al., 2006). Smith et al. (2011) detected three species belonging to Ixodes spp. are I. ricinus (72.1%), I. hexagonus (21.7%), and I. canisuga (5.6%). Livanova et al. (2018) identified 11 tick species, most commonly Dermacentor reticulates with a prevalence of 40.7%. Maurelli et al. (2018) observed that the tick samples collected from study dogs were belonged to four genera and 14 species, with a higher prevalence of Rhipicephalus spp. (10.8%), in particular R. sanguineus (63.6%), compared to Dermacentor spp. (0.6%), Haemophysalis spp. (0.2%), and Ixodes spp. (0.2%). Ramos et al. (2020) inspected 139 dogs from nine species, and collected tick species from three species including Amblyomma sculptum, R. sanguineus, and R. microplus with a dominance of A. sculptum. A number of authors reported that the brown dog tick (R. sanguineus) is the most common tick species in dogs, particularly in urban areas (Galaviz-Silva et al., 2013; Ojeda-Chi et al., 2019; Beristain-Ruiz et al., 2022). In Iraq, Obaid et al. (2023) showed significantly that the higher prevalence of Hyalomma spp. in different provinces was in Duhok (88.6%), Maysan (83.9%), Baghdad (83.47%), Erbil (78.3%), Basra (78.11%), Dhi-Qar (75.85%), Al-Qadisiyah (74.42%), Najaf (73.33%), and Karbala (70.42%). Other recent studies have confirmed morphologically and molecularly that different species of Ixodid ticks collected from ruminants were belong to the genus of Hyalomma (Karawan et al., 2021; Aziz, 2022; Makawi and Hadi, 2023). Therefore, a significant incidence of H. anatolicum in rural areas might explain its high existence in study dogs. Hard ticks of Hyalomma genus have been demonstrated as a vector for Babesia species in most endemic parts of Asia and Africa (Kumar et al., 2020; Karshima et al., 2022). In Iraq, despite the extensive work to elucidate patterns of Babesia spp. and abundance in various animals (AL-Shabbani and Faraj, 2023; Hossein, 2023; Al-Ani and Yousif, 2024; Al-Shammari et al., 2024), relatively two studies have systematically evaluated Babesia spp. in dogs (Otranto et al., 2019), as well as B. canis and B. gibsoni in dogs only (Badawi and Yousif, 2020a,b). In the present work, we showed molecularly that 3.79% of study ticks were having B. gibsoni distributed in R. sanguineus and H. anatolicum but not in R. turanicus. Globally, different Babesia species was demonstrated in various tick species for example B. microti (0%–0.9%), B. canis (0%–66.7%), B. venatorum (0%–0.4%), B. crassa (0%–4%), B. vogeli (0%–0.9%), and B. divergens (0%–0.4%) in Russia (43); and B. venatorum (84.3%), B. vulpes (10%), B. divergens / B. capreoli (2.9%), and B. microti (1.4%) in UK (Abdullah et al., 2018). Phylogenetically, analyzing of local B. gibsoni isolates revealed a close relation to the global NCBI-BLAST B. gibsoni strains isolated from Iraqi dogs by Badawi and Yousif (2020a,b). This indicates that local B. gibsoni isolates might be circulated between ticks and stray and domestic dogs in Iraq. The 18S rRNA gene, used in this study, is widely applied in Asia, Australia, America, and Europe to establish phylogenetic relationships as well as to differentiate the genotypes or subspecies of canine babesiosis (Schäfer et al., 2023; Yin et al., 2023; Zygner et al., 2023). ConclusionTo the best of our knowledge, this represents the first study in Iraq demonstrated molecularly and phylogenetically the presence of B. gibsoni in different tick species including H. anatolicum, R. sanguineus, and R. turanicus. Subsequently, we first indicate the high prevalence of H. anatolicum in stray dogs suggesting its role in the transmission of canine babesiosis. Current concerns over the potential impacts of climate change and the increased movement between countries of stray animals on the distribution of ticks highlight the need for an accurate understanding of existing prevalence patterns. Furthermore, epidemiological screening, surveillance, and monitoring of parasitic infection could be carried out broadly with the help of appropriate diagnostic assays such as molecular and phylogenetic techniques. In Iraq, there was limited available data to identify various parasites in ticks and the exact role of each tick species in the transmission of infection among different types of animals. AcknowledgmentThe authors grateful acknowledge the Head and staff of the Department of Parasitology (College of Veterinary Medicine, University of Basrah) and the Iraqi Natural History Research Center and Museum for approving and supporting the current study. Conflict of interestThe authors have no conflict of interest to disclose. Authors’ contributionsGYAA: Designation of the work, collection of tick samples their related data as well as information concerning stray dogs, and statistical analysis. IME: Molecular assaying of ticks and detection of B. gibsoni in different tick species. NKT: Phylogentic analysis of study local B. gibsoni isolates. All authors contributed equally in writing, reading, and approving the final copy of the manuscript. FundingNo external funding was received (private funding). Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbdullah, S., Helps, C., Tasker, S., Newbury, H. and Wall, R. 2016. Ticks infesting domestic dogs in the UK: a large-scale surveillance programme. Parasit. Vectors 9, 1–9. Abdullah, S., Helps, C., Tasker, S., Newbury, H. and Wall, R. 2018. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the UK. Med. Vet. Entomol. 32(1), 14–22. Aktas, M. 2014. A survey of Ixodid tick species and molecular identification of tick-borne pathogens. Vet. Parasitol. 200(3–4), 276–283. Al-Ani, A.N. and Yousif, A.A. 2024. Clinical and molecular study of Babesia caballi in racing horses in Baghdad. Iraqi J. Vet. Sci. 38(2), 477–483. AL-Shabbani, M.A.A. and Faraj, A.A. 2023. Molecular detection and phylogenetic analysis of Babesia species infected ticks of camels (Camelus dromedarius) In Al-Najaf Al-Ashraf Province, Iraq. Ann. Forr. Res. 66(1), 267–285. Al-Shammari, A.S., Almahdawi, M.K. and Al-Bayati, A.S. 2024. Prevalence of blood protozoa in cattle in babylon governorate, Iraq. Egypt. J. Vet. Sci. 55(3), 633–642. Arsalan, S.H., Daham, E., Al-Obaidi, Q.T. and Sulaiman, E.G. 2006. Study the percentage of infection with endo and ecto parasites in dogs in Mosul/Iraq. Iraqi J. Vet. Sci. 20(1), 125–137. Awad, A.H.H. and Abdul-Hussein, M.A. 2006. New record of two species of hard ticks from some domestic animals in Basrah-Iraq. J. Basrah Res. (Sci.) 32(1), 1–6. Aziz, K.J. 2022. Morphological and molecular identification of Ixodid ticks that infest ruminants in Erbil province, Kurdistan Region-Iraq. Passer J. Basic Appl. Sci. 4(1), 8–13. Aziz, K.J. and AL-barwary, L.T.O. 2019. Prevalence rate of Ixodid ticks in equids and some nearby farm animals in Erbil governorate, north of Iraq. Basrah J. Vet. Res. 18(1), 337–359. Badawi, N.M. and Yousif, A.A. 2020a. Survey and molecular study of Babesia gibsoni in dogs of Baghdad province, Iraq. Iraqi J. Vet. Med. 44(E0), 34–41. Badawi, N.M. and Yousif, A.A. 2020b. Babesia canis spp. in dogs in Baghdad Province, Iraq: First molecular identification and clinical and epidemiological study. Vet. World 13(3), 579–585. Baneth, G. 2018. Babesia of domestic dogs. In Parasitic protozoa of farm animals and pets. Springer Nature, Berlin, pp: 241–258. Baneth, G., Cardoso, L., Brilhante-Simões, P. and Schnittger, L. 2019. Establishment of Babesia vulpes n. sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Parasit. Vectors 12, 1–8. Beristain-Ruiz, D.M., Garza-Hernández, J.A., Figueroa-Millán, J.V., Lira-Amaya, J.J., Quezada-Casasola, A., Ordoñez-López, S. and Rodríguez-Alarcón, C.A. 2022. Possible association between selected tick-borne pathogen prevalence and Rhipicephalus sanguineus sensu lato infestation in dogs from Juarez City (Chihuahua), Northwest Mexico–US Border. Pathogens 11(5), 1–16. Birkenheuer, A.J. 2021. Babesiosis. In Greene’s infectious diseases of the dog and cat. Philadelphia, PA: WB Saunders, pp: 1203–1217. Chauvin, A., Moreau, E., Bonnet, S., Plantard, O. and Malandrin, L. 2009. Babesia and its hosts: adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet. Res. 40(2), 1–18. Claerebout, E., Losson, B., Cochez, C., Casaert, S., Dalemans, A.C., De Cat, A. and Lempereur, L. 2013. Ticks and associated pathogens collected from dogs and cats in Belgium. Parasit. Vectors 6, 1–9. Conesa, J.J., Sevilla, E., Terrón, M.C., González, L.M., Gray, J., Pérez-Berná, A.J. and Montero, E. 2020. Four-dimensional characterization of the Babesia divergens asexual life cycle, from the trophozoite to the multiparasite stage. MSphere 5(5), 1–15. Estrada-Peña, A., Bouattour, A., Camicas, J. and Walker, A.R. 2004. A guide to identification of species. London, UK: Bioscience Reports, pp: 29–34. Friedhoff, K.T. 2018. Transmission of Babesia. In Babesiosis of domestic animals and man. Boca Raton, FL: CRC Press, pp: 23–52. Galaviz-Silva, L., Pérez-Treviño, K.C. and Molina-Garza, Z.J. 2013. Distribution of Ixodid ticks on dogs in Nuevo León, Mexico, and their association with Borrelia burgdorferi sensu lato. Exp. Appl. Acarol. 61, 491–501. Gharban, H.A. 2023. Molecular prevalence and phylogenetic confirmation of bovine trichomoniasis in aborted cows in Iraq. Vet. World 16(3), 580–587. Ghosh, R., Tarafdar, A., Chobe, D.R., Sharath Chandran, U.S., Rani, S. and Sharma, M. 2019. Diagnostic techniques of soil borne plant diseases: recent advances and next generation evolutionary trends. Biol. Forum Int. J. 11(2), 1–13. Hadi, U.K., Soviana, S. and Pratomo, I.R.C. 2016. Prevalence of ticks and tick-borne diseases in Indonesian dogs. J. Vet. Sci. Technol. 7(3), 1–7. Hatem, A.N. 2020. Prevalence and ecology of the brown dog tick Rhipicephalus sanguineus in domestic mammals in Basrah province, Iraq, with the acaricidal effect of Quercus brantti acorns extract in adults. Iraqi J. Agric. Sci. 51(6), 1670–1677. Hossein, M.A. 2023. Identification of blood protozoa infestation transmitted by vector tikes among awassi sheep herds in Kifri City, Kurdistan Region of Iraq. UHD J. Sci. Technol. 7(2), 1–5. Hussain, N., Shabbir, R.M.K., Ahmed, H., Afzal, M.S., Ullah, S., Ali, A. and Cao, J. 2023. Prevalence of different tick species on livestock and associated equines and canine from different agro-ecological zones of Pakistan. Front. Vet. Sci. 9, 1–11. Kahlig, P., Paris, D.H. and Neumayr, A. 2021. Louse-borne relapsing fever-A systematic review and analysis of the literature: Part 1-Epidemiology and diagnostic aspects. PLOS Negl. Trop. Dis. 15(3), 1–28. Karasová, M., Tóthová, C., Grelová, S. and Fialkovičová, M. 2022. The etiology, incidence, pathogenesis, diagnostics, and treatment of canine babesiosis caused by Babesia gibsoni infection. Animals 12(6), 1–19. Karawan, A.C., Mohammed, N.Q., Ahmed, H.S. and Khudhair, O.H. 2021. Molecular study of ticks; Hyalomma anatolicum, isolated from sheep in Al-Diwaniyah Province, Iraq. Ann. Rom. Soc. Cell. Biol. 25(6), 8433–8440. Karshima, S.N., Karshima, M.N. and Ahmed, M.I. 2022. Infection rates, species diversity, and distribution of zoonotic Babesia parasites in ticks: a global systematic review and meta-analysis. Parasitol. Res. 121(1), 311–334. Kumar, B., Manjunathachar, H.V. and Ghosh, S. 2020. A review on Hyalomma species infestations on human and animals and progress on management strategies. Heliyon 6(12), 1–13. Lager, M. 2020. Molecular and serological tools for clinical diagnostics of Lyme borreliosis-can the laboratory analysis be improved? Linköping, Sweden: Linköping University Electronic Press, pp: 1744. Latrofa, M.S., Angelou, A., Giannelli, A., Annoscia, G., Ravagnan, S., Dantas-Torres, F. and Otranto, D. 2017. Ticks and associated pathogens in dogs from Greece. Parasit. Vectors 10, 1–7. Léger, E., Vourc’h, G., Vial, L., Chevillon, C. and McCoy, K.D. 2013. Changing distributions of ticks: causes and consequences. Exp. Appl. Acarol. 59(1), 219–244. Livanova, N.N., Fomenko, N.V., Akimov, I.A., Ivanov, M.J., Tikunova, N.V., Armstrong, R. and Konyaev, S.V. 2018. Dog survey in Russian veterinary hospitals: tick identification and molecular detection of tick-borne pathogens. Parasit. Vectors 11, 1–10. Mahlobo, S.I.C. 2018. Ticks and tick-borne zoonotic pathogens of domestic animals in Lesotho (Doctoral dissertation, North-West University). Available via http://hdl.handle.net/10394/31266. Makawi, Z.A. and Hadi, A.M. 2023. Identification of hard ticks from buffalo Bubalus bubalis (Linnaeus, 1758) in Iraq. Bull. Iraq Nat. Hist. Mus. 17(3), 423–434. Martínez-García, G., Santamaría-Espinosa, R.M., Lira-Amaya, J.J. and Figueroa, J.V. 2021. Challenges in tick-borne pathogen detection: the case for Babesia spp. identification in the tick vector. Pathogens 10(2), 1–31. Maurelli, M.P., Pepe, P., Colombo, L., Armstrong, R., Battisti, E., Morgoglione, M.E. and Zanet, S. 2018. A national survey of Ixodidae ticks on privately owned dogs in Italy. Parasit. Vectors 11, 1–10. Mohammad, M.K. 2015. Distribution of Ixodid ticks among domestic and wild animals in central Iraq. Bull. Iraq Nat. Hist. Mus. 13(3), 23–30. Obaid, H.H., Hasson, R.H., Al–Ani, M.O., Fayyad, E.J., Abbas, S.F. and Hamza, T.H. 2023. Geographical distribution of Ixodidae (hard ticks) in all provinces of Iraq. Iraqi J. Vet. Sci. 37 (Supplement I–IV), 197–201. Ojeda-Chi, M.M., Rodriguez-Vivas, R.I., Esteve-Gasent, M.D., Pérez de León, A.A., Modarelli, J.J. and Villegas-Perez, S.L. 2019. Ticks infesting dogs in rural communities of Yucatan, Mexico and molecular diagnosis of rickettsial infection. Transbound. Emerg. Dis. 66(1), 102–110. Onyiche, T.E., Răileanu, C., Fischer, S. and Silaghi, C. 2021. Global distribution of Babesia species in questing ticks: a systematic review and meta-analysis based on published literature. Pathogens 10(2), 1–27. Otranto, D., Iatta, R., Baneth, G., Cavalera, M.A., Bianco, A., Parisi, A. and Chomel, B. 2019. High prevalence of vector-borne pathogens in domestic and wild carnivores in Iraq. Acta Trop. 197, 1–31. Proctor, H.C., Smith, I.M., Cook, D.R. and Smith, B.P. 2015. Subphylum Chelicerata, Class Arachnida. In Thorp and Covich’s freshwater invertebrates. Cambridge, MA: Academic Press, pp: 599–660. Raghavan, M., Glickman, N., Moore, G., Caldanaro, R., Lewis, H. and Glickman, L. 2007. Prevalence of and risk factors for canine tick infestation in the United States, 2002–2004. Vector Borne Zoonotic Dis. 7(1), 65–75. Rajakaruna, R.S. and Eremeeva, M.E. 2023. Eco-epidemiology of Tick-borne pathogens: role of tick vectors and host animal community composition in their circulation and source of Infections. In One health: human, animal, and environment triad, Chapter 23, USA: Wiley, pp: 325–350. Ramos, V.N., Lemos, F.G., Azevedo, F.C., Arrais, R.C., Lima, C.F.M., Candeias, I.Z. and Szabó, M.P.J. 2020. Wild carnivores, domestic dogs and ticks: shared parasitism in the Brazilian Cerrado. Parasitol. 147(6), 689–698. Ravindran, R., Hembram, P.K., Kumar, G.S., Kumar, K.G.A., Deepa, C.K. and Varghese, A. 2023. Transovarial transmission of pathogenic protozoa and rickettsial organisms in ticks. Parasitol. Res. 122(3), 691–704. Sahu, A., Mohanty, B., Panda, M.R., Sardar, K.K. and Dehuri, M. 2013. Prevalence of tick infestation in dogs in and around Bhubaneswar. Vet. World 6(12), 982–985. Saleh, M.N., Allen, K.E., Lineberry, M.W., Little, S.E and Reichard, M.V. 2021. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet. Parasitol. 294, 1–25. Saleh, M.N., Sundstrom, K.D., Duncan, K.T., Ientile, M.M., Jordy, J., Ghosh, P. and Little, S.E. 2019. Show us your ticks: a survey of ticks infesting dogs and cats across the USA. Parasit. Vectors 12, 1–11. Schäfer, I., Helm, C.S., von Samson-Himmelstjerna, G., Krücken, J., Kottmann, T., Holtdirk, A. and Müller, E. 2023. Molecular detection of Babesia spp. in dogs in Germany (2007–2020) and identification of potential risk factors for infection. Parasit. Vectors 16(1), 1–12. Smith, F.D., Ballantyne, R., Morgan, E.R. and Wall, R. 2011. Prevalence, distribution and risk associated with tick infestation of dogs in Great Britain. Med. Vet. Entomol. 25(4), 377–384. Umesha, S. and Manukumar, H.M. 2018. Advanced molecular diagnostic techniques for detection of food-borne pathogens: Current applications and future challenges. Crit. Rev. Food Sci. Nutr. 58(1), 84–104. Yin, F., Guo, C., Tian, Z., Li, D., Mu, D., Liu, H. and Li, F. 2023. Analysis of genetic diversity and population structure of Babesia gibsoni. Front. Vet. Sci.10, 1–9. Zygner, W., Gójska-Zygner, O., Bartosik, J., Górski, P., Karabowicz, J., Kotomski, G. and Norbury, L.J. 2023. Canine Babesiosis caused by large Babesia species: global prevalence and risk factors-a review. Animals 13(16), 1–43. | ||
| How to Cite this Article |
| Pubmed Style Essa IM, Azzal GY, Thamer NK. First molecular sequencing of Babesia gibsoni in ticks, Iraq. Open Vet. J.. 2024; 14(8): 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 Web Style Essa IM, Azzal GY, Thamer NK. First molecular sequencing of Babesia gibsoni in ticks, Iraq. https://www.openveterinaryjournal.com/?mno=204579 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.32 AMA (American Medical Association) Style Essa IM, Azzal GY, Thamer NK. First molecular sequencing of Babesia gibsoni in ticks, Iraq. Open Vet. J.. 2024; 14(8): 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 Vancouver/ICMJE Style Essa IM, Azzal GY, Thamer NK. First molecular sequencing of Babesia gibsoni in ticks, Iraq. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 Harvard Style Essa, I. M., Azzal, . G. Y. & Thamer, . N. K. (2024) First molecular sequencing of Babesia gibsoni in ticks, Iraq. Open Vet. J., 14 (8), 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 Turabian Style Essa, Israa M., Ghazi Y. Azzal, and Nadia K. Thamer. 2024. First molecular sequencing of Babesia gibsoni in ticks, Iraq. Open Veterinary Journal, 14 (8), 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 Chicago Style Essa, Israa M., Ghazi Y. Azzal, and Nadia K. Thamer. "First molecular sequencing of Babesia gibsoni in ticks, Iraq." Open Veterinary Journal 14 (2024), 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 MLA (The Modern Language Association) Style Essa, Israa M., Ghazi Y. Azzal, and Nadia K. Thamer. "First molecular sequencing of Babesia gibsoni in ticks, Iraq." Open Veterinary Journal 14.8 (2024), 2029-2039. Print. doi:10.5455/OVJ.2024.v14.i8.32 APA (American Psychological Association) Style Essa, I. M., Azzal, . G. Y. & Thamer, . N. K. (2024) First molecular sequencing of Babesia gibsoni in ticks, Iraq. Open Veterinary Journal, 14 (8), 2029-2039. doi:10.5455/OVJ.2024.v14.i8.32 |