| Research Article | ||
Open Vet. J.. 2024; 14(8): 2049-2056 Open Veterinary Journal, (2024), Vol. 14(8): 2049–2056 Research Article Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to PlumbumMuhammad Hamdan1, Widjiati Widjiati2*, Priya Nugraha1 and Jovian Philip Swatan11Department of Neurology, Faculty of Medicine, Universitas Airlangga—Dr. Soetomo General Academic Hospital, Surabaya, Indonesia 2Department of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Widjiati Widjiati. Department of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: widjiati [at] fkh.unair.ac.id Submitted: 21/06/2024 Accepted: 18/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
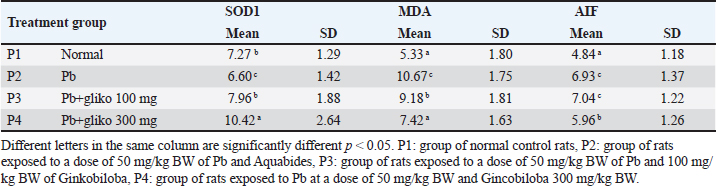
ABSTRACTBackground: Lead (Plumbum/Pb) has been identified as a potential cause of Parkinson’s disease as well as possibly contributing to disease progression. Ginkgo biloba extract has been widely used to prevent and treat stroke which prevents brain cell apoptosis and neuroinflammation. This has been shown to be beneficial in cognitive recovery in stroke incidents. Aim: This study aimed to determine the effect of G. biloba on the expression of superoxide dismutase (SOD), malondialdehyde (MDA), and apoptosis-inducing factor (AIF) in the brain cells of rats (Rattus novergicus) exposed to Plumbum. Methods: The experimental animals used were 36 male white rats divided into 4 groups with different treatments of Plumbum and G. biloba at varying doses for 42 days, after which the brains were collected for examination of SOD, MDA, and AIF expressions using immunohistochemical methods and analyzed using analysis of variance and Duncan’s test. Results: Plumbum administration caused a significant decrease in SOD expression and an increase in MDA and AIF expressions (p < 0.05). Ginkgo biloba administration significantly increased SOD expression and decreased MDA and AIF expressions (p < 0.05), with optimal increases in SOD, decreases in MDA, and modulation of AIF observed in the group exposed to 50 mg/kg BW Pb and 300 mg/kg BW G. biloba. Conclusion: Preventive administration of G biloba increased SOD expression, and reduced MDA and AIF expressions in Pb-exposed rats, with an optimal dose of 300 mg/kg BW, suggesting its potential as an affordable drug to prevent brain cell death-related diseases. Keywords: Affordable Medicines, Brain cells, Ginkgo biloba, Plumbum. IntroductionLead (Plumbum) has significant neurotoxic effects on brain cells which induce various pathological changes at the cellular and molecular levels. Chronic exposure to lead can result in impaired synaptogenesis which inhibits the formation and function of synapses vital for interneuronal communication; therefore, it is likely to cause cognitive deficits and impaired learning (Li et al., 2022). Plumbum moreover induces oxidative stress by increasing the production of reactive oxygen species (ROS) and decreasing cellular antioxidant capacity, subsequently causing critical oxidative damage to components in cellular cells (Del Rio et al., 2005). Furthermore, Plumbum disrupts intracellular calcium homeostasis which triggers apoptotic cascades and mitochondrial dysfunction (Kuang et al., 2018). Plumbum neurotoxicity is also manifested in impaired neuroplasticity, which is essential for learning and memory processes (Srivastav et al., 2017). In addition, Plumbum can interfere with neurotransmission systems, especially affecting the dopaminergic and glutamatergic systems, as well as triggering neuronal inflammatory responses that contribute to long-term neuronal damage (Biernacka et al., 2023). The accumulation of these effects can result in progressive neurodegeneration and significant cognitive dysfunction. The entry of Plumbum into the body will affect nearly every organ and system in the human body, including the digestive, cardiovascular, renal, and reproductive systems (Sajini et al., 2024). The Plumbum mechanism is clearly visible in the central nervous system which can be seen from a decrease in SOD activity which causes increased oxidative stress because Plumbum binds to the sulfhydryl group on the SOD enzyme (Gąssowska et al., 2016; Fan et al., 2020), increases MDA production as a marker of high lipid peroxidation and causes mitochondrial dysfunction because Plumbum induces free radicals by attacking the cell lipid membrane (Baranowska-Bosiacka et al., 2013; Han et al., 2021), which induces neuro-inflammation and disruption of calcium homeostasis, and it involves AIF-1 released from the cytosol and nucleus that induces caspase-independent pathways to mediate cell death (apoptosis) (Zhao et al., 2014; Bjørklund et al., 2018). Ginkgo biloba is a medicinal plant known in Asian cultures for thousands of years and is believed to originate from the Permian Era, approximately 250 million years ago (Jin et al., 2024). G. biloba has emerged as a promising herbal medicine for Parkinson’s patients. The active ingredients in G. biloba that could prevent oxidative stress and apoptosis in the brain primarily include quercetin, a flavonoid with strong antioxidant properties that can neutralize free radicals and protect cells from oxidative damage. Another flavonoid found in G. biloba, kaempferol, also possesses antioxidant and anti-apoptotic properties. Ginkgolides, terpenoid compounds, are well-known for their ability to inhibit platelet-activating factors and provide protective effects to nerve cells. Another terpenoid, bilobalides, also plays a role in protecting the brain from oxidative damage and apoptosis by enhancing the expression of antioxidant enzymes and reducing cell damage (Chan et al., 2007; Abdel-Zaher et al., 2009). Ginkgo biloba extract has been shown to have neuroprotective effects through various mechanisms: it increases the activity of SOD, which helps neutralize free radicals and reduces oxidative stress (Mohebbi et al., 2023), it decreases levels of MDA, a marker of lipid peroxidation which indicates a reduction in oxidative damage to nerve cells (Lorca et al., 2023), and it modulates the activity of AIF, which plays a role in regulating programmed cell death; therefore, it protects dopaminergic neurons from degeneration (Olufunmilayo et al., 2023). This research aimed to evaluate the impact of G. biloba on the expression levels of superoxide dismutase (SOD), malondialdehyde (MDA), and apoptosis-inducing factor (AIF) in the brain cells of rats (Rattus norvegicus) that were exposed to lead (Plumbum). It is hoped that the result of the research can become an affordable medicine to prevent diseases caused by brain cell death. Materials and MethodsMaterialsThe research materials used were rat brain, SOD antibody (polyclonal antibody with catalog number MBS20044173, MDA antibody (anti-malondialdehyde antibody with catalog number 243066, mouse monoclonal antibody, Abcam), and AIF antibody (AIF Polyclonal Antibody with catalog number –bs 0037R, Bioss). Lead(II) acetate basic (Merck KGaA, Darmstadt, Germany), Ginkgo biloba (Tebokan Forte-Willmar-schwabe-Straße 4, 76227 Karlsruhe, Germany). MethodThis research is a laboratory experiment using a Completely Randomized Design, with the assumption that all treatments are the same from sampling to processing as well as laboratory conditions. The stages of this research consist of acclimatization of mice for 1 week, administration of Plumbum solution, and G. biloba (tablet preparation dissolved in physiological NaCl 0.9%) every morning at 09.00 AM, once per day orally for 42 days. Collection of brain samples on day 43 and examination on SOD, MDA, and AIF expressions using the immnohistochemical method. The study utilized 36 male white rats (Rattus novergicus), 3-month-old mice weighing 160–180 g, comes from Pusat Veterinaria Farma Surabaya, Indonesia divided into 4 groups: group P1 as the normal control, group P2 exposed to Plumbum at 50 mg/kg BW and treated with Aquabides, group P3 exposed to Plumbum at 50 mg/kg BW and administered G. biloba 100 mg/kg BW, and group P4 exposed to Plumbum at 50 mg/kg BW and administered Ginkgo biloba 300 mg/kg BW (Huang et al., 2007). Ginkgo biloba and Plumbum were administered orally for 42 days, with preventive G. biloba administration preceding Plumbum exposure. On day 43, brains were collected for examination of SOD, MDA, and AIF expressions using the immunohistochemical (IHC) method under an Olympus® CX-41 microscope at 400x magnification. Immnohistochemical stainingFixed brain samples underwent IHC staining. After treatment with 3% hydrogen peroxide and washing with PBS, the sections were incubated with 0.025% trypsin at 37°C, washed again, and then blocked with Ultra V block. Samples were incubated with primary antibodies against SOD/MDA/AIF for 1 hour at room temperature, followed by incubation with biotinylated link and streptavidin. The color was developed using 3,3-Diaminobenzidine tetrahydrochloride chromogen, and sections were counterstained with methylene green (Kim et al., 2016). Observations were made under an Olympus® CX-41 microscope at 400x magnification. Expression of SOD/MDA/AIF was appraised using the modified semiquantitative IRS scale according to Remmele and Stegner, 1987. The method takes into account both the proportion of positively stained cells and the intensity of the reaction color, while its final result represents the product of the parameters, with values ranging from 0 to 12 points (no reaction=0 points (–); weak reaction=1-2 points (+), moderate reaction=3–4 points (++), intense reaction=6–12 points (+++). Evaluated quantitatively by stimation of the percentage of positive cells (0%–5%=no reaction (–), 6%–25%=weak reaction (+), 26%–50%=moderate reaction (++), above 50%=intense reaction (+++). Statistical analysisThe data on SOD, MDA, and AIF expression were analyzed using SPSS software. One-way analysis of variance was employed to assess differences between groups, with a p-value ≤ 0.05 considered statistically significant. In cases where differences were detected, post-hoc analysis was performed using Duncan’s multiple range test. Ethical approvalThis research has received ethical approval from the Faculty of Veterinary Medicine, Universitas Airlangga with number: 3.KE.123.07.2018. ResultsThe results of examining the expression of SOD, MDA, and AIF between the control and treatment groups after statistical analysis was carried out can be seen in Table 1. The results of the study showed that the SOD expression of P1 was higher compared to that of the positive control (P2) (p < 0.05). MDA expression of the normal control group (P1) was lower than that of the positive control group (P2), likewise, AIF expression of the normal control group (P1) was lower than that of the positive control group (P2) (p < 0.05). The SOD expression of rats exposed to Plumbum was lower (6.06 ± 1.42) than that of normal rats (7.27 ± 1.29). The MDA expression of rats exposed to Plumbum was higher (10.67 ± 1.75) when it was compared with normal rats (Table 1). AIF expression of rats exposed Plumbum was higher (6.93 ± 1.37) when compared with that of normal rats (4.84 ± 1.18). The positive control group was not significantly different from the P3 group, that is rats exposed to Plumbum and administered with 100 mg/kg BW G. biloba (p > 0.05); however, there was a significant difference in group P4, that is rats exposed to Plumbum and administered with G. biloba 300 mg/kg BW (p < 0.05) (Table 1). The results of statistical analysis from a group of rats exposed to Plumbum and G. biloba 100 mg/kg BW showed that there was an increase in SOD expression compared to normal rats, a decrease in MDA expression compared to a positive control treatment group, that is rats exposed to Plumbum and AIF expression was not significantly different from treatment group administered with only Plumbum. SOD expression of rats exposed to Plumbum and G. biloba 300 mg/kg BW increased compared to other treatment groups and there was a decrease in MDA and AIF expressions compared to positive control treatment exposed to only Plumbum (p < 0.05) (Table 1). The Remmele method provides a score value based on staining intensity and the percentage of positive cells. IHC staining shows the expression of SOD, MDA, and AIF in the brain cells of rats with Plumbum and G. biloba 100 mg/Kg BW and 300 mg/Kg BW. Yellow arrows indicate the presence of SOD, MDA, and AIF expressions in the rat brain cells shown by brown chromogen (Figs. 1–3). DiscussionExposure to heavy metals such as Plumbum is a risk factor for neurotransmitter disorders in brain cells. These heavy metals can accumulate in the substantia nigra and cause oxidative stress, therefore it inhibits neurotransmitters and change basal ganglion function. This oxidative stress will then induce mitochondrial function, disrupt DNA synthesis, and cause damage to lipids and membrane proteins (Olufunmilayo et al., 2023). The entry of heavy metals into the body can deactivate various antioxidant enzymes by binding to the activity of thiol groups and thus contributing to damage of the pro-oxidant environment. In addition, this study shows that increasing levels of Plumbum are positively correlated with glutathione disulfide, glutathione peroxidase (GPx), and ROS then negatively correlated with glutathione reductase (GR), SOD, and glutathione S-transferase. Thus, it is certain that high doses of Plumbum will produce an oxidant environment and it takes time for the damage caused by this heavy metal and it depends on the dose exposed to the body (Fan et al., 2020). Under conditions of neurotoxicity, intracellular Plumbum affects mitochondria. Normally the mitochondrial permeability transition pore (MPTP) allows free passage of substances with a molecular weight of less than 1.5 kDa. However, under pathological conditions, the accumulation of Plumbum leads to the free migration of substances with a molecular weight of more than 1.5 kDa without selection. Mitochondrial membrane potential (MMP), the main prerequisite for MPTP opening, will decrease significantly with different Plumbum concentrations. Opening of the MPTP causes swelling and makes mitochondria to release Cyt-c, AIF, and DNA endonuclease from the mitochondria to the cytoplasm and induce cell apoptosis (Han et al., 2021). Table 1. Mean and standard deviation of SOD, MDA and AIF expression from rat brain exposed to Pb dan Glinkobiloba for 42 days.
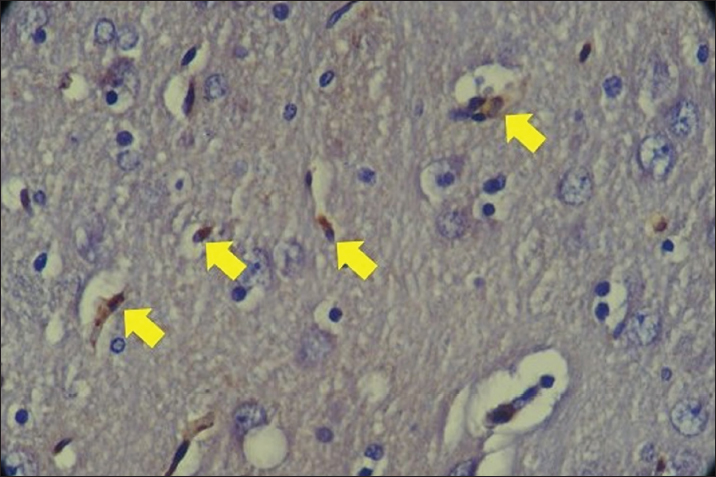
Fig. 1. Staining of SOD1 expression in rat brain cells with Plumbum and Ginkgo biloba 100 mg. The yellow arrow indicates the presence of SOD one expression in rat brain cells characterized by brown chromogen. IHC. Original magnification at 400x The results of the study showed that the SOD expression of the normal control group was higher compared to that of the positive control. The lowest levels were found in the positive control group with Plumbum exposure. The highest expression of SOD was shown by the group exposed to Plumbum + G. biloba 300 mg/kgBW (10.42) and was significantly different from the other groups (p < 0.05). This shows that exposure to Plumbum can damage the bonds of thiol groups and deactivate the antioxidant enzyme SOD which leads to an oxidant environment (Fan et al., 2020). At a low dose (100 mg/kg BW), G. biloba provides sufficient bioactive compounds such as flavonoids and terpenoids, which possess antioxidant properties to neutralize free radicals generated by lead exposure. This helps in the recovery or maintenance of SOD levels comparable to the control group. At a high dose (300 mg/kg BW), the amount of available bioactive compounds is significantly higher, providing stronger antioxidant protection. This allows for a significant increase in SOD expression, surpassing the control group. SOD is a crucial enzyme that catalyzes the dismutation of superoxide anions into oxygen and hydrogen peroxide, so more of this enzyme means a greater capacity to neutralize oxidative stress induced by lead (Chan et al., 2007). Ginkgo biloba contains ginkgolides and bilobalide that protect against ischemia-reperfusion injury via Nrf2 activation. Ginkgolide B attenuates oxidative stress by inhibiting ROS and inducing antioxidants like HO-1, NQO-1, and SOD1 through Akt/Nrf2 signaling (Li et al., 2022). Previous studies showed G. biloba increased SOD activity, reduced apoptosis (Zhao et al., 2014), decreased Keap1, and induced Nrf2 nuclear translocation upregulating SOD1 and GPx1 (Zhao et al., 2014; Fan et al., 2020). Thus, G. biloba mitigated Plumbum -induced oxidative stress by restoring SOD. Regarding MDA, Plumbum exposure elevated MDA levels compared to controls, indicating increased oxidative stress. MDA, a lipid peroxidation marker, can modify amino acids and form adducts with nucleic acids and lipids under Plumbum-induced stress (Del Rio et al., 2005). The optimal G. biloba dose (100 mg/kg BW) co-administered with Plumbum significantly reduced MDA levels compared to Plumbum alone (p < 0.05). Ginkgo biloba’s flavonoids (24%) and terpenoids (6%) with low molecular weights enable blood–brain barrier penetration. These antioxidant compounds eliminate free radicals, mitigating Plumbum-induced oxidative stress and lipid peroxidation evidenced by reduced MDA.
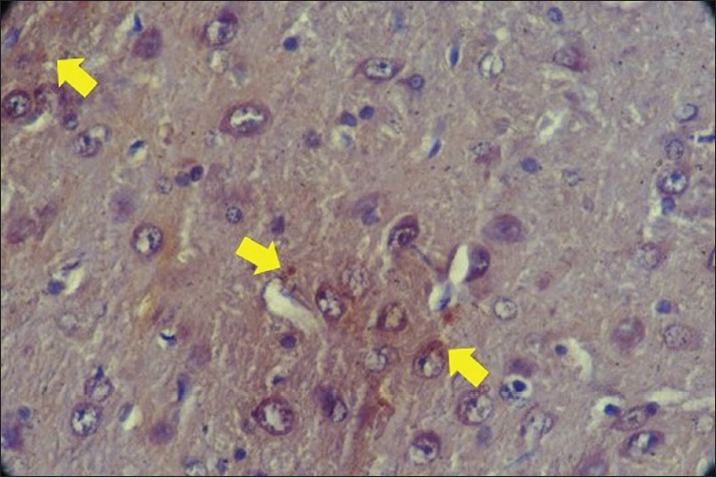
Fig. 2. MDA expression with Plumbum and Ginkgo biloba 300 mg in rat brain cells. The yellow arrow indicates the presence of MDA expression in rat brain cells characterized by brown chromogen. IHC. Original magnification at 400x. Kuang et al. (2018) demonstrated that G. biloba administration increased SOD and glutathione peroxidase activity, inhibited MDA expression, and reduced oxidative damage in A53T α-synuclein transgenic mice. The combination of levodopa with optimized doses of G. biloba may provide better therapeutic efficacy than either agent alone, as G. biloba stabilizes redox status, rejuvenates mitochondrial function, and improves locomotor activity in Parkinson’s disease (Srivastav et al., 2017). Regarding AIF, this study revealed the highest AIF expression in the group exposed to Plumbum + G. biloba 100 mg/kg BW, while the lowest levels were observed in the normal control group. As previously discussed, Plumbum induces MPTP opening, mitochondrial swelling, and the release of cytochrome-c, AIF, and DNA endonucleases, leading to cell apoptosis (Han et al., 2021). Although Plumbum exposure resulted in high AIF expression (6.93), the 100 mg/kg BW G. biloba dose was suboptimal, unable to improve cell apoptosis, exhibiting the highest AIF expression (7.04). Conversely, the 300 mg/kg BW G. biloba dose inhibited cell apoptosis more effectively than the Plumbum-only positive control and the Pb + 100 mg/kg BW G. biloba group. AIF was a mitochondrial protein involved in cell death through the apoptosis pathway. Although a high dose of G. biloba (300 mg/kg BW) is sufficient to increase SOD expression and decrease MDA, it may not fully restore AIF expression to control levels. This could be due to the fact that AIF is involved in a complex cell death pathway that does not solely depend on the reduction of free radicals. The decrease in AIF may require a longer time or additional interventions to reach levels equivalent to the control group. Additionally, although oxidative stress is reduced, there may be other mechanisms affecting AIF expression that are not fully addressed by the antioxidant protection from G. biloba (Abdel-Zaher et al., 2009). This is related to the Ginkgolide and bilobalide compounds in G. biloba (Biernacka et al., 2023). The terpenoid components are still not well known regarding their biosynthesis. One of the enzymes most often involved in ginkgolide biosynthesis is levopimaradiene synthase (Gąssowska et al., 2016). Ginkgolide and bilobalide have the same three-step biosynthetic pathway. In the final step is cyclization and oxidation catalyzed by terpenoid synthase and cytochrome P450 (CYP-450)-dependent monooxygenase. It is known that the complex of Cyt-c (cytochrome P450 (CYP-450), AIF and DNA endonuclease originates from the mitochondria to the cytoplasm and induces cell apoptosis. (Han et al., 2021; Biernacka et al., 2023).
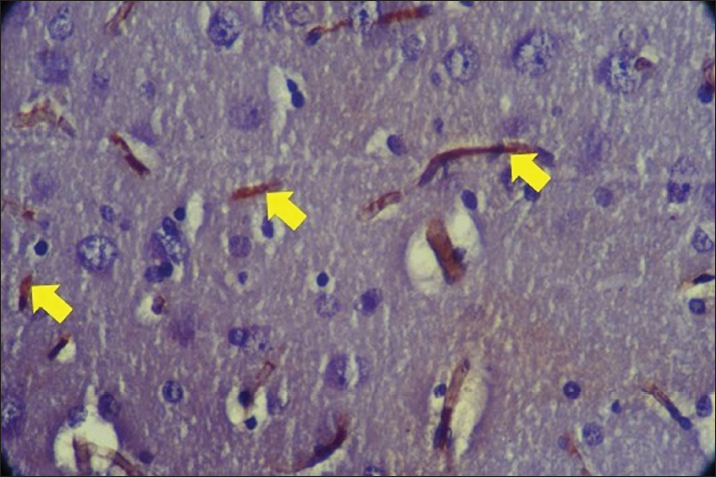
Fig. 3. Expression of AIF with Plumbum and Ginkgo biloba 300 mg. The yellow arrow indicates the presence of AIF expression in rat brain cells characterized by brown chromogen. IHC. Original magnification at 400x. The results of this study were relevant in showing that G. biloba could reduce oxidative stress induced by lead through the increase in SOD expression and decrease in MDA expression. Oxidative stress is a primary mechanism of cell damage caused by heavy metals such as lead. By decreasing AIF expression, this study demonstrates that G. biloba could help reduce cell apoptosis induced by oxidative stress. This research was relevant in developing interventions to protect cells from damage caused by toxic environmental exposures. The study also identifies the optimal dose of G. biloba that is effective (300 mg/kg BW) in increasing SOD expression and decreasing MDA and AIF expression, which is highly relevant for determining a safe and effective dose in clinical applications or preventive therapy. Previous research has shown that G. biloba has antioxidant and neuroprotective properties that could reduce oxidative damage and apoptosis in animal models exposed to various toxins. Chan et al. (2007) demonstrated that G. biloba can reduce oxidative damage in the brains of rats induced by oxidative stress. Several studies have explored the use of G. biloba in preventing cellular damage due to various toxic agents, showing findings consistent with this research. The study by Smith et al. (2005) demonstrated that G. biloba can enhance antioxidant activity in cells and tissues. This research adds preclinical evidence that G. biloba can be beneficial in reducing damage induced by heavy metals such as lead, as reported in several studies. Sastre et al. (1998) reported that G. biloba can reduce lipid peroxidation and enhance antioxidant status in rats exposed to toxic agents. ConclusionIt was concluded that preventive administration of G. biloba could increase SOD expression, and reduce MDA and AIF expressions in rats exposed to Plumbum. Administration of Ginkgo biloba at a dose of 300 mg/kg BW as a neuroprotective agent was able to increase SOD expression and decrease MDA and AIF expressions. AcknowledgmentsThis research is part of a dissertation and was funded through a scholarship from the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia. The author acknowledges the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia and the Faculty of Medicine, Universitas Airlangga, for their financial support and research facilities. Conflict of interestAll authors declare that there is no conflict of interest. FundingThis research is part of a dissertation and funded through a scholarship from the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia. Data availabilityThe information backing the discoveries of this research is not openly accessible due to sensitivity concerns but can be obtained from the corresponding author upon reasonable inquiry. Authors’ contributionMH, WW, PN, and JPS designed this research. MH, WW, PN, and JPS conducted a survey and took samples at the samples field. All authors examined samples in the research laboratory. All authors compiled, read, revised, and approved the final manuscript. ReferencesAbdel-Zaher, A. O., Abdel-Rahman, M. S., Hafez, M. M., and Omran, G. A. 2009. Protective effect of Ginkgo biloba extract against lead-induced oxidative stress and apoptosis in rat brain. Neurotoxicology 30(4), 677–684. Baranowska-Bosiacka, I., Gutowska, I., Rybicka, M., Nowacki, P., and Chlubek, D. 2013. Neurotoxicity of lead. Hypothetical molecular mechanisms of synaptic function disorders. Neurol. Neurochir. Pol. 46(6), 569–78. Biernacka, P., Adamska, I., and Felisiak, K. 2023. The Potential of Ginkgo biloba as a source of biologically active compounds-a review of the recent literature and patents. Molecules 28(10), 3993. Bjørklund, G., Tinkov A. A., Hosnedlová, B., Kizek R., Ajsuvakova, O. P., Chirumbolo, S., Skalnaya, M. G, Peana, M., Dadar M., El-Ansary A., Qasem H., Adams J. B., Aaseth J., and Skalny A. V. 2018. The role of glutathione redox imbalance in autism spectrum disorder: a review. Free Radic. Biol. Med. 121, 185–193. Chan, A., Butterworth, R. F., and Boucher, D. 2007. Neuroprotective effects of Ginkgo biloba extract EGb 761 in a model of neurodegeneration induced by thiamine deficiency. Pharmacol. Res. 56(4), 451–458. Del Rio, D., Stewart, A. J., and Pellegrini, N. 2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15(4), 316–328. Fan, Y., Zhao, X., Yu, J., Xie, J., Li, C, Liu, D., Tang, C., and Wang, C. 2020. Lead-induced oxidative damage in rats/mice: a meta-analysis. J. Trace Elem. Med. Biol. 58, 126443. Gąssowska M., Baranowska-Bosiacka I., Moczydłowska J., Tarnowski M., Pilutin A., Gutowska I, Strużyńska L., Chlubek D., and Adamczyk A. 2016. Perinatal exposure to lead (Plumbum) promotes Tau phosphorylation in the rat brain in a GSK-3β and CDK5 dependent manner: Relevance to neurological disorders. Toxicology 347-349, 17–28. Han, Q., Zhang, W., Guo, J., Zhu, Q., Chen, H., Xia, Y., and Zhu, G. 2021. Mitochondrion: a sensitive target for Pb exposure. J. Toxicol. Sci. 46(8), 345–358. Huang, X., Whitworth, C. A., and Rybak, L. P. 2007. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otol. Neurotol. 28(6), 828–833. Jin, X., Dong, W., Chang, K., and Yan Y. 2024. Research on the signaling pathways related to the intervention of traditional Chinese medicine in Parkinson’s disease:a literature review. J. Ethnopharmacol. 326, 117850. Kim, S.W., Roh, J. and Park, C.S. 2016. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 50(6), 411-418. Kuang, S., Yang, L., Rao, Z., Zhong, Z., Li, J., Zhong, H., Dai, L., and Tang, X. 2018. Effects of Ginkgo biloba extract on A53T α-synuclein transgenic mouse models of Parkinson’s disease. Can. J. Neurol. Sci. 45, 182–187. Li, Y., Zhu, X., Wang, K., Zhu, L., Murray, M., and Zhou, F. 2022. The potential of Ginkgo biloba in the treatment of human diseases and the relationship to Nrf2-mediated antioxidant protection. J. Pharm. Pharmaco. 74(12), 1689–1699. Lorca, C., Mulet M., Arévalo-Caro, C., Sanchez, M. Á., Perez, A., and Perrino, M. 2023. Plant-derived nootropics and human cognition: a systematic review. Crit. Rev. Food Sci. Nutr. 63(22), 5521–5545. Mohebbi, N., Kalantar M. A., Mousavi, M., Taghizadeh-Ghehi, M., Rezaie, M., and Hooshyari, Z. 2023. Ginkgo biloba efficacy in the treatment of drug-induced Parkinsonism: a randomized clinical trial. Iran J. Pharm. Res. 22(1), 1–8. Olufunmilayo, E. O., Gerke-Duncan, M. B, and Holsinger, R. M. D. 2023. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 12(2), 517. Remmele, W., and Stegner, H. E. 1987. Proposal for a uniform definition of an immunoreactive score (IRS) for the immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologie 8, 138–140. Sajini, D. V, Krishnamurthy, P. T, Chakkittukandiyil, A., and Mudavath, R. N. 2024. Orientin Modulates Nrf2-ARE, PI3K/Akt, JNK-ERK1/2, and TLR4/NF-kB pathways to produce neuroprotective benefits in Parkinson’s Disease. Neurochem. Res. 49(6), 1577–1587. Sastre, J., Millán, A., and García de la Asunción, J. 1998. A Ginkgo biloba extract (EGb 761) prevents mitochondrial aging by protecting against oxidative stress. Free. Radic. Biol. Med. 24(5), 769–778. Srivastav, S., Fatima, M., and Mondal, A. C. 2017. Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed. Pharmacother. 92, 856–863. Smith, J. V., Burdick, A. J., Golik, P., Khan, I., Wallace, D., and Henck, J. W. 2005. Anti-apoptotic properties of Ginkgo biloba extract EGb 761 in differentiated PC12 cells. Phytother. Res. 19(8), 666–669. Zhao, M., Wang, X. X., and Wan, W. H. 2014. Effects of the Ginkgo biloba extract on the superoxide dismutase activity and apoptosis of endothelial progenitor cells from diabetic peripheral blood. Genet. Mol. Res. 13(1), 220–227. | ||
| How to Cite this Article |
| Pubmed Style Hamdan M, Widjiati W, Nugraha P, Swatan JP. Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. Open Vet. J.. 2024; 14(8): 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 Web Style Hamdan M, Widjiati W, Nugraha P, Swatan JP. Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. https://www.openveterinaryjournal.com/?mno=206004 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.34 AMA (American Medical Association) Style Hamdan M, Widjiati W, Nugraha P, Swatan JP. Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. Open Vet. J.. 2024; 14(8): 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 Vancouver/ICMJE Style Hamdan M, Widjiati W, Nugraha P, Swatan JP. Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 Harvard Style Hamdan, M., Widjiati, . W., Nugraha, . P. & Swatan, . J. P. (2024) Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. Open Vet. J., 14 (8), 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 Turabian Style Hamdan, Muhammad, Widjiati Widjiati, Priya Nugraha, and Jovian Philip Swatan. 2024. Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. Open Veterinary Journal, 14 (8), 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 Chicago Style Hamdan, Muhammad, Widjiati Widjiati, Priya Nugraha, and Jovian Philip Swatan. "Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum." Open Veterinary Journal 14 (2024), 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 MLA (The Modern Language Association) Style Hamdan, Muhammad, Widjiati Widjiati, Priya Nugraha, and Jovian Philip Swatan. "Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum." Open Veterinary Journal 14.8 (2024), 2049-2056. Print. doi:10.5455/OVJ.2024.v14.i8.34 APA (American Psychological Association) Style Hamdan, M., Widjiati, . W., Nugraha, . P. & Swatan, . J. P. (2024) Effect of Ginkgo biloba administration to apoptosis in rat (Rattus novergicus) brain cells exposed to Plumbum. Open Veterinary Journal, 14 (8), 2049-2056. doi:10.5455/OVJ.2024.v14.i8.34 |