| Research Article | ||
Open Vet. J.. 2024; 14(10): 2651-2661 Open Veterinary Journal, (2024), Vol. 14(10): 2651-2661 Research Article Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male ratsSarah Hussein Abdulwahid Al-hafidh and Ammar Ahmed Abdulwahid*Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Ammar Ahmed Abdulwahid. Department of Physiology, Biochemistry, and Pharmacology. College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: ammar.a [at] covm.uobaghdad.edu.iq Submitted: 25/07/2024 Accepted: 26/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
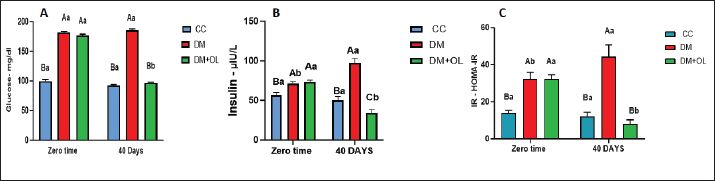
AbstractBackground: Diabetes mellitus (DM) is a common metabolic disorder with well-known serious secondary complications. It is also associated with central nervous system damage. This damage is characterized by impairments in brain functions, with neurochemical and structural abnormalities. Aim: The study was conducted to clarify the neuroprotective effects of olive leaf supplements on the brain and brain histological structure of diabetic adult rats. Methods: Thirty adult male rats were allocated into three groups, the first group (CC), received an oral supplement containing olive leaves supplements (OLS) and served as a control; in the other group , DM was induced in these animals and left for 40 days; and the third group was DM+OL, which induced DM, then treated with oral OLS for 40 days. The investigation included serum glycemic index measurements, in addition to the level of malondialdehyde (MDA), glutathione (GSH), dopamine (DOP), acetylcholinesterase (AchE) in brain tissue, and histopathology of brain and pancreas. Results: We demonstrated a significant increase in glycemic index measurements in diabetic groups (DM, DM+OL) at the beginning of a trial; however, the same parameters were significantly decreased after treatment with OLS in only the DM+OL group after 40 days. The study also showed differences in the levels of MDA, and GSH, in the diabetic groups, which returned to normal levels after being treated with OLS. Moreover, AchE and DOP exhibited a significant decline in diabetic rats. However, OLS induced a considerable rise in these neurotransmitters after treatment with it in the DM+OL group. The histopathological section of the pancreas and brain showed histopathological changes in DM groups; whereas, the tissue was shielded from chemical damage from DM by the OLS treatment in DM+OL animals. Conclusion: Overall, diabetes impairs glucose hemostasis by affecting glucose concentration, insulin level, and insulin resistance. However, olive leaf supplements restored the glucose hemostasis close to normal in diabetic rats. Furthermore, diabetes affects neurotransmitters by increasing the level of oxidative stress in brain tissue, and brain cell damage. Nevertheless, olive leaf supplements can ameliorate DM harmful effects by retrieving the normal oxidative environment in the brain. Keywords: Diabetes, Olive leaves, Brain, Dopamine, Alloxan. IntroductionA form of metabolic syndrome characterized by persistently high blood sugar levels due to dysregulated glucose metabolism brought on by abnormalities in the activity of the pancreatic β-cell is diabetes mellitus (DM). Type 1 DM and type 2 diabetes (T2DM) are caused by insulin insufficiency or insulin resistance (IR), respectively. They are pathologically caused by abnormalities in glucose metabolism resulting from deficiencies in pancreatic β-cell activity (Abbas and Abbas, 2018; Boye et al., 2021). DM causes substantial health and financial consequences globally, making it a major public health concern (Zhao et al., 2024). Ten percent of adults globally have T2DM, which is the most common kind of the disease (Sun et al., 2022). DM is pervasive microvascular and macrovascular pathology, which accelerates atherosclerotic disease and produces retinopathy, nephropathy, peripheral neuropathy, and nephritis. It was formerly believed that the avascular cornea was impervious to the vascular consequences of DM; yet, a substantial body of research now indicates that DM causes structural, functional, and biochemical damage to the cornea’s endothelial cells (Goldstein et al., 2020; Abdulghafoor et al., 2021). It is another ailment marked by elevated blood glucose, ineffective insulin, and other metabolism-related issues. It also has a connection to hyperglycemia, which causes oxidative stress. DM is a prevalent disease in cats, with estimates ranging from 1:100 to 1:500 in first-opinion practices. Additionally, there is evidence that the prevalence of feline DM has been rising, which may be partially attributed to the growth in the frequency of obesity (McCann et al., 2007; Sallander et al., 2012). According to studies, cats with DM typically live between 13 and 29 months. Better-stabilized cats experience higher survival periods, and many cats with DM pass away from illnesses other than DM. Cats with DM have a fair outlook as long as the illness is well-controlled (Sparkes et al., 2015). Most cats with DM are similar to people with Type 2 DM, which is caused by a combination of IR, reduced β-cell activity, and other hereditary and environmental variables. In cats, hypersomatotropism (acromegaly) is thought to account for 25% of instances of diabetes in the UK; hyperadrenocorticism, pancreatic illness, and the use of diabetogenic medications are other known reasons (O’neill et al., 2016). Bădescu and College stated a depressive mood illness combines several symptoms that affect a person’s ability to function, research suggests that the prevalence of depression is higher in people with prediabetes, in patients with undiagnosed diabetes, and in patients with diabetes who have already received a diagnosis than in people with normal glucose metabolism. In 40% of patients with type 1 or T2DM, anxiety increases the likelihood of developing T2DM by 60% and worsens prognosis, treatment non-compliance, quality of life, and mortality (Bădescu et al., 2016). Moreover, DM is connected with decrements in cognitive function and alterations in brain anatomy. Neuropsychological testing has demonstrated mild-to-moderate decreases in cognitive performance in people with both type 1 and T2DM when compared to non-diabetic controls. A 50% higher incidence of dementia has also been linked to T2DM. It is unknown at this time if persons with type 1 diabetes experience this kind of relationship (Moheet et al., 2015). Although the brain needs reactive oxygen species (ROS) for signaling, an excessive accumulation of ROS can lead to oxidative damage to cells (Cobley et al., 2018). Most vulnerable regions of the brain belong to regions with high cognitive activity, like the hippocampus (Wang and Michaelis, 2010). The hippocampus is extremely susceptible due to its complex structure, and any disruption or atrophy results in a decline in cognition (Njan et al., 2020). Because oxidative stress leads to a decrease in antioxidant defenses and an increase in free radical formation, it is a significant factor in the development of diabetes complications, including learning and memory problems. These free radicals cause DNA damage by protein oxidation and accelerated cell death in several brain regions, including the hippocampus (Bhupatiraju et al., 2023). The main driver of oxidative stress is an excess production of free radicals brought on by a blockage in the normal operation of the antioxidant response system, which reduces the activity of antioxidant enzymes (Salim, 2017). Furthermore, an azo molecule called AAPH (2,20-azobis (2 amidinopropane) dihydrochloride) can be thermally broken down in vitro to yield peroxyl radicals, which are frequently the result of chain reactions in oxidative processes in biological systems (Lins et al., 2018). The oxidative damage caused by hyperglycemia is what causes these changes (Pham-Huy et al., 2008). Olive leaves are abundant sources of a wide range of bioactive substances, such as verbascoside, luteolin, and luteolin-7-O-glucoside, phenolic alcohols like hydroxytyrosol, and secoiridoids like oleuropein (Romero-Márquez et al., 2023). Herbal remedies are widely used in many countries, with approximately 80% of the global population still relying on conventional medicine, and medicinal plants have undergone extensive research (Khalil and Shakir, 2015). The olive tree is one of the species with the highest antioxidant activity among naturally occurring antioxidants, notwithstanding its oil, fruits, and leaves (Servili et al., 1999; Obaid et al., 2015). The pharmacological effects are thought to be caused by oleuropein. Oleuropein has been shown to have biological activity mostly related to antioxidant and anti-inflammatory properties and the potential to treat inflammatory and oxidant-associated diseases, including diabetes, hepatic disorders, cardiovascular disease, and obesity. Oleuropein can scavenge free radicals, exhibit anti-clastogenic capabilities, and prevent the growth of certain tumor cell types. This polyphenol can prevent lipoxygenases and low-density lipoprotein oxidation. It also exhibits hypoglycemic and hypocholesterolemia properties (Şahin et al., 2011; Mohammadi et al., 2016). Additionally, possesses neuroprotective qualities, meaning that it can be used to prevent neurological conditions such as epilepsy, depression, anxiety, Parkinson’s disease, and Alzheimer’s. By reducing the quantity of inflammatory cytokines and chemokines and deactivating astrocytes and microglia, it appears that OP lessens the damage to neurons (Butt et al., 2021). Prior research elucidated the function of olive leaves in antioxidant and glucose regulation, but it was unclear how they related to brain histology and neurotransmitters. Therefore, in addition to examining the histological alterations in the brain brought on by DM type 2, our current study sought to investigate the toxic consequences of the disease on the activity of neurotransmitters like dopamine (DOP) and acetylcholinesterase (AchE) in brain supernatant. Materials and MethodsAnimalsThirty Wister-adult male rats with an average weight of 230 ± 50 g were housed in special plastic cages (15 × 70 × 60 cm) in a room at the animal house of the College of Veterinary Medicine, University of Baghdad, Iraq. Rats were acclimatized for 2 weeks before the study which lasted for 40 days after diabetes induction. Rats were randomly divided into three equal groups (10 rats per group). The first 10 animals were the control group (CC), in which the rats were supplied with olive leaves supplement 150 mg/kg B.W. The left 20 rats served as diabetic animals (DM and DM+OL) with an intraperitoneal (IP) injection of alloxan monohydrate and nicotinamide to induce DM. The DM+OL group received 150 mg/kg.BW of olive leaves supplement (now—USA) was treated. Induction of DMDiabetes mellites induced by a single IP injection of 120 mg/kg of (alloxan monohydrate) alongside nicotinamide 50 mg/kg (Vattam et al., 2016), twenty rats were the only ones in which type II DM was induced after overnight fasting. After receiving nicotinamide injections and alloxan, blood glucose levels were measured twice weekly to monitor diabetes. Following DM induction for 7 days, the experiment was started. Blood samplingFollowing the DM induction and treatment administration at (0 time and 40 days) of the trial, blood samples were collected. Using intramuscular injections of Xylazine 40 mg/kg B.W. and Ketamine 60 mg/kg B.W. to induce anesthesia, the rats’ hearts were punctured to extract blood directly from them. Blood was placed in a gel test tube and left to stand for 15 minutes at room temperature to allow clots to form. The serum samples were centrifugated at 3,000 rpm for 10 minutes to estimate the levels of complement components of serum glucose and serum insulin. Tissue collection and homogenizationAt the termination of the experiment, after collecting the blood samples, all animals were decapitated and their brains were removed. The brain was dissected and divided into two hemispheres. One of these parts was placed in a plastic container for the ELISA test and frozen at −20oC. Brains were homogenized in 1.5 ml extraction buffer (containing 10 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100), and homogenized tissue then centrifuged for 5 minutes at 5,000 rpm. The samples were used to measure malondialdehyde (MDA), glutathione (GSH), AchE, and DOP levels. However, the other part of the brain was kept in polypropylene containers filled with 10% formalin for a standard histological examination. Histopathological studyFollowing animal sacrifice, brains were stored in 10% formalin at a ratio of 10:1 of the specimen’s volume. After 3 or 4 hours, the tissues were washed with tap water to remove the formalin solution from the samples. To prepare the tissue for histopathological analysis, the samples went through several procedures. These procedures consist of cutting, staining, blocking, clearing, embedding, and dehydrating (Suvarna et al., 2018). Studied parametersAssessing the glucose levels in serum (mg/dl) by use of glucose oxidase and a specialist glucose kit (Biosam/UAE). Moreover, the determination of serum Insulin concentration (μIU/l) was conducted by the rat insulin ELISA kit which was used to measure the insulin concentration in the serum in μIU/l. However, the determination of IR is performed by The homeostasis model assessment (HOMA) index which relies on the concentration of fasting serum glucose mg/dl and fasting serum insulin to determine IR (Matthews et al., 1985). Assessment of oxidative markers in rat brains to an evaluation of MDA and GSH concentrations in the male rat brain (ng/l) was measured by ELISA kits, (Cloud-Clone Corp/USA). The evaluation of DOP level and AchE in a male rat’s brain (ng/l) were measured using ELISA kit as well, (Cloud-Clone Corp/USA). Statistical analysisEvery statistical test was carried out with GraphPad Prism (Ver 10.1.0). The obtained data are displayed as mean ± SEM. Two-Way and One-Way ANOVA were used to assess the statistical significance of sample differences. Statistical significance was considered when p values < 0.05 (Unno et al., 2024). Ethical approvalEthical approval was granted through the local committee of animal care and use at the College of Veterinary Medicine within the University of Baghdad (Number 12614 on 23/11/2023) before starting this study. ResultsGlycemic index: serum Glucose concentration, serum insulin level, and IRThe present study claims a significant (p < 0.05) increase in serum glucose levels in diabetic groups (DM and DM+OL) at zero time compared with the CC group, whereas at 40 days the glucose concentration was significantly (p < 0.05) higher in the DM group compared with control and treated groups (Fig. 1A). Interestingly, treating diabetic rats for 40 days with olive leaves supplement significantly decreased glucose levels as in the DM+OL group. Similarly, there was a significant (p < 0.05) elevation in serum insulin level in diabetic groups (DM and DM+OL) at zero time, in comparison with control animals (Fig. 1B); however, at day 40, insulin levels were significantly (p < 0.05) decreased in animals treated with olive leaves supplement (DM+OL) compared with diabetic DM and CC groups (Fig. 1B). Furthermore, considering the periods of the experiment, it can be noticed there is a significant difference in insulin levels in diabetic groups (DM and DM+OL) at 40 days compared with 0 day of trial (Fig. 1B). Figure 1C illustrates serum IR which was significantly (p < 0.05) increased in the DM group and DM+OL group at zero time compared with the CC group, while, at the end of the experiment, significantly (p < 0.05) elevation was observed only in the diabetic group DM compared with DM+OL and CC animals at the same period and with the same group at day zero of study (Fig. 1C). However, DM+OL animals recorded a significant decline at 0 day compared with the end of the experiment (Fig. 1C).
Fig. 1. A: Effect of olive leaf extract on Serum glucose level in induced diabetic adult male rats. B: Effect of olive leaf extract on Serum insulin level in induced diabetic adult male rats. C: Effect of olive leaf extract on Serum insulin resentence in induced diabetic adult male rats. Data represented as mean ± SEM. * The differences between groups throughout time are denoted by capital letters. * The differences between the two time periods are denoted by the use of lowercase letters. * Data represented as mean ± SE N=10 per group. Table 1. Effect of diabetes and olive leaves extract on MDA (ng/ml) in brain tissue of adult male rats.
Table 2. Effect of diabetes and olive leaves extract on GSH (μg/ml) in brain tissue of adult male rats.
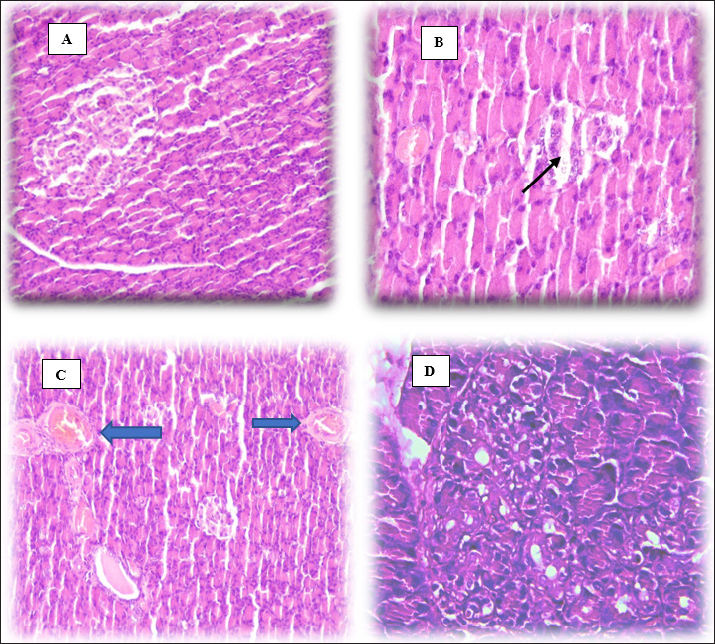
Malondialdehyde in brain tissueMalondialdehyde levels in brain tissue at 40 days reveal a significant increase (p < 0.05) in this parameter in the DM group compared with CC and DM+OL groups (Table 1). However, the olive leaf supplement brings the level of MDA in the brain closely to the normal level in DM+OL experiment animals, when the rapprochement of the CC rats and DM+OL rats showed no significant result (p < 0.05). Glutathione in brain tissueThe level of GSH was assessed at the end of the study. The results indicate there is a significant (p < 0.005) decrease in brain GSH in the DM group compared with two other groups. On the other hand, diabetic rats in the DM+OL were treated with olive leaf supplements and exhibited a significant (p < 0.05) increase in GSH level compared with CC groups; however, it is still significantly higher than in DM animals (Table 2). Dopamine level in brain tissueThe effect of olive leaf supplements on DOP levels in diabetic and control animals is displayed in Table 3. After 40 days of the experiment, DM rats showed a significant (p < 0.05) decline in DOP concentration compared with CC and DM+OL groups, whereas, non-significant differences were noticed between CC and DM+OL animals (Table 3). Acetylcholine esterase in brain tissueA statistical analysis of the obtained data revealed that AchE in brain tissue was significantly different(p < 0.05) in all experiment groups compared with each other. The lowest value of AchE was found in DM experiment animals, whereas, the highest value was in control rats. On the other hand, AchE of the DM+OL group was significantly (p < 0.05) higher than in DM but still significantly (p < 0.05) lower than CC group (Table 4). Histological examinationThe histopathological investigation of pancreatic tissue in experimental animals showed no clear lesion of the tissues obtained from the control group, (Fig. 2A); however, DM-induced pancreatic damage represented by a marked reduction in size, necrosis, and a fragment of the pancreatic islets (black arrow) (Fig. 2B), as well as, congested blood vessels (blue arrows) (Fig. 2C). Interestingly, treating diabetic rats with olive leaves supplement protected the pancreatic tissue from the harmful effects of DM, and that is what has been shown in Figure 2D of no pathological lesion in the pancreas of DM+OL animals. Table 3. Effect of diabetes and olive leaves extract on DOP (pg/ml) in brain tissue of adult male rats.
Table 4. Effect of diabetes and olive leaves extract on Acetylcholine estras (pg/ml) in brain tissue of adult male rat.
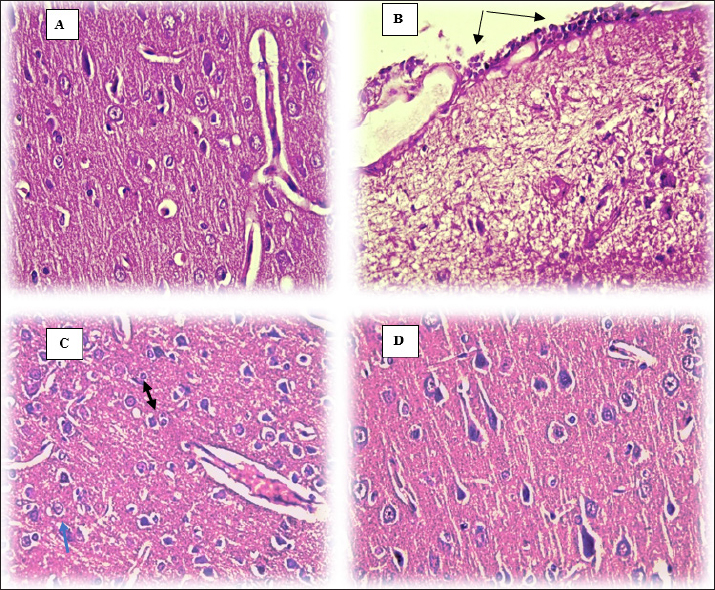
The brain section that belongs to control animals showed a normal appearance of different sizes and shapes of neurons and blood vessels (Fig. 3A), moreover, sections of DM rats show mononuclear cell infiltration in the pia mater (black arrows) (Fig. 3B), likewise, Figure 3C in diabetic rats shows proliferation of glial cells with Alzheimer’s cells (blue arrow) consisting of two astrocytes in one space (black arrow: Fig. 3C) in the brain parenchyma. Remarkably, administering supplements containing olive leaf extract to diabetic rats shielded the brain tissue from the deleterious effects of diabetes. This is demonstrated by Figure 3D, which displays the absence of any pathological lesions in the brain of DM+OL animals. DiscussionIn the present study, DM-induced neuropathy and the investigated olive leaves supplement effects on the brain and oxidative stress were investigated. Results of the current study reveal a significant elevation in glucose concentration, insulin level, and IR in diabetic animals immediately after diabetes induction; however, olive leaves supplement restores the parameters mentioned above close to the control ranges. Many studies have looked into how OL affects blood sugar levels in diabetic rats (Acar-Tek and Ağagündüz, 2020; Nosić et al., 2023). One useful indicator for confirming the onset of DM is the glycemic index, which includes glucose level, insulin level, and IR. The central symptom of diabetes, elevated blood glucose, impacts every bodily function (Taher and Arrak, 2016). A study by Jimenez-Garcia, and collaborators shows that using olive leaves supplement (OL) may regulate blood glucose levels in diabetic rats by improving the regeneration of β-cells in the pancreas (Jimenez-Garcia et al., 2021), what the histological section of the current study proven, which is a response to the decreased insulin responsiveness of target tissues, which increases the workload of the pancreatic β-cells and stimulates insulin over-secretion (Shahen et al., 2020). In T2DM, this research emphasizes the link between hyperglycemia and hyperinsulinemia together. As mentioned earlier, both the DM and DM+OL groups showed an increase in insulin concentration. However, in the case of DM+OL rats, the insulin concentration reverted to a near-control value after treatment with olive leaves. In the early stages of T2DM, hyperinsulinemia occurs when pancreatic beta cells respond to high blood sugar and IR by secreting more insulin than is normally necessary to keep glucose levels stable. By destroying insulin receptors on many cell surfaces, including pancreatic beta cells, hyperinsulinemia exacerbates IR. In the latter stages of T2DM, it lowers insulin secretion (Oza and Kulkarni, 2018). Researchers achieved their aim of reducing hyperglycemia and hyperinsulinemia in an alloxan-induced diabetic rabbit model by using oleuropein, the most abundant active phenolic component in olive leaf extract (Boaz et al., 2011). In addition, IR spiked in the diabetic mice (DM, DM+OL) before returning to normal in the treated group (DM+OL), suggesting that olive leaves may have this effect. In this study, it has been found that oleuropein supplements dramatically improved insulin sensitivity and decreased serum insulin activity in DM+OL diabetic rats. Researchers observed that olive leaf extract was more effective in restoring insulin, glucose, and HOMA-IR levels. According to the researchers, OLE enhanced the secretory capacity of pancreatic β-cells and insulin sensitivity (De Bock et al., 2013). Pancreatic histopathology samples from diabetic animals showed necrotic and atrophying islets of Langerhans lesions. There was no discernible histological damage in diabetic animals after treatment with a supplement containing olive leaves at 150 mg/kg. Evidence suggests that taking a supplement containing olive leaf extract may prevent harm to pancreatic tissues. Previous research by Bansal (2002) that the pancreatic degeneration seen in diabetic rats induced by alloxan monohydrate is a result of the necrotic action of the drug on the β cells (Bansal, 2002). The present study recorded higher MDA levels and lower levels of antioxidant enzymes. One of the most hazardous byproducts of lipid oxidation is MDA, which is partially produced by free radicals. Numerous investigations have demonstrated that DM significantly increases its concentration (Slatter et al., 2000). Therefore, Deciphering the connection between hyperglycemia and elevated ROS production could be crucial in understanding the etiology of neurodegeneration, a potential DM consequence (Haorah et al., 2007). It was also established that in diabetic rats, the decline in an antioxidant system, both enzymatic and nonenzymatic, may be reflected in the elevation of lipid peroxidation. According to earlier research, the brain and sciatic nerve of diabetic rats had higher levels of MDA (Cui et al., 2008; Arnal et al., 2010). The current study found that when OLE (150 mg/kg) was administered to DM rats, their antioxidant status improved and their MDA levels significantly decreased compared to the DM group. Results by (Mostafa-Hedeab et al., 2015; Abd El-Rahman, 2016; Navarro et al., 2017) and (Soliman et al., 2019) are presented here. Oleuropein is the primary ingredient in olive leaf extract. Strong anti-inflammatory and antioxidant properties of oleuropein have been demonstrated (Ahamad et al., 2019). OLE’s bioactive components, such as flavonoids and flavones, which are excellent hydroxyl radical scavengers and peroxidation inhibitors, may be the cause of its antioxidant action (Arora et al., 1998; Nasser and Khudair, 2022). In diabetic animals, GSH levels in the brain decline drastically, indicating increased consumption due to oxidative stress. Hyperglycemia-induced reductions in GSH levels or activities may increase ROS formation, which in turn may lead to oxidative stress and the release of inflammatory cytokines and chemokines. Our findings show that T2DM rats have lower supernatant GSH contents than non-diabetic controls. Therefore, similar to earlier research (Forrester et al., 1990; Nguyen et al., 2014). GSH levels are brought back to normal by supplementing with olive leaves. The antioxidant activity of oleuropein found in supplements made from olive leaves may be demonstrated by raising the activities of GSH peroxidase and superoxide dismutase (Pourkhodadad et al., 2016). Oxidative stress intensifies lipid peroxidation and ROS generation. Proteins get glycated, glucose autoxidation occurs, and polyol metabolism is triggered by hyperglycemia. These alterations quicken the production of ROS, which raises oxidative changes to proteins and lipids (Selim and Selim, 2013). Furthermore, hyperglycemia significantly increases the amount of substrate available for brain acidosis-causing aerobic glycolysis (Biessels et al., 1994). Baydas, et al. (2002) claimed that elevated free radical generation was caused by a drop in the body’s natural antioxidant defense levels (Baydas et al., 2002). In Ghareeb and Hussen (2008) discovered Neuronal activity is inhibited due to a decrease in the glycemic process. An inhibitory characteristic was found in the cerebrum of diabetic rats, as evidenced by the considerable inhibition of normal animals’ brain AChE activity that the cerebral extract from alloxan-diabetic rats showed (Ghareeb and Hussen, 2008). Comparing the diabetic rats in the current study to the CC group, there was a noticeable drop in serum AChE, followed by a decrease in supplement administration. Moreover, our results concur with earlier research showing that OL taken orally has shown to be highly protective against AChE inhibition (Albasher et al., 2020). A sharp decline in DOP levels in DM groups was rectified by using an olive leaf supplement. Thoughts of researchers (that reduced VMAT2 levels in diabetic mice may indicate compromised synaptic vesicle size, leading to DOP accumulation in the cytosol, contributing to neurodegeneration and oxidative stress-induced neurodegeneration (Pérez-Taboada et al., 2020). Moreover, an etiological mechanism for variations in DOP neurotransmission in response to salient stimuli may be provided by leptin, which regulates neuronal function. Messages containing leptin are sent to the brain’s reward centers, where the DOP system reduces the value of foods as rewards (Rayshan et al., 2023). Current findings concur with Sarbishegi and co-workers that oral administration of oleuropein may protect the dopaminergic neuron loss (Sarbishegi et al., 2014). After diabetic induction administration of olive leaves supplement antioxidant-rich largely stimulates antioxidant enzyme activity in the midbrain during aging; thus, it modifies the damage caused by free radicals to dopaminergic neurons.
Fig. 2. Histopathological sections of pancreas tissues following 40 days (H&E stain, 40×). (A) Control group (CC). (B) Diabetic group (DM). (C) DM group. (D) DM+OL group.
Fig. 3. Brain histology of rats following 40 days (H&E stain, 40×). (A) Control group (CC). (B) Diabetic group DM. (C) DM group. (D) DM+OL group. Sections of the brain show damaged brain cells in DM rats, the free radical theory suggests mitochondrial oxidative damage, particularly irreversible damage, causes aging and cellular malfunction, leading to neurological and cognitive diseases due to their vulnerability. Damage repair after supplementation with OLS. As mentioned, olive leaf supplements act as a neuroprotective (Pourkhodadad et al., 2016), by minimizing the effects of age and free radicals on the central nervous system, improving nerve transmission, and shielding nerve cells from oxidative stress. ConclusionThe present data demonstrate that OLS administration decreased oxidative stress MDA and increased antioxidant agent GSH, acted as neuroprotective by increased (DOP) and (AchE) in brain tissue in diabetic rats. AcknowledgmentsThe authors would like to acknowledge College of Veterinary Medicine/University of Baghdad for providing the facilities to perform this project. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no external funding. Authors’ ContributionsConceptualization and design: SA; Practical work: SA and AA; formal analysis and interpretation of data: SA, AA; writing-original draft preparation: SA and AA; All authors revised and approved the final manuscript for publication. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. ReferencesAbd El-Rahman, H. 2016. The effect of olive leaf extract and Α-Tocopherol on nephroprotective activity in rats. J. Nutr. Food Sci. 6, 479. Abbas, A.B. and Abbas, D.A. 2018. Evaluation of lipid profile and inflammatory parameters in female diabetes type 2 induced rabbits treated with glimepride, bromocriptine and fluoxtein. Iraqi J. Vet. 42(2), 97–104. Abdulghafoor, H.A., Ramadhan, S.J. and Nawfal, A.J. 2021. Therapeutic effects of allicin against the diabetes mellitus induced by streptozotocin in male rats. NVEO 8934–8945. Acar-Tek, N. and Ağagündüz, D. 2020. Olive leaf (Olea europaea L. folium): potential effects on glycemia and lipidemia. ANM. 76(1), 10–15. Ahamad, J., Toufeeq, I., Khan, M.A., Ameen, M.S.M., Anwer, E.T., Uthirapathy, S., Mir, Sh.R. and Ahmad, J. 2019. Oleuropein: a natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 33(12), 3112–3128. Albasher, G., Albrahim, T., Alsultan, N., Alfaraj, S., Alharthi, M.S., Kassab, R.B. and Abdel Moneim, A.E. 2020. Red beetroot extract mitigates chlorpyrifos-induced reprotoxicity associated with oxidative stress, inflammation, and apoptosis in rats. ESPR 27, 3979–3991. Arnal, E., Miranda, M., Barcia, J., Bosch-Morell, F. and Romero, F. 2010. Lutein and docosahexaenoic acid prevent cortex lipid peroxidation in streptozotocin-induced diabetic rat cerebral cortex. Neurosci. J. 166(1), 271–278. Arora, A., Nair, M.G. and Strasburg, G.M. 1998. Structure–activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol. Med. 24(9), 1355–1363. Bansal, R. 2002. Alloxan and streptozotoan action. Acta Diabetol. Lat. 17, 214. Bădescu, S., Tătaru, C., Kobylinska, L., Georgescu, E., Zahiu, D., Zăgrean, A. and Zăgrean, L. 2016. The association between diabetes mellitus and depression. J. Med. Life 9(2), 120–125. Baydas, G., Canatan, H. and Turkoglu, A. 2002. Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J. Pineal Res. 32(4), 225–230. Bhupatiraju, L., Bethala, K., Goh, K. W., Dhaliwal, J. S., Siang, T. C., Menon, S., Menon, B., Anchu, K.B., Chan, S.Y., Ming, L.C. and Khan, A. 2023. Influence of Murraya koenigii extract on diabetes induced rat brain aging. J. Med. Life 16(2), 307–316. Biessels, G.-J., Kappelle, A., Bravenboer, B., Erkelens, D. and Gispen, W. 1994. Cerebral function in diabetes mellitus. Diabetologia 37, 643–650. Boaz, M., Leibovitz, E., Dayan, Y.B. and Wainstein, J. 2011. Functional foods in the treatment of type 2 diabetes: olive leaf extract, turmeric and fenugreek, a qualitative review. Funct. Food Health Dis. 1(11), 472–481. Boye, A., Barku, V.Y.A., Acheampong, D.O. and Ofori, E.G. 2021. Abrus precatorius leaf extract reverses alloxan/nicotinamide-induced diabetes mellitus in rats through hormonal (Insulin, GLP-1, and glucagon) and enzymatic (α-amylase/α-glucosidase) modulation. Biomed Res. Int. 2021, 9920826. Butt, M.S., Tariq, U., Naz, A. and Rizwan, M. 2021. Neuroprotective effects of oleuropein: recent developments and contemporary research. J. Food Biochem. 45(12), e13967. Cobley, J.N., Fiorello, M.L. and Bailey, D.M. 2018. 13 reasons why the brain is susceptible to oxidative stress. Redox. Biol. 15, 490–503. Cui, X.P., Li, B.Y., Gao, H.Q., Wei, N., Wang, W.l. and Lu, M. 2008. Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J. Nutr. Sci. Vitaminol. 54(4), 321–328. De Bock, M., Derraik, J.G., Brennan, C.M., Biggs, J.B., Morgan, P.E., Hodgkinson, S.C., Hofman, P.L. and Cutfield, W.S. 2013. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: a randomized, placebo-controlled, crossover trial. PLoS One 8(3), e57622. Forrester, T.E., Badaloo, V., Bennett, F.I. and Jackson, A.A. 1990. Excessive excretion of 5-oxoproline and decreased levels of blood glutathione in type II diabetes mellitus. Eur. J. Clin. Nutr. 44(11), 847–850. Ghareeb, D.A. and Hussen, H.M. 2008. Vanadium improves brain acetylcholinesterase activity on early stage alloxan-diabetic rats. Neurosci. Lett. 436(1), 44–47. Goldstein, A.S., Janson, B.J., Skeie, J.M., Ling, J.J. and Greiner, M.A. 2020. The effects of diabetes mellitus on the corneal endothelium: a review. Surv. Ophthalmol. 65(4), 438–450. Haorah, J., Ramirez, S.H., Schall, K., Smith, D., Pandya, R. and Persidsky, Y. 2007. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood–brain barrier dysfunction. J. Neurochem. 101(2), 566–576. Jimenez-Garcia, S.N., Garcia-Mier, L., Vazquez-Cruz, M.A., Ramirez-Gomez, X.S., Guevara-Gonzalez, R.G., Garcia-Trejo, J.F. and Feregrino-Perez, A.A. 2021. Role of natural bio-active compounds as antidiabetic agents. Bioactive natural products for pharmaceutical applications. Springer International Publishing, pp: 535–561. Khalil, L.W. and Shakir, W.A. 2015. The protective role of alcoholic extract of (Anethum graveolens) seeds on renal function in alloxan induced diabetic rabbits. Iraqi J. Vet. 39(2), 1–6. Lins, P.G., Pugine, S.M.P., Scatolini, A.M. and de Melo, M.P. 2018. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon 4(9), e00805. Matthews, D.R., Hosker, J.P., Rudenski, A.S., Naylor, B., Treacher, D.F. and Turner, R. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. McCann, T.M., Simpson, K.E., Shaw, D.J., Butt, J.A. and Gunn-Moore, D.A. 2007. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire-based putative risk factor analysis. J. Feline Med. Surg. 9(4), 289–299. Mohammadi, A., Jafari, S.M., Esfanjani, A.F. and Akhavan, S. 2016. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 190, 513–519. Moheet, A., Mangia, S. and Seaquist, E.R. 2015. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 1353(1), 60–71. Mostafa-Hedeab, G., Sati, L.M., Elnaggar, H.M., Elgatlawey, Z.O., Eltwab, A.A., Elsaghayer, W.A. and Ali, H. 2015. Ameliorating effect of olive leaf extract on cyclosporine-induced nephrotoxicity in rats. Iran. J. Kidney Dis. 9(5), 361–368. Navarro, M., Morales, F.J. and Ramos, S. 2017. Olive leaf extract concentrated in hydroxytyrosol attenuates protein carbonylation and the formation of advanced glycation end products in a hepatic cell line (HepG2). Food Funct. 8(3), 944–953. Nasser, K.M. and Khudair, K.K. 2022. Effect of acarbose and olive leaf extract (Oleuropein) on glycemic index and antioxidant status in high fructose and H2 O2 exposed rats (Parts-II). Kufa J. Vet. Sci. 13(2), 42–55. Nguyen, D., Hsu Jean, W., Jahoor, F. and Sekhar Rajagopal, V. 2014. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 99(1), 169–177. Njan, A.A., Adenuga, F.O., Ajayi, A.M., Sotunde, O., Ologe, M.O., Olaoye, S.O., Erdogan, O.N. and Iwalewa, O.E. 2020. Neuroprotective and memory-enhancing effects of methanolic leaf extract of Peristrophe bicalyculata in rat model of type 2 diabetes mellitus. Heliyon 6(5), e04011. Nosić, M., Waisundara, V.Y. and Banjari, I. 2023. Olive oil, fruit and leaves in diabetes mellitus type 2 treatment. Expl. Food Foodomics 1(4), 192–205. Obaid, A.I., Alol, L.H., Al-Mzaien, A.K. and Omer, H.K.H. 2015. Effect of crude polyphenol extracted from black olive fruit (Olea europae) on male reproductive system of rats. Iraqi J. Vet. 39(1), 62–69. O’neill, D., Gostelow, R., Orme, C., Church, D., Niessen, S., Verheyen, K. and Brodbelt, D. 2016. Epidemiology of diabetes mellitus among 193,435 cats attending primary-care veterinary practices in England. J. Vet. Inter. Med. 30(4), 964–972. Oza, M.J. and Kulkarni, Y.A. 2018. Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front. Pharmacol. 9, 739. Pérez-Taboada, I., Alberquilla, S., Martín, E.D., Anand, R., Vietti-Michelina, S., Tebeka, N.N., Cantley, J., Cragg, S.J., Moratalla, R. and Vallejo, M. 2020. Diabetes causes dysfunctional dopamine neurotransmission favoring nigrostriatal degeneration in mice. Mov. Disord. 35(9), 1636–1648. Pham-Huy, L.A., He, H. and Pham-Huy, C. 2008. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4(2), 89–96. Pourkhodadad, S., Alirezaei, M., Moghaddasi, M., Ahmadvand, H., Karami, M., Delfan, B. and Khanipour, Z. 2016. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J. Physiol. Sci. 66, 397–405. Rayshan, A.R., Abdulwahid, A.A. and Alsaedi, A.A. 2023. The protective effects of melatonin against brain disorders induced by the western diet in male rats. World Vet. J. 13(2), 264–284. Romero-Márquez, J.M., Forbes-Hernández, T.Y., Navarro-Hortal, M.D., Quirantes-Piné, R., Grosso, G., Giampieri, F., Lipari, V., Sánchez-González, C., Battino, M. and Quiles, J.L. 2023. Molecular mechanisms of the protective effects of olive leaf polyphenols against Alzheimer’s disease. Int. J. Mol. Sci. 24(5), 4353. Şahin, S., Bilgin, M. and Dramur, M.U. 2011. Investigation of oleuropein content in olive leaf extract obtained by supercritical fluid extraction and soxhlet methods. Sep. Sci. Technol. 46(11), 1829–1837. Salim, S. 2017. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 360(1), 201–205. Sallander, M., Eliasson, J. and Hedhammar, Å. 2012. Prevalence and risk factors for the development of diabetes mellitus in Swedish cats. Acta Vet. Scand. 54, 1–6. Sarbishegi, M., Mehraein, F. and Soleimani, M. 2014. Antioxidant role of oleuropein on midbrain and dopaminergic neurons of substantia nigra in aged rats. Iran. Biomed. J. 18(1), 16. Selim, S.A. and Selim, A.O. 2013. Effect of streptozotocin-induced diabetes mellitus on the cerebellar cortex of adult male albino rats: histological and immunohistochemical study. Egypt. J. Histol. 36(1), 103–113. Servili, M., Baldioli, M., Selvaggini, R., Macchioni, A. and Montedoro, G. 1999. Phenolic compounds of olive fruit: one-and two-dimensional nuclear magnetic resonance characterization of nüzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 47(1), 12–18. Shahen, V., Gerbaix, M., Koeppenkastrop, S., Lim, S., McFarlane, K., Nguyen, A.N., Peng, X.Y., Weiss, N.B. and Brennan-Speranza, T. 2020. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 55, 109–118. Slatter, D., Bolton, C. and Bailey, A. 2000. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia, 43, 550–557. Soliman, G.A., Saeedan, A.S., Abdel-Rahman, R.F., Ogaly, H.A., Abd-Elsalam, R.M. and Abdel-Kader, M.S. 2019. Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: molecular and biochemical study. Saudi Pharm. J. 27(3), 326–340. Sparkes, A.H., Cannon, M., Church, D., Fleeman, L., Harvey, A., Hoenig, M., Peterson, M.E., Reusch, C.E., Taylor, S. and Rosenberg, D. 2015. ISFM consensus guidelines on the practical management of diabetes mellitus in cats. J. Feline Med. Surg. 17(3), 235–250. Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B.B. Stein, C., Basit, A., Chan, J.C., Mbanya, J.C. and Pavkov, M.E. 2022. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. Suvarna, K.S., Layton, C. and Bancroft, J.D. 2018. Bancroft’s theory and practice of histological techniques. London, UK: Elsevier Health Sciences. Taher, I.A. and Arrak, J.K. 2016. The effect of melatonin on adrenal gland and pancreas function in alloxan–induced diabetes in adult female rabbits. Iraqi J. Vet. Med. 40, 38–46. Unno, J., Mills, K.F., Ogura, T., Nishimura, M. and Imai, S.I. 2024. Absolute quantification of nicotinamide mononucleotide in biological samples by double isotope-mediated liquid chromatography-tandem mass spectrometry (dimeLC-MS/MS). NPJ Aging 10(1), 2. Vattam, K., Raghavendran, H., Murali, M., Savatey, H. and Kamarul, T. 2016. Coadministration of alloxan and nicotinamide in rats produces biochemical changes in blood and pathological alterations comparable to the changes in type II diabetes mellitus. Hum. Exp. Toxicol. 35(8), 893–901. Wang, X. and Michaelis, E.K. 2010. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2, 1224. Zhao, Y., Li, D., Shi, H., Liu, W., Qiao, J., Wang, S., Liu, R., Han, F., Li, J. and Li, J. 2024. Associations between type 2 diabetes mellitus and chronic liver diseases: evidence from a Mendelian ranldomization study in Europeans and East Asians. Front. Endocrinol. 15, 1338465. | ||
| How to Cite this Article |
| Pubmed Style Al-hafidh SHA, Abdulwahid AA. Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Vet. J.. 2024; 14(10): 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 Web Style Al-hafidh SHA, Abdulwahid AA. Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. https://www.openveterinaryjournal.com/?mno=211419 [Access: January 02, 2026]. doi:10.5455/OVJ.2024.v14.i10.15 AMA (American Medical Association) Style Al-hafidh SHA, Abdulwahid AA. Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Vet. J.. 2024; 14(10): 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 Vancouver/ICMJE Style Al-hafidh SHA, Abdulwahid AA. Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Vet. J.. (2024), [cited January 02, 2026]; 14(10): 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 Harvard Style Al-hafidh, S. H. A. & Abdulwahid, . A. A. (2024) Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Vet. J., 14 (10), 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 Turabian Style Al-hafidh, Sarah Hussein Abdulwahid, and Ammar Ahmed Abdulwahid. 2024. Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Veterinary Journal, 14 (10), 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 Chicago Style Al-hafidh, Sarah Hussein Abdulwahid, and Ammar Ahmed Abdulwahid. "Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats." Open Veterinary Journal 14 (2024), 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 MLA (The Modern Language Association) Style Al-hafidh, Sarah Hussein Abdulwahid, and Ammar Ahmed Abdulwahid. "Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats." Open Veterinary Journal 14.10 (2024), 2651-2661. Print. doi:10.5455/OVJ.2024.v14.i10.15 APA (American Psychological Association) Style Al-hafidh, S. H. A. & Abdulwahid, . A. A. (2024) Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats. Open Veterinary Journal, 14 (10), 2651-2661. doi:10.5455/OVJ.2024.v14.i10.15 |