| Research Article | ||
Open Vet. J.. 2024; 14(8): 2040-2048 Open Veterinary Journal, (2024), Vol. 14(8): 2040–2048 Research Article Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluentRagil Angga Prastiya1*, Trilas Sardjito1, Amung Logam Saputro1, Darmawan Setia Budi1, Styuderil Imaniro Maxdhameta2, Elis Sulistiyawati2, Deny Sulistyowati3, Anny Amaliya3, Samira Musa Sasi4 and Nining Haryuni51Division of Veterinary Reproduction, Faculty of Health, Medicine, and Life Sciences (FIKKIA), Universitas Airlangga, Banyuwangi, Indonesia 2Bachelor of Veterinary Medicine, Faculty of Health, Medicine, and Life Sciences (FIKKIA), Universitas Airlangga, Banyuwangi, Indonesia 3Singosari National Artificial Insemination Center, Directorate General of Livestock and Animal Health, Malang City, Indonesia 4Department of Zoology, Faculty of Science, University of Tripoli, Tripoli, Libya 5Nahdlatul Ulama Blitar University, Blitar City, Indonesia *Corresponding Author: Ragil Angga Prastiya. Division of Veterinary Reproduction, Faculty of Health, Medicine, and Life Sciences (FIKKIA), Universitas Airlangga, Banyuwangi, Indonesia. Email: ragilap [at] fkh.unair.ac.id Submitted: 13/06/2024 Accepted: 19/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
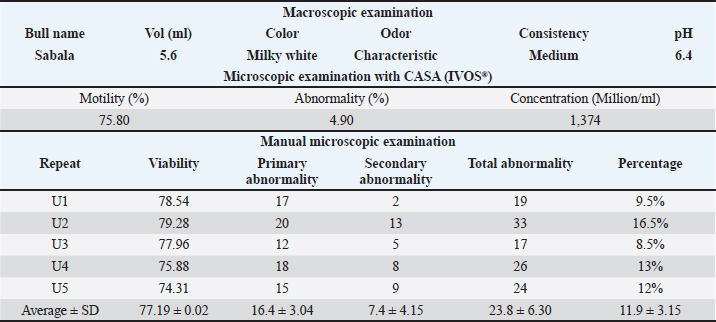
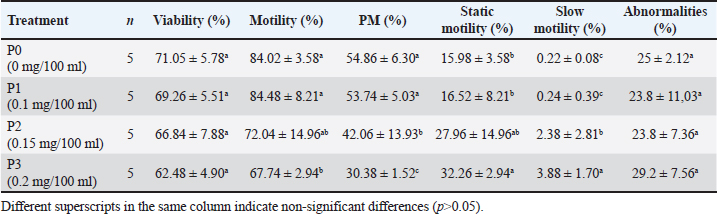
ABSTRACTBackground: The preservation of semen quality and kinematic characteristics during cryopreservation is crucial for the reproductive success and genetic management of livestock, particularly in Bali bulls. Aim: This study aimed to investigate the effect of adding purified green tea extract antioxidant Epigallocatechin-3-gallate (EGCG) in tris egg yolk diluent on the quality and kinematic characteristics of frozen semen from Bali bulls. Methods: Fresh and frozen semen samples were obtained from Bali bull and divided into four different treatment groups. P0 contained semen samples + diluent, while P1 to P3 consisted of semen samples + diluent supplemented with EGCG levels of 0.1, 0.15, and 0.2 mg/100 ml, respectively. Data were analyzed using One-way ANOVA and followed by Duncan’s test if significant differences were found (p<0.05). Parameters observed included the assessment of fresh semen quality, kinematic analysis, post-thawing sperm viability, and abnormality. Results: The results indicated that the assessment of fresh semen quality showed macroscopic and microscopic semen quality according to SNI 4869-1:2021. Kinematic analysis revealed significant differences in DSL and STR parameters between P0 and P3 (p<0.05). EGCG supplementation also caused significant differences in motility between P0 and P3 (p<0.05). Viability and spermatozoa abnormality with EGCG supplementation did not show significant differences (p>0.05). Conclusion: The best results for motility, kinematics, and sperm morphology variables were found in P1 as it did not exhibit a decrease in motility, kinematics, and sperm morphology. Viability did not show significant differences between P1, P2, and P3, but the best results were found in P2 as it did not exhibit a decrease in viability with mean and standard deviation (66.84 ± 7.88). Abnormality variables also did not show significant differences between P1, P2, and P3, but the best results were found in P2 as it did not exhibit a decrease in abnormality with mean and standard deviation (23.80 ± 7.36). Keywords: Bali bull, EGCG, Kinematics, Livestock and gene bank, Sperm quality. IntroductionThe livestock sector in Indonesia currently encompasses a wide range of operations, from small-scale to large-scale enterprises (Asikin et al., 2020). Among the various types of livestock farming, cattle farming, particularly Bali cattle, is being extensively developed (Rusdiana and Soeharsono, 2017). This is due to Bali cattle’s superior adaptability, commendable production performance, and high reproductive capacity. Bali cattle are domesticated descendants of the wild banteng (Baco et al., 2020). These cattle hold substantial potential for development as they represent the largest population among local cattle breeds (Scholtz et al., 2013). The high population and widespread distribution of Bali cattle across Indonesia indicate their excellent adaptability, making them a suitable candidate for development by farmers as a national food source (Widyas et al., 2022). Efforts to increase their population can be achieved through various means, including genetic improvement and reproductive efficiency, particularly through artificial insemination (AI) programs (DeJarnette et al., 2022). Given the significant role of Bali cattle in the livestock sector, enhancing the quality of their semen is essential to further increase their population in Indonesia (Prastowo et al., 2019). Despite their relatively smaller size compared to other cattle breeds, Bali cattle exhibit superior reproductive adaptation even within the same habitat (Sutarno and Setyawan, 2016). However, the semen quality of Bali bull has been found to be suboptimal in some instances, requiring interventions to enhance its quality (Sutarno and Setyawan, 2015). This suboptimal semen quality is often attributed to oxidative stress, which can impair sperm motility and viability (Kaltsas, 2023). (Suyadi et al., (2020) emphasized that reproductive factors, particularly through AI, are crucial for successful genetic improvement. One method to enhance semen quality, thereby facilitating genetic improvement in Bali cattle, is the inclusion of antioxidant compounds in the diluent during the semen freezing process (Petruska et al., 2014). One potent antioxidant is epigallocatechin-3-gallate (EGCG), a catechin derivative from green tea (Kim et al., 2014). The four primary catechins are EGCG, which makes up approximately 59% of the total catechins, epigallocatechin (EGC), epicatechin 3-gallate (ECG), and epicatechin (Narotzki et al., 2013). Catechin derivatives possess the ability to prevent damage from free radicals and are abundant and exceptionally potent antioxidants, being 200 times more effective than other antioxidants such as vitamins C and E (Du et al., 2012; Mondal and De, 2018; Baldi et al., 2019). These antioxidants are naturally found only in tea (Xu et al., 2021). The addition of EGCG to the tris-egg yolk diluent can influence the quality of sperm. The motility and viability of sperm can be maintained in tris-egg yolk diluent due to its buffering agents and substantial nutritional content (Susilowati et al., 2021). Given the high antioxidant potential of EGCG, research is needed to evaluate the effect of EGCG in tris-egg yolk diluent on the quality of Bali bull sperm. The combination of EGCG with tris-egg yolk diluent aims to maintain kinematic parameters and assess the impact of EGCG on the morphological improvement of Bali bull sperm as evaluated by CASA (Prastiya et al., 2023). According to Gallagher et al. (2019), sperm kinematics refers to the dynamic movement of the sperm head along its trajectory. This kinematic measurement predicts spermatozoa fertility since the success of fertilization is influenced by the speed and pattern of sperm movement. Maylem et al. (2018) stated that sperm kinematic parameters provide an objective assessment of motility, a primary quality indicator crucial for spermatozoa fertility. Various treatments, such as diluent addition, freezing, and cooling. Materials and MethodsSemen sampleThe study was conducted at the Singosari National AI Center (SNAIC) in Malang, East Java, Indonesia. The study was carried out from December 12, 2023, to December 23, 2023. Bali bull semen was collected once a week in the morning. The ejaculate was processed into frozen semen following the guidelines of the Indonesian National Standard SNI: 4869-1:2017 (production and analysis of frozen semen) by the National Standardization Agency of Indonesia. The semen samples were obtained from a seven-year-old Bali bull named Sabala, with bull code 116132, sourced from SNAIC. Semen collection was performed using an artificial vagina, and the quality of the Bali bull semen was assessed at the SNAIC Laboratory. Semen quality evaluation included both macroscopic and microscopic examinations. Macroscopic evaluation involved assessing color, consistency, pH level, volume, and odor, while microscopic evaluation assessed concentration, motility, viability, abnormality, and mass movement. Purified EGCG from green tea extractThe EGCG purification process from green tea extract to be added to the Bali bull frozen semen diluent involved several steps. First, 10 grams of commercial green tea extract were dissolved in 100 ml of 70% ethanol. The mixture was homogenized using a vortex mixer for 10 minutes and then centrifuged at 4,000 rpm for 15 minutes to separate the solids. The supernatant was collected and concentrated using a rotary evaporator at 40°C until the volume was reduced to approximately 20 ml. The concentrated extract was then subjected to column chromatography using a C18 stationary phase, conditioned with methanol. The concentrated extract was applied to the column and eluted with a gradient solvent system of water and methanol (starting from 90:10 to 50:50). Fractions were collected separately and analyzed using a UV-Vis spectrophotometer at around 280 nm to detect the presence of EGCG. Fractions containing EGCG were pooled and further concentrated using a rotary evaporator. If additional purification was needed, high-performance liquid chromatography was employed. The purified EGCG solution was then diluted in deionized water to achieve the desired concentrations of 0.1/100, 0.15/100, and 0.2 mg/100 ml. The purified EGCG was added to the tris egg yolk diluent for the frozen semen. The EGCG-enriched diluent was stored under cold conditions until it was used for the cryopreservation of Bali bull semen. This process ensured the acquisition of pure EGCG from green tea extract for enhancing the quality of Bali bull frozen semen. Experimental design and assessmentBali bull semen that met both macroscopic and microscopic quality criteria was diluted using a tris-egg yolk extender with the addition of EGCG compound, followed by the process of making frozen semen. Fresh semen from Bali bulls that meets both macroscopic and microscopic criteria will proceed to the next stage, which is dilution. The semen will be diluted using a tris egg yolk diluent composed of several ingredients: 1.6% Tris aminomethane, 0.9% citric acid, 1.4% lactose, 80% distilled water, 2.5% raffinose, 20% egg yolk, 100,000 IU/100 ml penicillin, 0.1 g/100 ml streptomycin, and 13% glycerol. The study design employed a completely randomized design since the only variable was the treatment. Randomization was applied to all components in the experiment, which included four treatments and five replicates: the treatments consisted of Bali bull semen + 13% glycerol + tris-egg yolk diluent with varying concentrations of EGCG: (P0) 0/100, (P1) 0.1/100, (P2) 0.15/100, and (P3) 0.2 mg/100 ml. Data collection from each treatment was conducted by assessing parameters after sperm freezing. Motility assessment of Bali bull semen involved placing a drop of spermatozoa on a glass slide, covering it with a cover glass, and observing under a microscope at 400× magnification to evaluate the progressive movement of spermatozoa. Viability assessment used Eosin-Nigrosin staining. Dead spermatozoa before slide preparation absorbed the stain and appeared purple, while live spermatozoa remained white and transparent. The process involved mixing one drop each of Eosin-Nigrosin stain and Bali bull semen on a glass slide, homogenizing, air drying over a Bunsen burner flame for up to 15 seconds, and observing under a microscope. Viability was expressed as a percentage. Abnormality assessment used previously stained slides to observe under a microscope at 400× magnification, with the percentage of abnormal spermatozoa recorded. Freezing procedureFresh semen that is deemed to have good quality, with progressive motility (PM) of not less than 70%, proceeds to the glycerolization process by adding a tris egg yolk diluent. Two types of diluent solutions are prepared: solution A with EGCG compound, and solution B containing diluent and EGCG compound with 13% glycerol. The solutions are gradually mixed along the walls of the tube in varying volumes according to a predetermined diluent formula. After the diluent is added, the semen solution is placed into a cool top at 4°C–5°C for a maximum equilibration time of 4 hours to allow the sperm cells to equilibrate with the added cryoprotectant (glycerol). In the “before freezing” examination stage, if the liquid semen meets the standard, it proceeds to the freezing process. Semen that does not meet the quality standard is discarded. The acceptable quality standard for liquid semen is a minimum of 60% PM with a speed of 3. Once the semen meets the quality standard in the “before freezing” stage, the straw printing is carried out simultaneously with the dilution process to determine the number of straws to be printed. The printed straws are labeled with information about the bull type, bull name, bull code, batch number, and the producer of the frozen semen. The number of straws depends on the number of spermatozoa in the ejaculate. Bali bull straws are marked with a red color. The next step is filling and sealing, where the diluted semen is filled into the straws using an automated filling and sealing machine. This machine automatically fills each straw with 0.25 mL of liquid semen and seals the straw’s end with a laboratory plug. This process is conducted in a cool top. After printing, filling, and sealing, the process continues with prefreezing and freezing. Prefreezing involves exposing the straws to nitrogen vapor at −160°C for 9–12 minutes to prevent cold shock. The freezing stage involves immersing the semen straws in liquid nitrogen, bringing the temperature down to −196°C. During the thawing stage, previously filled straws are thawed in a water bath at 37°C for 30 seconds. The straw is held horizontally, with the filter end down and the seal end up, and the end of the straw is cut. The straw is placed in an Eppendorf tube; if it does not spill, the straw is in good condition. One drop of semen is placed on a glass slide and examined under a microscope to assess post-thawing quality. Sperm kinematics and morphology variablesThe study of sperm morphology and kinematic parameters was conducted using the Computer-Assisted Semen Analysis (CASA) software from the Sperm Analyzer IVOS IITM (Hamilton Thorne Inc., USA). The CASA settings included a frame rate of 60 Hz, 30 frames obtained, minimum contrast set to 35, minimum cell size at 5 pixels, cell size at 9 pixels, and cell intensity at 110 pixels. These settings were applied to evaluate the motility of Bali bull sperm. The frozen Bali bull sperm samples were thawed at 37°C for 30 seconds (Zhong et al., 2019). For each semen sample, 5 μl was placed on a pre-warmed (38°C) counting chamber. Sperm motility was observed using a 10× negative phase and 100× brightfield for morphology with values ranging from 0% to 100% (Chen et al., 2019). At least 1000 sperm were analyzed. The kinematic and motility parameters assessed included total motility (TM), PM, average path velocity (VAP), straight-line velocity (VSL), curvilinear velocity (VCL), straightness (STR), linearity (LIN), amplitude of lateral head displacement (ALH), beat/cross-frequency (BCF), and wobble (WOB). Sperm morphology was observed and measured for normal morphology (NM), abnormal morphology (AM), bent tail (BT), coiled tail (CT), proximal droplets (PD), distal droplets (DDs), and distal midpiece reflex (DMR). MDA level testTo assess malondialdehyde (MDA) levels in frozen Bali bull semen samples treated with varying concentrations of EGCG, each sample was thawed to room temperature and processed accordingly. A 0.5 ml aliquot of each thawed semen sample was mixed with 1 ml of 0.67% Thiobarbituric acid (TBA) solution (in 20% acetic acid, pH 3.5) in reaction tubes. The mixture was heated in a water bath at 95°C for 60 minutes to form MDA-TBA adducts, followed by rapid cooling in ice water for 10 minutes to halt the reaction. The reaction mixture was then extracted with 2 ml of n-butanol, vortexed, and centrifuged at 3000 rpm for 10 minutes to separate the upper n-butanol layer containing the MDA-TBA complex. The absorbance of the n-butanol layer was measured at 532 nm using a spectrophotometer, and MDA levels were quantified using a calibration curve prepared with MDA standards. Statistical analysis, such as analysis of variance (ANOVA) followed by post-hoc tests as needed, was performed to compare MDA levels among different EGCG treatment groups. Results were reported in nmol/ml of semen, with discussions on the implications of MDA measurements for semen quality and the efficacy of EGCG treatment. Data analysisData obtained from this study were analyzed using the statistical product and service solutions (SPSS) version 29 for Windows. Analysis was performed using one-way ANOVA. If significant differences were detected, Duncan’s multiple range test was conducted to identify specific differences between treatments. Ethical approvalThis study was conducted at the Singosari National AI Center (SNAIC) in Malang, East Java, Indonesia, adhering to the standard operating procedures of SNI ISO 9001:2015 and SNI ISO 37001:2016. The research was overseen by veterinarians affiliated with these organizations. Ethical guidelines and approval for the responsible use of bulls in the collection of fresh sperm were granted by the Ethics Committee. ResultsMacroscopic and microscopic examinationThe macroscopic and microscopic examinations of fresh semen from Bali bulls were conducted to determine the suitability of the semen for further treatment. The results of these examinations are presented in Table 1. The macroscopic and microscopic observations aimed to assess the quality of the fresh semen from Bali bulls through macroscopic parameters such as volume, color, odor, pH, and consistency. If the semen was deemed suitable, it proceeded to microscopic examination. Microscopic examinations were conducted using Computer-Assisted Sperm Analysis (CASA, IVOS®), evaluating motility, abnormality, and concentration. Additionally, manual assessments determined the percentage of viable spermatozoa and abnormal spermatozoa in each sample. The fresh semen of the Bali bull named Sabala has a volume of 5.6 ml, a milky white color, and a characteristic odor typical of the bull. The pH of Sabala’s semen is 6.4, and its consistency is medium, which can be observed by gently tilting the test tube containing the semen. The microscopic examination using CASA (IVOS®) includes the assessment of motility, abnormality, and concentration. The motility of Sabala’s semen is 75.80%, the abnormality rate is 4.90%, and the concentration is 1,374 million/ml. In the manual examination, the sperm viability result was 77.19%. For sperm abnormality assessment using both manual methods and IVOS, there was a difference of 7% between the two methods. Sperm quality assessmentThe post-thaw motility (PTM) of frozen Bali bull semen after adding the antioxidant compound EGCG, with three treatments are detailed in Table 2. Statistical analysis using one-way ANOVA followed by Duncan’s test indicated significant differences in motility with the addition of EGCG. Specifically, there was a significant decrease in motility between the control group P0 and P3. However, no significant decrease was observed between the control and P1 and P2. For total and PM, there was a significant decrease between the control P0 and both P2 and P3, while there are no significant between the control and P1. Static motility showed a significant increase between the control P0 and P3, with non-significant increases observed between the control and P1 and P2. Slow motility showed a significant increase between the control P0 and both P2 and P3, with a non-significant observed between the control and P1. Table 1. Results of macroscopic and microscopic examination of fresh Bali bull semen.
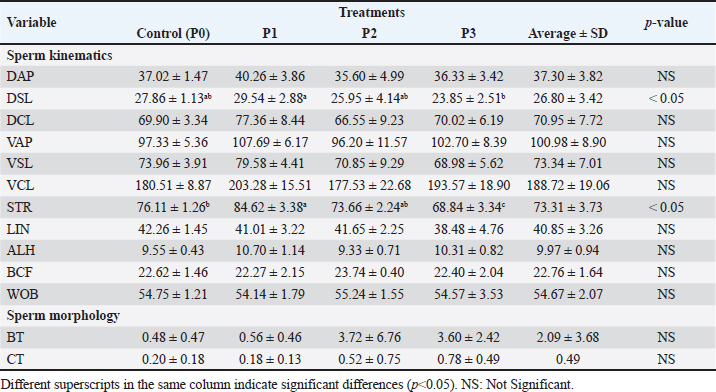
Table 2. Mean and standard deviation of viability and PTM in frozen Bali bull semen.
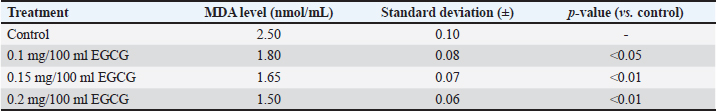
Statistical analysis using one-way ANOVA followed by Duncan’s test indicated no significant differences in viability between the control (P0) and the EGCG treatments (P1, P2, and P3). In this study, the addition of EGCG 0.1 mg/100 ml (P1) resulted in the highest sperm viability, closely approximating the control (P0). The high viability of spermatozoa in P1 could be due to the quality of the frozen semen enhanced by EGCG, which contains unique components not present in the tris-egg yolk extender. The average viability percentage of Bali bull semen in this study was 66.66 ± 6.42, indicating that the semen used was suitable. The observations on the addition of the EGCG compound to the abnormality of Bali bull sperm PTM after treatment showed non-significant differences (p>0.05). The data on sperm abnormality, based on the average results from Table 2, indicate that P1 and P2 have the lowest average abnormality compared to P0 and P3. Sperm kinematics assessmentThe observation results of frozen semen kinematics from Bali Bulls after the addition of the antioxidant compound EGCG, given in three treatments, showed the mean and standard deviation can be seen in Table 3. The observation results of frozen semen from Bali Bulls PTM after the addition of the antioxidant compound EGCG with three treatments showed the mean and standard deviation as follows: distance average path (DAP) was 37.30 ± 3.82, distance straight line (DSL) was 26.80 ± 3.42, distance curvilinear (DCL) was 70.95 ± 7.72, VAP was 100.98 ± 8.90, VSL was 73.34 ± 7.01, VCL was 188.72 ± 19.06, STR was 73.31 ± 3.73, LIN was 40.85 ± 3.26, ALH was 9.97 ± 0.94, BCF was 22.76 ± 1.64, and WOB was 54.67 ± 2.07. The mean and standard deviation of sperm morphology (BT) were 2.09 ± 3.68 and (CT) was 0.42 ± 0.49. The observation results of adding EGCG compound to sperm kinematics and morphology after treatment showed that there were significant differences (p < 0.05) in the DSL and STR parameters. Meanwhile, in other kinematic parameters such as DAP, DCL, VAP, VSL, LIN, ALH, BCF, WOB, and sperm morphology such as BT and CT, there were no significant differences (p > 0.05). The significant results from the DSL and STR parameters could be influenced by differences in the quality of frozen semen added with EGCG compound acting as an antioxidant. The pattern of sperm kinematics and morphology results showed that the EGCG compound dosage with the best results was 0.1 mg/100 ml in (P1) because there was an improvement in sperm quality, and the results approached those of the control treatment (P0). The high pattern of sperm kinematics and morphology obtained from treatment P1 could be influenced by differences in the quality of frozen semen due to the antioxidant effect of the EGCG compound. MDA levelsThe results of the MDA levels indicate a significant reduction in oxidative stress when EGCG is added to the egg yolk tris diluent. The control group, which did not receive any EGCG, exhibited the highest MDA level at 2.50 nmol/ml. This level of MDA is indicative of higher lipid peroxidation (LPO), reflecting greater oxidative stress. With the addition of 0.1 mg/100 ml EGCG, the MDA level decreased to 1.80 nmol/ml. This reduction is statistically significant, with a p-value of less than 0.05, indicating that the addition of EGCG at this dosage effectively reduces oxidative stress compared to the control. These findings are detailed in Table 4. Increasing the EGCG dosage to 0.15 mg/100 ml resulted in a further decrease in MDA level to 1.65 nmol/ml. This decrease is highly significant, with a p-value of less than 0.01, demonstrating a stronger antioxidant effect at this dosage. At the highest dosage of 0.2 mg/100 mL EGCG, the MDA level was the lowest at 1.50 nmol/ml. This reduction is also highly significant, with a p-value of less than 0.01, indicating that higher concentrations of EGCG provide the greatest protection against oxidative stress. Table 3. Means ± SD of sperm kinematic and sperm morphologic of Bali bull in diluent post thawed with different doses of EGCG.
Table 4. MDA levels with different dosages of EGCG.
DiscussionSperm, unlike other cells, are characterized by distinct structure, function, and sensitivity to oxidative stress (Drevet et al., 2022). It is well-documented that sperm quality deteriorates significantly during prolonged incubation and cryopreservation, with a clear link between reactive oxygen species (ROS) levels and LPO under these conditions (Kameni et al., 2021). ROS induces significant damage during the freezing and thawing processes, making the inclusion of exogenous antioxidants in cryopreservation media essential to protect sperm (Tariq et al., 2015). Previous studies have shown that adding EGCG to cryo-diluent media enhances sperm quality and fertility potential while reducing oxidative stress in bovine sperm (Li et al., 2022). Research on the effect of EGCG on extenders or diluents for sperm quality and kinematic parameters is crucial in the field of reproductive health, especially in livestock farming. The findings suggest that EGCG, when used as a diluent, can have a positive impact on sperm quality and kinematic parameters at lower concentrations (Mustofa et al., 2021). However, higher concentrations may negatively affect sperm motility and viability (Bucci et al., 2017). EGCG’s antioxidant properties can help protect sperm from oxidative stress, which can damage sperm DNA and affect sperm motility and kinematic parameters, potentially leading to improved sperm quality (Alqawasmeh et al., 2021). The addition of 50 µM EGCG in diluents has been shown to increase TM, PM, and decrease static motility compared to other doses (Akarsu et al., 2023). The addition of antioxidants did not impact sperm viability and abnormality, consistent with previous findings (Gadani et al., 2017). Although the current study used flow cytometry and the previous one employed an epifluorescence microscope, both studies observed a decrease in viable spermatozoa during the post-thaw incubation period (Gadani et al., 2017). EGCG has been shown to inhibit proteasomal activity, which is involved in protein degradation and turnover (Bonfili et al., 2011). This inhibition can affect sperm motility and kinematic parameters by altering the regulation of protein phosphorylation and ubiquitination (Zigo et al., 2019). Proteasomal inhibition by MG-132, a proteasome inhibitor, has been shown to negatively impact velocity-based kinematic sperm parameters (VSL, VAP, and VCL) in a dose-dependent manner (Hackerova et al., 2023). This suggests that EGCG, which also inhibits proteasomal activity, might have similar effects on sperm velocity (Hung et al., 2022). In this study, there were no negative effects of EGCG on velocity (VSL, VAP, and VCL) because the concentrations used were not high. EGCG has been shown to inhibit proteasomal activity, which is involved in protein degradation and turnover (Bonfili et al., 2011). This inhibition can affect sperm motility and kinematic parameters by altering the regulation of protein phosphorylation and ubiquitination (Zigo et al., 2019). Proteasomal inhibition by MG-132, a proteasome inhibitor, has been shown to negatively impact velocity-based kinematic sperm parameters (VSL, VAP, and VCL) in a dose-dependent manner (Hackerova et al., 2023). This suggests that EGCG, which also inhibits proteasomal activity, might have similar effects on sperm velocity (Hung et al., 2022). In this study, there were no negative effects of EGCG on velocity (VSL, VAP, and VCL) because the concentrations used were not high. The effect of EGCG on kinematic sperm parameters, specifically DAP, DSL, DCL, and STR, is not explicitly stated in the provided sources (Setumo et al., 2022). In this study, there was no effect of EGCG on DAP and DCL, but there was a significant decrease in DSL and STR at the P3 dose, indicating that this dose negatively affects PM, which could negatively impact fertilization success. Sperm with progressive and straight-line movement are more likely to reach the ovum and fertilize it successfully, as this movement allows spermatozoa to navigate through cervical mucus and reach the fallopian tube, where fertilization occurs (Nordhoff and Wistuba, 2023). Different results were reported by Li et al. (2022), who found that adding 0.6 mg/ml EGCG to bovine diluent positively affected TM, distance average path, distance straight line, distance curved line, VAP, VCL, VSL, ALH and beat/cross frequency, as well as sperm CAT, GSH-Px, and SOD levels. Further research is needed on other parameters to determine the positive impact of EGCG as a strong antioxidant with antimicrobial effects. This includes exploring the effects of low dose of EGCG on apoptotic markers, and SOD, GSH, and GPx levels, which have not been extensively studied. Additionally, testing related to the seminal microbiome is necessary to evaluate the positive effects of EGCG’s antimicrobial properties, potentially substituting the use of antibiotics in diluents. ConclusionBased on the research findings, it can be concluded that the addition of the EGCG compound at a dosage of 0.1 mg/100 ml in egg yolk tris diluent did not show a decrease in motility, viability, or abnormality compared to the control treatment, indicating that this dosage can maintain semen quality. The addition of the EGCG compound at a dosage of 0.2 mg/100 ml in egg yolk tris diluent showed a decrease in motility, suggesting that higher concentrations may have detrimental effects. In the kinematic observation with parameters DAP, DCL, VAP, VSL, LIN, ALH, BCF, and WOB, there were no changes compared to the control treatment, showing that EGCG at these dosages does not negatively impact these kinematic parameters. However, in the DSL and STR parameters, a dosage of 0.1 mg/100 ml in egg yolk tris diluent showed an increase in kinematics, indicating a potential improvement in PM and straight-line movement, which are crucial for fertilization success. All treatments did not affect sperm morphology, highlighting that EGCG does not induce morphological abnormalities in sperm. Importantly, EGCG exhibits a dose-dependent reduction in MDA levels, a marker of oxidative stress, with significant decreases observed at all concentrations tested. These results underscore EGCG’s role as a potent antioxidant in protecting semen from oxidative damage during cryopreservation. AcknowledgmentThe researcher extends gratitude to the Singosari Artificial Insemination Center (BBIB) for their assistance and facilitation throughout the research process. The researcher also expresses appreciation to the research supervisor, Dr. Denny Sulistyowati, for guidance and direction during the research period. Additionally, thanks are due to the other academic supervisors who provided valuable advice and feedback in the preparation of this scientific article. The researcher also acknowledges the involvement and support of all parties who contributed to and assisted in the research process. Conflict of interestThe authors declare that there are no conflicts of interest regarding the publication of this manuscript. Authors’ contributionsAll authors contributed to the conception and design of the study. R.A. Prastiya and T. Sardjito supervised the entire research project. S.I. Maxdhameta, and E. Sulistiyawati were responsible for conducting the experiments and collecting the data. D. Sulistyowati and A. Amaliya provided technical support and guidance in the methodology. S.M. Sasi contributed to the data analysis and interpretation. N. Haryuni helped in drafting and revising the manuscript. All authors have read and approved the final manuscript. FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. It was supported by the collaborative efforts of the research group. Data availabilityAll data that underpin the findings of this study are included within the manuscript. ReferencesAkarsu, S.A., Koca, R.H., Cihangiroğlu, A.Ç., Acısu, T.C., Güngör, İ.H., Sönmez, M., Türk, G., Gür, S., Ömür, A.D. and Duruel, H.E.E. 2023. Determination of the effect of epigallocatechin gallate on oxidative stress, apoptosis and sperm quality in rabbit semen. New Trends Med. Sci. 4, 156–162. Alqawasmeh, O., Zhao, M., Chan, C., Leung, M., Chow, K., Agarwal, N., Mak, J., Wang, C., Pang, C., Li, T., Chu, W. and Chan, D. 2021. Green tea extract as a cryoprotectant additive to preserve the motility and DNA integrity of human spermatozoa. Asian J. Androl. 23, 150–156. Asikin, Z., Baker, D., Villano, R. and Daryanto, A. 2020. Business models and innovation in the Indonesian smallholder beef value chain. Sustain. 12, 7020. Baco, S., Malaka, R. and Hatta, M. 2020. The body condition and reproduction performances of bali cattle cows through the improved feeding in the intensive management system. In IOP Conference Series: Earth and Environmental Science, pp. 012101. Baldi, A., Abramovič, H., Poklar Ulrih, N. and Daglia, M. 2019. Tea catechins. In Handbook of dietary phytochemicals, pp: 1–46. Bonfili, L., Cuccioloni, M., Mozzicafreddo, M., Cecarini, V., Angeletti, M. and Eleuteri, A.M. 2011. Identification of an EGCG oxidation derivative with proteasome modulatory activity. Biochim. 93, 931–940. Bucci, D., Spinaci, M., Mislei, B., Gadani, B., Rizzato, G., Love, C.C., Tamanini, C., Galeati, G. and Mari, G. 2017. Epigallocatechin-3-gallate (EGCG) and green tea polyphenols do not improve stallion semen parameters during cooling at 4°C. Reprod. Domest. Anim. 52, 270–277. Chen, X., Chen, T., Sun, J., Luo, J., Liu, J., Zeng, B., Zhang, Y. and Xi, Q. 2019. Lower methionine/cystine ratio in low-protein diet improves animal reproductive performance by modulating methionine cycle. Food Sci. Nutr. 7, 2866–2874. DeJarnette, J.M., Harstine, B.R., McDonald, K. and Marshall, C.E. 2022. Commercial application of flow cytometry for evaluating bull sperm. Anim. Reprod. Sci. 246, 106838. Drevet, J.R., Hallak, J., Nasr-Esfahani, M.-H. and Aitken, R.J. 2022. Reactive oxygen species and their consequences on the structure and function of mammalian spermatozoa. Antioxid. Redox Signal. 37, 481–500. Du, G.-J., Zhang, Z., Wen, X.-D., Yu, C., Calway, T., Yuan, C.-S. and Wang, C.-Z. 2012. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 4, 1679–1691. Gadani, B., Bucci, D., Spinaci, M., Tamanini, C. and Galeati, G. 2017. Resveratrol and epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology 90, 88–93. Gallagher, M.T., Cupples, G., Ooi, E.H., Kirkman-Brown, J.C. and Smith, D.J. 2019. Rapid sperm capture: high-throughput flagellar waveform analysis. Hum. Reprod. 34, 1173–1185. Hackerova, L., Klusackova, B., Zigo, M., Zelenkova, N., Havlikova, K., Krejcirova, R., Sedmikova, M., Sutovsky, P., Komrskova, K. and Postlerova, P. 2023. Modulatory effect of MG-132 proteasomal inhibition on boar sperm motility during in vitro capacitation. Front. Vet. Sci. 10, 1116891. Hung, S.W., Li, Y., Chen, X., Chu, K.O., Zhao, Y., Liu, Y., Guo, X., Man, G.C.-W. and Wang, C.C. 2022. Green tea epigallocatechin-3-gallate regulates autophagy in male and female reproductive cancer. Front. Pharm. 13, 906746. Kaltsas, A. 2023. Oxidative stress and male infertility: the protective role of antioxidants. Medicina 59, 1769. Kameni, S.L., Meutchieye, F. and Ngoula, F. 2021. Liquid storage of ram semen: associated damages and improvement. Open J. Anim. Sci. 11, 473–500. Kim, H.-S., Quon, M.J. and Kim, J. 2014. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2, 187–195. Li, Z., Wang, H., Yuan, C., Lu, P., Zhou, Y., Lu, W., Zhao, J., Liu, H. and Wang, J. 2022. Epigallocatechin 3-gallate improves the quality of bull semen cryopreservation. Andrologia 54, 1–7. Maylem, E.R.S., Aquino, F.P., Ocampo, L.C., Atabay, E.P. and Atabay, E.C. 2018. The use of computer assisted sperm analyzer in evaluating the sperm kinematics of fresh and frozen-thawed buffalo semen. Philipp. J. Vet. Anim. Sci. 44, 42–50. Mondal, M. and De, S. 2018. Enrichment of (−) epigallocatechin gallate (EGCG) from aqueous extract of green tea leaves by hollow fiber microfiltration: modeling of flux decline and identification of optimum operating conditions. Sep. Purif.Technol. 206, 107–117. Mustofa, I., Susilowati, S., Wurlina, W., Hernawati, T. and Oktanella, Y. 2021. Green tea extract increases the quality and reduced DNA mutation of post-thawed Kacang buck sperm. Heliyon 7, e06372. Narotzki, B., Levy, Y., Aizenbud, D. and Reznick, A.Z. 2013. Green tea and its major polyphenol EGCG increase the activity of oral peroxidases. In Respiratory regulation-the molecular approach. New York, NY: Springer, pp. 99–104. Nordhoff, V. and Wistuba, J. 2023. Physiology of sperm maturation and fertilization. In Andrology: male reproductive health and dysfunction. New York, NY: Springer, pp. 55–75. Petruska, P., Capcarova, M. and Sutovsky, P. 2014. Antioxidant supplementation and purification of semen for improved artificial insemination in livestock species. Turk. J. Vet. Anim. Sci. 38, 643–652. Prastiya, R.A., Suprayogi, T.W., Debora, A.E., Wijayanti, A., Amalia, A., Sulistyowati, D. and Nugroho, A.P. 2023. Green tea extract addition into a Tris-based egg yolk extender improves Bali bull sperm quality. Anim. Biosci. 36, 209–217. Prastowo, S., Widyas, N., Ratriyanto, A., Kusuma, M.S.T., Dharmawan, P., Setiawan, I.A. and Bachtiar, A. 2019. Individual variance component of fresh semen quality in Bali cattle (Bos javanicus) Bull. In IOP Conference Series: Earth and Environmental Science, pp: 012025. Rusdiana, S. and Soeharsono, S. 2017. Farmer group performance bali cattle in luwu district east: the economic analysis. The Int. J. Trop. Vet. Biomed. Res. 2, 18–29. Scholtz, M.M., Maiwashe, A., Neser, F.W.C., Theunissen, A., Olivier, W.J., Mokolobate, M.C. and Hendriks, J. 2013. Livestock breeding for sustainability to mitigate global warming, with the emphasis on developing countries. S. Afr. J. Anim. Sci. 43, 269–281. Setumo, M.A., Choma, S.S., Henkel, R. and Opuwari, C.S. 2022. Green tea (Camellia sinensis) aqueous extract improved human spermatozoa functions in vitro. J. Med. Plants Econ. Dev. 6, a166. Susilowati, S., Sardjito, T., Mustofa, I., Widodo, O.S. and Kurnijasanti, R. 2021. Effect of green tea extract in extender of Simmental bull semen on pregnancy rate of recipients. Anim. Biosci. 34, 198–204. Sutarno, S. and Setyawan, A.D. 2015. Review: Genetic diversity of local and exotic cattle and their crossbreeding impact on the quality of Indonesian cattle. Biodiversitas 16, 327–354. Sutarno, S. and Setyawan, A.D. 2016. The diversity of local cattle in Indonesia and the efforts to develop superior indigenous cattle breeds. Biodiversitas 17, 275–295. Suyadi, S., Herwijanti, E., Septian, W.A., Furqon, A., Nugroho, C.D., Putri, R.F. and Novianti, I. 2020. Some factors affecting the semen production continuity of elite bulls: reviewing data at Singosari National Artificial Insemination Center (SNAIC), Indonesia. In IOP Conference Series: Earth and Environmental Science, pp: 012080. Tariq, M., Khan, M.S., Shah, M.G., Nisha, A.R., Umer, M., Hasan, S.M., Rahman, A. and Rabbani, I. 2015. Exogenous antioxidants inclusion during semen cryopreservation of farm animals. J. Chem. Pharm. Res. 7, 2273–2280. Widyas, N., Widi, T.S.M., Prastowo, S., Sumantri, I., Hayes, B.J. and Burrow, H.M. 2022. Promoting sustainable utilization and genetic improvement of Indonesian local beef cattle breeds: a review. Agric. 12, 1566. Xu, H., Feng, G., Wang, L., Zhang, C., Liu, Y., Zhang, X., Lin, C., Liu, G., Zu, Z. and Zhang, Y. 2021. Fresh and cryopreserved semen, minerals, hormones and health characteristics in response to reciprocal combinations of vitamin D3 and 25-hydroxyvitamin D3 in the mature and prepubertal Holstein bulls’ diet. Anim. Feed Sci. Technol. 281, 115094. Zhong, H.-X., Li, G.-G., Xiong, F., Chen, P.-L., Wan, C.-Y., Yao, Z.-H., Ma, Z.-H., Zeng, Y. and Sun, Q. 2019. [IVOS Ⅱ versus Sperm Class Analyzer in the results of semen analysis]. Zhonghua Nan Ke Xue 25, 124–128. Zigo, M., Manaskova-Postlerova, P., Jonakova, V., Kerns, K. and Sutovsky, P. 2019. Compartmentalization of the proteasome-interacting proteins during sperm capacitation. Sci. Rep. 9, 12583. | ||
| How to Cite this Article |
| Pubmed Style Prastiya RA, Sardjito T, Saputro AL, Budi DS, Maxdhameta SI, Sulistiyawati E, Sulistyowati D, Amaliya A, Sasi SM, Haryuni N. Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Vet. J.. 2024; 14(8): 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 Web Style Prastiya RA, Sardjito T, Saputro AL, Budi DS, Maxdhameta SI, Sulistiyawati E, Sulistyowati D, Amaliya A, Sasi SM, Haryuni N. Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. https://www.openveterinaryjournal.com/?mno=205609 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.33 AMA (American Medical Association) Style Prastiya RA, Sardjito T, Saputro AL, Budi DS, Maxdhameta SI, Sulistiyawati E, Sulistyowati D, Amaliya A, Sasi SM, Haryuni N. Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Vet. J.. 2024; 14(8): 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 Vancouver/ICMJE Style Prastiya RA, Sardjito T, Saputro AL, Budi DS, Maxdhameta SI, Sulistiyawati E, Sulistyowati D, Amaliya A, Sasi SM, Haryuni N. Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 Harvard Style Prastiya, R. A., Sardjito, . T., Saputro, . A. L., Budi, . D. S., Maxdhameta, . S. I., Sulistiyawati, . E., Sulistyowati, . D., Amaliya, . A., Sasi, . S. M. & Haryuni, . N. (2024) Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Vet. J., 14 (8), 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 Turabian Style Prastiya, Ragil Angga, Trilas Sardjito, Amung Logam Saputro, Darmawan Setia Budi, Styuderil Imaniro Maxdhameta, Elis Sulistiyawati, Deny Sulistyowati, Anny Amaliya, Samira Musa Sasi, and Nining Haryuni. 2024. Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Veterinary Journal, 14 (8), 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 Chicago Style Prastiya, Ragil Angga, Trilas Sardjito, Amung Logam Saputro, Darmawan Setia Budi, Styuderil Imaniro Maxdhameta, Elis Sulistiyawati, Deny Sulistyowati, Anny Amaliya, Samira Musa Sasi, and Nining Haryuni. "Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent." Open Veterinary Journal 14 (2024), 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 MLA (The Modern Language Association) Style Prastiya, Ragil Angga, Trilas Sardjito, Amung Logam Saputro, Darmawan Setia Budi, Styuderil Imaniro Maxdhameta, Elis Sulistiyawati, Deny Sulistyowati, Anny Amaliya, Samira Musa Sasi, and Nining Haryuni. "Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent." Open Veterinary Journal 14.8 (2024), 2040-2048. Print. doi:10.5455/OVJ.2024.v14.i8.33 APA (American Psychological Association) Style Prastiya, R. A., Sardjito, . T., Saputro, . A. L., Budi, . D. S., Maxdhameta, . S. I., Sulistiyawati, . E., Sulistyowati, . D., Amaliya, . A., Sasi, . S. M. & Haryuni, . N. (2024) Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Veterinary Journal, 14 (8), 2040-2048. doi:10.5455/OVJ.2024.v14.i8.33 |