| Original Article | ||
Open Vet. J.. 2022; 12(2): 231-241 Open Veterinary Journal, (2022), Vol. 12(2): 231–241 Original Research Outcome of radioiodine therapy for feline hyperthyroidism: Fixed dose versus individualized dose based on a clinical scoring systemJoana Matos1†, Bérénice Lutz1*†, Lisa-Maria Grandt1, Felix Meneses2, Daniela Schweizer-Gorgas2, Thierry Francey1 and Miguel Campos11Small Animal Internal Medicine Division, Department of Clinical Veterinary Medicine, Vetsuisse Faculty University of Bern, Bern, Switzerland 2Clinical Radiology, Department of Clinical Veterinary Medicine, Vetsuisse Faculty University of Bern, Bern, Switzerland †These authors contributed equally to this work. *Corresponding Author: Bérénice Lutz. Small Animal Internal Medicine Division, Department of Clinical Veterinary Medicine, Vetsuisse Faculty University of Bern, Bern, Switzerland. Email: berenice.lutz [at] gmail.com Submitted: 26/11/2021 Accepted: 21/03/2022 Published: 05/04/2022 © 2022 Open Veterinary Journal
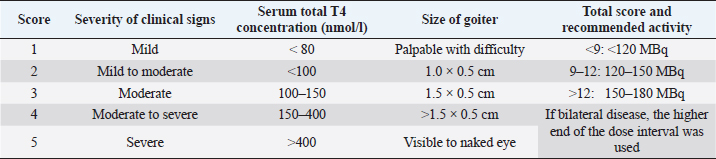
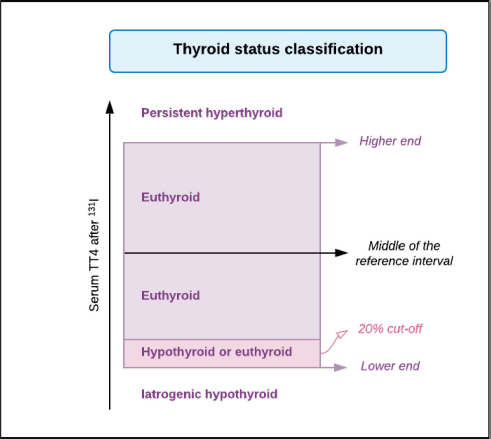
AbstractBackground: Hyperthyroidism is the most frequent endocrinopathy in older cats. To date, there is no consensus on how to best calculate the dose of radioiodine to administer to hyperthyroid cats. Aim: The goals of this study were to compare thyroid function, renal function, and survival time between hyperthyroid cats receiving a fixed dose of radioiodine and those receiving an individualized dose calculated using a clinical scoring system. Methods: Medical records of 110 cats treated with radioiodine therapy at the University of Bern between 2010 and 2020 were reviewed. Thyroid function, renal function, and survival of cats treated with a fixed dose of radioiodine (2010–2015; n=50) were compared to those of cats treated with an individualized dose (2015–2020; n=60) at different time points after therapy. Results: Treatment with a fixed dose of radioiodine (mean=168 ± 26 MBq) was associated with 69% of euthyroidism, 19% persistent hyperthyroidism, and 12% hypothyroidism, whereas treatment with an individualized dose (mean=120 ± 30 MBq) led to 54% euthyroidism, 23% hyperthyroidism, and 23% hypothyroidism (p=0.73). More than 12 months after treatment, the incidence of azotemia was comparable between cats treated with a fixed dose (37%) and those treated with an individualized dose (31%) (p=0.77). No factors were found to be predictive of treatment failure (hypothyroidism or hyperthyroidism) after therapy. Median survival time after radioiodine therapy was 44 months. In a multivariate analysis, persistent hyperthyroidism was the only variable independently associated with a shorter survival time (HR=6.24, p=0.002). Conclusion: The method of calculating the dose of radioiodine (fixed vs. individualized) to treat feline hyperthyroidism does not appear to be decisive for posttreatment thyroid function, renal function, or survival. Keywords: Cat, Dose, Hyperthyroidism, Radioiodine. IntroductionHyperthyroidism is the most frequent endocrinopathy in older cats, reported in up to 12.3% of cats over the age of 8 (Stephens et al., 2014; Köhler et al., 2016; McLean et al., 2017). It is a multisystemic disease that affects the cardiovascular, gastrointestinal, and renal systems, as well as the metabolic rate (Peterson, 1984). In the hyperthyroid state, the renal function is affected by several mechanisms, including the activation of the renin–angiotensin–aldosterone system and increased renal blood flow, leading to an increase in glomerular filtration rate (GFR) (Vaske et al., 2016). The increased GFR in addition to decreased creatinine production due to muscle loss contributes to the masking of renal disease in hyperthyroid cats. There are several therapeutic options available for feline hyperthyroidism: antithyroid drugs, radioiodine therapy, thyroidectomy, and nutritional management (Carney et al., 2016). Many authors consider radiodioine-131 as the best curative treatment option (Okosieme et al., 2020). It is considered safe, effective, and durable, regardless of the method of dosage (Peterson and Becker, 1995; Milner et al., 2006; Lucy et al., 2017; Finch et al., 2019). However, the literature describes approximately 2.5%–9% of persistent hyperthyroidism and 2%–40% of iatrogenic hypothyroidism after therapy (Peterson and Becker, 1995; Lucy et al., 2017; Morré et al., 2018; Fernandez et al., 2019; Finch et al., 2019). The development of iatrogenic hypothyroidism and azotemia after radioiodine treatment has been associated with a shorter survival time (Williams et al., 2010). Methods of dosing the activity of radioiodine to be administered include using a fixed dose or calculating an individualized dose either based on a clinical scoring system or based on scintigraphy tracer studies. At the moment, there is no consensus on which method is most appropriate. To the authors’ knowledge, there are no studies comparing thyroid function, renal function, and survival between cats treated with a fixed dose of radioiodine and cats treated with an individualized dose based on a clinical scoring system. The main objectives of this study were to compare thyroid function, renal function, and survival between hyperthyroid cats treated with a fixed dose of radioiodine and cats treated with an individualized dose based on a clinical score in the same institution. Secondary aims were to identify factors associated with treatment failure and survival after radioiodine treatment. Materials and MethodsStudy designRetrospective study. Data collectionThe medical records of all cats treated with radioiodine between 2010 and 2020 at the Small Animal Hospital of the University of Bern were reviewed. Follow-up after treatment was carried out at the referring veterinary clinics. If information regarding thyroid and renal function before and after therapy was lacking, referring veterinarians were contacted. If the additional information was not sufficient to assess thyroid and renal function, a recheck appointment was recommended, if the cat was still alive. Several laboratories using different analytical methods and reference intervals for serum T4 concentration determination were involved. The majority of T4 measurements were performed by chemiluminescent immunoassay (CEDIA T4, TermoFischer, USA; Cobas 8000 c701, Roche Switzerland) at external laboratories (IDEXX Switzerland and Laboklin, Germany, respectively) or by immunoassay in dry-slide format with a point-of-care analyzer (IDEXX Catalyst Dx, IDEXX Laboratories, Westbrook). In most cases, T4 before and after radioiodine treatment was measured by the same laboratory. Thyroxine-stimulating hormone (TSH) was measured using a canine TSH chemiluminescence assay (IMMULITE 2000 Canine TSH, Siemens Healthcare Diagnostics Products Ltd; ADVIA Centaur, Siemens Healthcare Diagnostics Products Ltd) at external laboratories (IDEXX Switzerland and Laboklin, Germany, respectively). Cats were divided in two groups according to the method of radioiodine dosage: the “fixed dose” (F) group (2010–2015) and the “individualized dose” (I) group (2015–2020). The fixed dose was changed from 180 MBq to 165 MBq in 2014 and then to 150 MBq in 2015. In 2015, the occurrence of iatrogenic hypothyroidism after radioiodine treatment and its consequences raised concerns. In the hope of limiting this treatment complication, while maintaining treatment success, a decision was made to change from using a fixed dose to an individualized dose. The individualized dose of each cat was calculated according to a clinical scoring system, as previously described (Table 1) (Mooney, 1994). Briefly, the dose to be administered is calculated by summing the scores assigned to the severity of clinical signs, size of the goiter, and (untreated) total T4. In all cats, radioiodine was injected subcutaneously. Following the injection, the cats were hospitalized in isolation for a minimum period of 9 days and were only discharged when the measured radiation level was below 0.25 uSv/h at 1 m distance using a Geiger counter.Follow-up information for each cat was grouped as follows: 1–3 months, 4–12 months, and more than 12 months after radioiodine treatment. Clinical and laboratory information was assessed in each of these time intervals. Thyroid function was classified as described in Figure 1. Persistent hyperthyroidism was defined as T4 concentration above the reference interval. Euthyroidism was defined as T4 within the reference interval and TSH under 0.3 ng/ml. If TSH was not available, T4 within the reference interval but above its 20th percentile was accepted for euthyroidism, as in one study, where thyroid function was determined by scintigraphy in all cats, no hypothyroid cat had a T4 concentration above the 18th percentile of the reference range (Peterson et al., 2017). Iatrogenic hypothyroidism was defined as T4 below the reference interval and/or TSH > 0.3 ng/ml. The fourth group was defined as eu- or hypothyroidism if T4 was within the 20th percentile of the reference interval and TSH was not available. Although thyroid function was assessed separately for each of the follow-up time points, a “definitive thyroid function” category was determined. This corresponded to the most recent and complete evaluation of thyroid function, allowing a clear classification, at least 3 months after treatment. When comparing the thyroid status after radioiodine treatment between the F group and the I group, cats classified as “eu- or hypothyroid” were excluded. Cats with values of creatinine concentration above reference interval for the respective laboratory at the second or the third follow-up (i.e., at least 4 months after treatment) were classified as azotemic. Table 1. Clinical scoring system used to determine the individualized activity of radioiodine to be administered (Mooney, 1994).
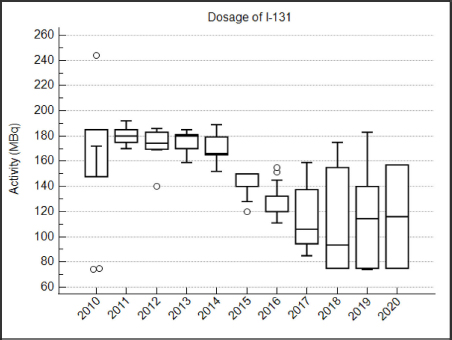
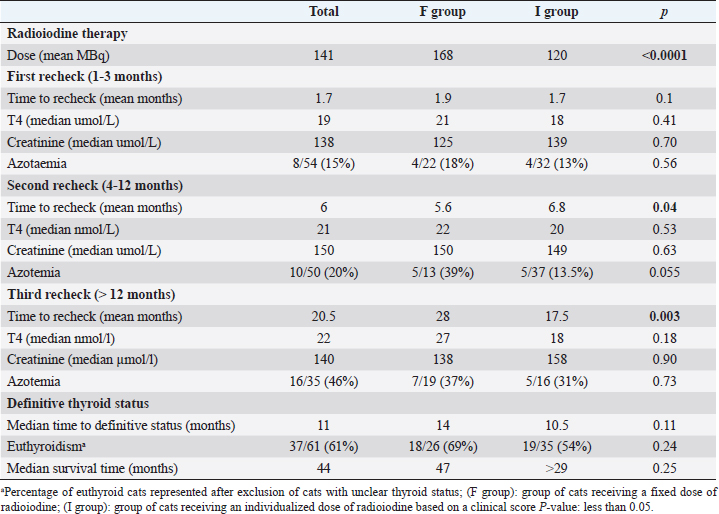
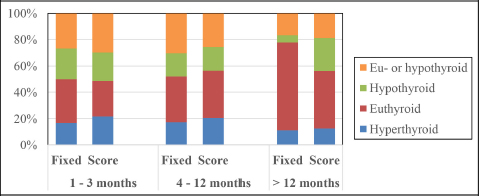
Fig. 1. Classification for thyroid function according to serum T4 concentration after radioiodine treatment. Statistical analysisData were analyzed using MedCalc Statistical Software version 20.009 (MedCalc Software Ltd, Ostend, Belgium). The D’Agostino–Pearson test was used to assess if continuous variables were normally distributed. These are presented as mean ± standard deviation (SD), whereas continuous variables that were not normally distributed are presented as median and interquartile range (IQR). Multiple comparisons were performed using analysis of variance tests (for normally distributed data) and Kruskal–Wallis test (for non-normally distributed data). Comparison of proportions between categorical variables was performed with the chi-squared test. Comparison of continuous variables was performed with the t-test for normally distributed data and with the Mann–Whitney test for non-normally distributed data. To identify potential risk factors for treatment failure, a univariate logistic regression was performed. In this analysis, cats classified as “eu- or hypothyroid” were excluded. All-cause-mortality was used for the survival analysis. Owners and veterinarians were contacted to determine if each cat was alive or dead, the date of death (if relevant) or last known date when the cat was alive. Cats alive at the time of the study and cats that were lost to follow-up were censored in the survival analysis. A univariate Cox proportional hazard regression was performed to determine the effect of the following parameters on survival: age at presentation, method of radioiodine dosage (fixed dose vs. individualized dose), definitive thyroid status (after exclusion of cats with undetermined thyroid function), presence of azotemia more than 4 months after treatment, and creatinine serum concentration more than 4 months after treatment. To identify independent predictors of survival, the univariate analysis was followed up by a multivariate Cox proportional hazard regression. Ethical approvalThis work involved the use of nonexperimental animals only. Established and internationally recognized high standards (“best practice”) of individual veterinary clinical patient care were followed. ResultsSignalmentA total of 110 hyperthyroid cats received radioiodine at our institution between 2010 and 2020. The median age of treated cats was 13 years (IQR: 11–14 years). Cats in the I group were significantly older at the time of treatment than cats in the F group (median=13 years (IQR: 11–13) versus 12 years (IQR: 12–14; p=0.01). Fifty-five cats were male (50%) and 55 were female (50%). There were significantly more male cats in the I group (36/60; 60%) compared to the F group (19/50; 38%) (p=0.02). Median weight at admission was 3.9 kg in the F group and 4.2 kg in the I group (p=0.63). Most common breeds were Domestic Shorthairs (92/110; 84%) and Norwegian Forest cats (3/110; 3%). There were two (2%) of each of the following breeds: Persian, Chartreux, Maine Coon, British Shorthair, and Ragdoll; and one (0.9%) of each of the following: Siamese, Angora Turk, Siberian, and Balinese. The most common clinical sign was weight loss, present in 12/22 (55%) cats in the F group and 17/23 (74%) cats in the I group (p=0.17). Hyperactivity was present in 2/4 (50%) cats in the F group and 3/4 (75%) cats in the I group (p=0.47). Polyphagia was present in 10/25 (40%) cats in the F group and 12/25 (48%) cats in the I group (p=0.57). Polyuria–polydipsia was present in 4/19 (21%) cats in the F group and 6/23 (26%) cats in the I group (p=0.7). Vomiting was significantly more frequent in the F group (8/12; 67%) compared to the I group (9/28; 32%; p=0.04). 56/64 (88%) cats were treated before radioiodine treatment with methimazole, whereas 8/64 (12%) were treated with carbimazole. The percentage of cats treated medically was not different between the two groups (p=0.80). Baseline clinical and clinicopathological analysisBefore radioiodine therapy, the mean body condition score (BCS) was 4.4/9 ± 1.7 (n=73). A cervical nodule was palpable in 57/61 (93%) cats and heart murmur was audible in 41/49 (84%) cats. The disease was unilateral in 29/44 (66%) cats. Those parameters were not different between the two groups (p=0.24; p=0.19 and p=0.22, respectively). Alopecia was significantly more present in the I group (28/41; 68%) compared to the F group (1/3; 33%, p < 0.001). On biochemistry, ALT and ALP were increased in 23/60 (38%) and 11/62 (18%) cats, respectively. This difference was not statistically significant between the two groups (p=0.28 and p=0.95, respectively). Mean creatinine concentration was 100 ± 41 µmol/l in the F group and 96 ± 47 µmol/L in the I group (p=0.63). Mean T4 concentration was 133 ± 74 nmol/l in the F group and 134 ± 69 nmol/l in the I group (p=0.53). Hypertrophic cardiomyopathy was diagnosed on cardiac ultrasound in 16/45 (36%) of the cats. The occurrence was not different between cats in the F and I groups (p=0.56). Evolution over the yearsFrom 2010 to 2020, there were no obvious changes in body weight, BCS, and T4 in the cats referred for radioiodine therapy. TreatmentFigure 2 shows the activity of radioiodine administered at our institution from 2010 to 2020. Fifty (45%) cats received a fixed activity of radioiodine and 60 (55%) received an individualized activity based on a clinical scoring system. Before 2013, while using a fixed dose, two cats received a lower dose of radioiodine (75 MBq) because the untreated T4 value was only mildly increased. One cat received a higher dose (245 MBq) because the untreated T4 value was severely increased. The mean dose of radioiodine administered in the F group (168 ± 26 MBq; IQR: 150–181) was significantly higher than the mean dose administered in the I group (120 ± 30 MBq; IQR: 90–140; p < 0.0001). Follow-upThe outcomes of the cats after radioiodine therapy according to the method of radioiodine dosage are presented in Table 2. Median body weight increased from 4.1 kg (IQR: 3.4–5.0; n=96) at moment of treatment to 4.8 kg (IQR: 4.3–6.0; n=11) more than 12 months after treatment (p=0.78). In the F group, the median T4 serum concentration significantly decreased from 133 nmol/l (IQR: 82–163; n=26) at treatment to 21 nmol/l (IQR: 10–28; n=30) for 1–3 months, 27 23 nmol/l (IQR: 17–28; n=23) for 4–12 months, and nmol/l (IQR: 21–31; n=18) more than 12 months after radioiodine therapy (p < 0.001). In the I group, median T4 serum concentration also significantly decreased from 134 nmol/l (IQR: 91–188; n=59) at treatment to 18 nmol/l (IQR: 15–38; n=37) for 1–3 months, 20 nmol/L (IQR: 17–29; n=39) for 4–12 months, and 18 nmol/L (IQR: 16–29; n=17) for more than 12 months after radioiodine (p < 0.001). In the F group, median creatinine serum concentration significantly increased from 110 µmol/l (IQR: 83–146; n=24) at treatment to 125 µmol/l (IQR: 96–167; n=26) for 1–3 months, 150 µmol/l (IQR: 115–198; n=17) for 4–12 months, and 138 µmol/l (IQR: 122–183; n=20) more than 12 months after radioiodine therapy (p=0.016). In the I group, median creatinine serum concentration also significantly increased from 96 µmol/l (IQR: 76–138; n=45) at treatment to 140 µmol/l (IQR: 105–185; n=31) for 1–3 months, 149 µmol/l (IQR: 119–179; n=37) for 4–12 months, and 158 µmol/l (IQR: 112–191; n=16) more than 12 months after radioiodine (p < 0.001). Assessment of thyroid function after radioiodine therapy is shown in Figure 3. The proportion of euthyroid cats increased at each follow-up. Definitive thyroid status could be assessed in 81/110 (74%) cats and a clear thyroid function could be determined in 61/110 (55%) cats. Median time to assessment of definitive thyroid status was 11 months (IQR: 5–20). Of those cats, 11 (14%) were hypothyroid, 37 (46%) were euthyroid, and 13 were persistently hyperthyroid (15%). The remaining 20 (25%) cats were eu- or hypothyroid. After excluding cats classified as eu- or hypothyroid, the overall percentages of euthyroidism and iatrogenic hypothyroidism were 61% (37/61) and 18% (11/61), respectively. One persistently hyperthyroid cat received a second dose of radioiodine, while the remaining hyperthyroid cats were treated with antithyroid medications.
Fig. 2. Box plots showing the activity (MBq) of radioiodine administered to 110 hyperthyroid cats from 2010 to 2020 (○ outliers). Table 2. Comparison of outcome parameters in 110 hyperthyroid cats after radioiodine treatment, according to the radioiodine dosing method .
Fig. 3. Bar chart illustrating the percentile distribution of thyroid status of cats at different time points after radioiodine treatment according to the method of radioiodine dosage (fixed dose vs. individualized dose).
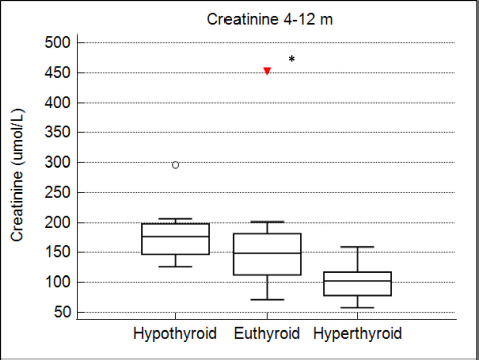
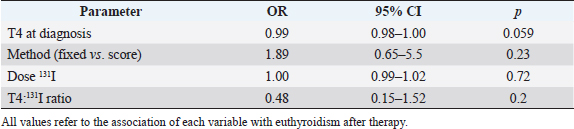
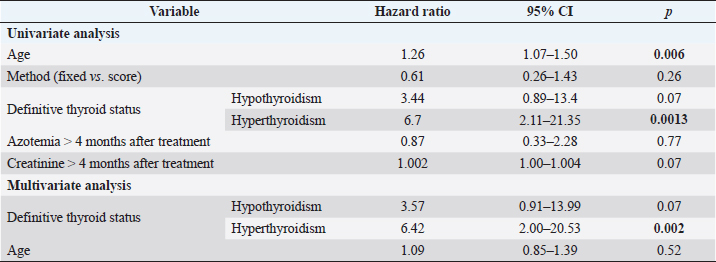
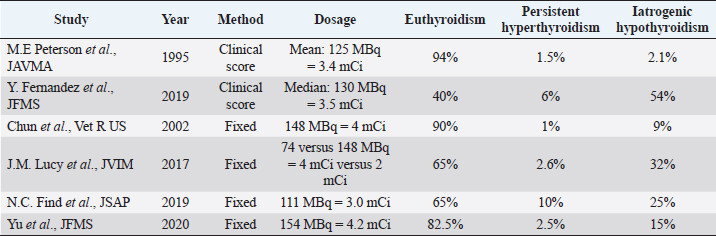
Fig. 4. Box plots comparing serum creatinine conce-ntrations (µmol/l) for 4–12 months after radioiodine therapy between hypothyroid, euthyroid, and hyperthyroid cats. The circle and the triangle represent outliers. * p < 0.05 compared to hypothyroid and euthyroid groups. After 4–12 months of therapy, serum creatinine concentration was significantly lower in cats with persistent hyperthyroidism compared to euthyroid and hypothyroid cats (n=40; p=0.002) (Fig. 4). However, more than 12 months after therapy, serum creatinine concentration was no longer significantly associated with thyroid status (n=27; p=0.12). After 4–12 months of therapy, azotemia was present in 38% of hypothyroid cats, 18% of euthyroid cats, and in no hyperthyroid cat (n=35; p=0.11). After 12 months, azotemia was present in 75% of hypothyroid cats, 26.3% of euthyroid cats, and in no hyperthyroid cat (n=27, p=0.057). Comparison of thyroid and renal functions between cats receiving a fixed activity and an individualized activity of radioiodineComparing the definitive thyroid function (most complete thyroid function assessment at least 3 months after radioiodine), in the F group (n=26) there were 18 (69%) euthyroid cats, 5 (19%) persistent hyperthyroid cats, and 3 (12%) iatrogenic hypothyroid cats. In the I group (n=35), there were 19 (54%) euthyroid cats, 8 (23%) persistent hyperthyroid cats, and 8 (23%) iatrogenic hypothyroid cats. These differences were not statistically significant (p=0.427). Of the 22 cats with unclear thyroid status, 10 belonged to the F group and 12 to the I group. After 4–12 months of therapy and more than 12 months after therapy, serum creatinine concentration was not significantly associated with method of radioiodine dose calculation (p=0.63 and p=0.90, respectively). The incidence of azotemia at any time point (1–3 months, 4–12 months, and more than 12 months) was also not significantly associated with method of radioiodine dose calculation (p=0.6, p=0.056, and p=0.73, respectively). Investigation of the causes for treatment failureThe results of the logistic regression are presented in Table 3. No association was found between the investigated variables and treatment failure (absence of euthyroidism) after treatment. Survival analysisMedian survival time was 44 months (n=102). The results of the survival analysis are presented in Table 4. In the univariate Cox proportional hazard regression, age at presentation (n=102; HR=1.26;p=0.006) and hyperthyroid definitive status ( n=60; HR=6.7; p=0.0013) were significantly associated with a shorter survival time. Serum creatinine concentration (n=69; HR=1.002; p=0.072) and the presence of azotemia (n=66; HR=0.869; p=0.773) more than 4 months after treatment were not associated with survival time. In the multivariate analysis, hyperthyroid definitive status was the only variable independently associated with a shorter survival time (n=60; HR=6.42; p=0.002). The Kaplan–Meier survival curves according to the definitive thyroid status and method of radioiodine dosage are shown in Figures 5 and 6. DiscussionThere is no consensus on how best to determine the dose of radioiodine to administer to hyperthyroid cats. The methods reported to date include using a fixed dose or calculating an individualized dose using either a clinical score or scintigraphy tracer studies (Morré et al., 2018). Given the increased awareness of the prevalence of iatrogenic hypothyroidism after radioiodine and concerns that a fixed dose may increase its frequency, our group decided to change from using a fixed dose to an individualized dose in 2015. To the authors’ knowledge, this is the first study comparing the two dosing methods. We could not identify significant differences in thyroid function, renal function, or survival time between cats treated with a fixed dose and cats treated with an individualized dose calculated using a clinical scoring system. The rates of euthyroidism and iatrogenic hypothyroidism in the F group of our study seem comparable to other studies using a fixed dose of radioiodine, when TSH is measured during follow-up (Lucy et al., 2017; Finch et al., 2019). Table 5 summarizes the outcomes of radioactive iodine reported in previous studies. However, the rate of persistent hyperthyroidism in the F group was higher than previously reported. Although results of different studies are difficult to compare, this is interesting because the mean dose of radioiodine administered in the F group was higher compared to previous reports. Studies using a fixed dose reporting a higher incidence of euthyroidism after treatment did not include TSH measurement during follow-up and could have underestimated the incidence of iatrogenic hypothyroidism (Chun et al., 2002; Vagney et al., 2018; Yu et al., 2020). Table 3. Univariate logistic regression to investigate factors associated with treatment failure.
Table 4. Univariate and multivariate Cox proportional hazard regression to investigate factors associated with survival.
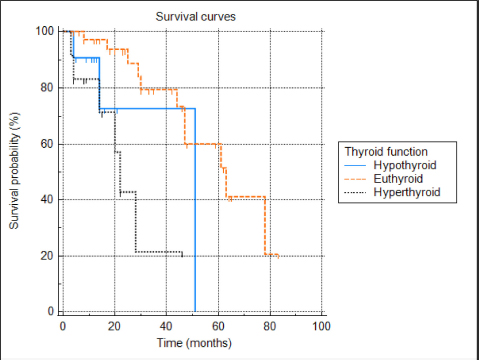
Fig. 5. Kaplan–Meier survival curves for 60 cats treated with radioiodine and divided in three groups according to definitive thyroid status (hypothyroid n=11; euthyroid n=37; and hyperthyroid n=12).
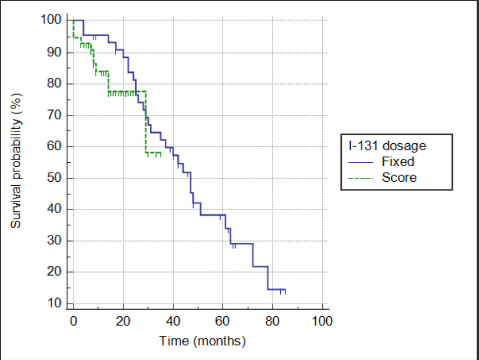
Fig. 6. Kaplan–Meier survival curves for 102 cats treated with radioiodine according to method of radioiodine dose calculation (F group: n=45; I group: n=57). A previous study evaluating an individualized dose of radioiodine to treat feline hyperthyroidism and measuring TSH during follow-up reported a markedly higher incidence of iatrogenic hypothyroidism and a lower rate of euthyroidism compared to the I group in our study (Fernandez et al., 2019) (Table 5). The mean dose of radioiodine administered in that study was slightly higher compared to the mean dose administered to the I group. As mentioned previously, some studies did not include TSH measurement during follow-up and likely underestimated the incidence of iatrogenic hypothyroidism (Mooney, 1994; Peterson and Becker, 1995; Vagney et al., 2018). TSH has been shown to be a more sensitive and specific marker for the identification of feline hypothyroidism compared to total T4 and free T4 (Peterson et al., 2017; Wakeling et al., 2020). A recent study on radioiodine treatment of hyperthyroid cats, using the same clinical score as in our study and including routine measurement of TSH during follow-up, reported an incidence of iatrogenic hypothyroidism of 40% (Fernandez et al., 2019). Recognizing iatrogenic hypothyroidism after radioiodine treatment is very important, especially in azotemic cats, as the combination of azotemia and hypothyroidism is associated with a shorter survival time (Williams et al., 2010). In our study, however, neither hypothyroidism nor azotemia after treatment was significantly associated with survival. Table 5. Outcome of radioiodine treatment in previous reports using either a fixed dosing method or an individualized dose based on a clinical score to treat feline hyperthyroidism.
In one study, the use of an individualized dose based on thyroid scintigraphy did not show superiority compared to a fixed dose of 167 MBq (Morré et al., 2018). It is interesting to note that the outcome reported in the group receiving the individualized activity (58% euthyroidism, 16% persistent hyperthyroidism, and 26% iatrogenic hypothyroidism) is very similar to the outcome reported for the I group in our study. In future studies, it would also be interesting to compare the outcome of radioiodine treatment dosed using either thyroid scintigraphy or a clinical score within the same institution (Peterson and Becker, 1995; Morré et al., 2018). In our study, we could not demonstrate the benefit of administering an individualized dose of radioiodine based on a clinical scoring system, compared to a fixed dose, to treat feline hyperthyroidism. Because the use of a clinical score makes the planning of radioiodine treatment more difficult, the results of our study should lead to a reflection of what is indeed the best solution. Although the use of fixed dose is easier to plan, using a clinical score has the theoretical advantage of allowing adaptation of the dose to the needs of a particular patient. Even if our study did not show an advantage of using an individualized activity, refinement of the way in which the clinical score system is applied over time could still lead to improved treatment success in the future. In our study, no factors predictive of treatment failure could be identified. In previous studies, higher T4 serum concentration at presentation (Morré et al., 2018; Yu et al., 2020) and higher dose of radioiodine have been associated with treatment failure (Lucy et al., 2017). Although we did not evaluate the effect of thyroid nodule size and bilateral disease on the outcome, in one study these were not associated with thyroid function after radioiodine treatment (Volckaert et al., 2016). In another study, an association was reported between larger goiter and persistent hyperthyroidism after radioiodine treatment (Morré et al., 2018). In our survival analysis, the only factor independently associated with shorter survival time was persistent hyperthyroidism. The effect of persistent hyperthyroidism on survival could be related to progressive clinical signs, namely weight loss, cardiac disease, and worsening quality of life. Most of these cats were likely treated medically or left untreated given the fact that many had been referred because of side effects of medical treatment. The owners’ refusal of a second radioiodine treatment could be due to costs and duration of hospitalization. In a survey, owners of cats with persistent or relapsing hyperthyroidism after radioiodine treatment were more likely to be unhappy with the outcome and their decision to choose radioiodine therapy (Boland et al., 2014). The main limitations of this study are associated with its retrospective nature and limited sample size. Some cats were also lost to follow-up. Unfortunately, thyroid function after therapy could not be clearly determined in all cats, hence the introduction of the category “eu- or hypothyroid”, when TSH measurement was not available and T4 serum concentration was under the 20th percentile of the reference interval. Moreover, although the incidence of azotemia after radioiodine treatment was not significantly associated with thyroid function, this analysis was likely underpowered. Finally, the dose of radioiodine in the F group was not uniform over the years as mentioned previously. In conclusion, the method of determining the activity of radioiodine to administer to hyperthyroid cats was not associated with thyroid function, renal function, or survival time after therapy. No predictors of treatment failure were identified and persistent hyperthyroidism was the only parameter independently associated with shorter survival time. Further improvement of the clinical scoring system is needed before an individualized dose of radioiodine can be recommended over the use of a fixed dose as a method of limiting iatrogenic hypothyroidism while maintaining treatment efficacy. AcknowledgmentsThe authors would like to thank Laboklin (Germany) for their collaboration in the more recent follow-up biochemical analyses. They would also like to thank the small animal clinic of the University of Bern for funding selected recheck appointments. Finally, they would like to thank the referring veterinarians and owners of treated cats for their help collecting data. Conflicts of interestThe authors declare that there are no conflicts of interest. Informed consentInformed consent was obtained from the owners or legal custodian of all animals described in this work for all procedures undertaken. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required. ReferencesBoland, L.A., Murray, J.K., Bovens, C.P.V. and Hibbert, A. 2014. A survey of owners’ perceptions and experiences of radioiodine treatment of feline hyperthyroidism in the UK. J. Feline Med. Surg. 16, 663–670. Carney, H.C., Ward, C.R., Bailey, S.J., Bruyette, D., Dennis, S., Ferguson, D., Hinc, A. and Rucinsky, A.R. 2016. 2016 AAFP Guidelines for the management of feline hyperthyroidism. J. Feline Med. Surg. 18, 400–416. Chun, R., Garrett, L.D., Sargeant, J., Sherman, A. and Hoskinson, J.J. 2002. Predictors of response to radioiodine therapy in hyperthyroid cats. Vet. Radiol. Ultrasound 43, 587–591. Fernandez, Y., Puig, J., Powell, R. and Seth, M. 2019. Prevalence of iatrogenic hypothyroidism in hyperthyroid cats treated with radioiodine using an individualised scoring system. J. Feline Med. Surg. 21, 1149–1156. Finch, N.C., Stallwood, J., Tasker, S. and Hibbert, A. 2019. Thyroid and renal function in cats following low-dose radioiodine (111Mbq) therapy. J. Small Anim. Pract. 60, 523–528. Köhler, I., Ballhausen, B.D., Stockhaus, C., Hartmann, K. and Wehner, A. 2016. Prevalence of and risk factors for feline hyperthyroidism among a clinic population in Southern Germany. Tierarztl. Prax. Ausg. K. Kleintiere. Heimtiere. 44, 149–157. Lucy, J.M., Peterson, M.E., Randolph, J.F., Scrivani, P.V., Rishniw, M., Davignon, D.L., Thompson, M.S. and Scarlett, J.M. 2017. Efficacy of low-dose (2 millicurie) versus standard-dose (4 millicurie) radioiodine treatment for cats with mild-to-moderate hyperthyroidism. J. Vet. Intern. Med. 31, 326–334. McLean, J.L., Lobetti, R.G., Mooney, C.T., Thompson, P.N. and Schoeman, J.P. 2017. Prevalence of and risk factors for feline hyperthyroidism in South Africa. J. Feline Med. Surg. 19, 1103–1109. Milner, R.J., Channell, C.D., Levy, J.K. and Schaer, M. 2006. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996–2003). J. Am. Vet. Med. Assoc. 228, 559–563. Mooney, C.T. 1994. Radioactive iodine therapy for feline hyperthyroidism: efficacy and administration routes. J. Small Anim. Pract. 35, 289–294. Morré, W.A., Panciera, D.L., Daniel, G.B., Monroe, W.E. and Were, S. 2018. Investigation of a novel variable dosing protocol for radioiodine treatment of feline hyperthyroidism. J. Vet. Intern. Med. 32, 1856–1863. Okosieme, O.E., Taylor, P.N. and Dayan, C.M. 2020. Should radioiodine now be first line treatment for Graves’ disease? Thyroid Res. 13, 3. Peterson, M.E. 1984. Feline hyperthyroidism. Vet. Clin. North Am. Small Anim. Pract. 14, 809–826. Peterson, M.E. and Becker, D.V. 1995. Radioiodine treatment of 524 cats with hyperthyroidism. J. Am. Vet. Med. Assoc. 207, 1422–1428. Peterson, M.E., Nichols, R. and Rishniw, M. 2017. Serum thyroxine and thyroid-stimulating hormone concentration in hyperthyroid cats that develop azotaemia after radioiodine therapy. J. Small Anim. Pract. 58, 519–530. Stephens, M.J., O’ Neill, D.G., Church, B.D., McGreevy, P.D., Thomson, P.C. and Brodbelt, D.C. 2014. Feline hyperthyroidism reported in primary-care veterinary practices in England: prevalence, associated factors and spatial distribution. Vet. Rec. 175(18), 458. Vagney, M., Desquilbet, L., Reyes-Gomez, E., Delisle, F., Devauchelle, P., Rodriguez-Piñeiro, M.I., Rosenberg, D. and de Fornel-Thibaud, P. 2018. Survival times for cats with hyperthyroidism treated with a 3.35 mCi iodine-131 dose: a retrospective study of 96 cases. J. Feline Med. Surg. 20, 528–534. Vaske, H.H., Schermerhorn, T. and Grauer, G.F. 2016. Effects of feline hyperthyroidism on kidney function: a review. J. Feline Med. Surg. 18, 55–59. Volckaert, V., Vandermeulen, E., Dobbeleir, A., Duchateau, L., Saunders, J.H. and Peremans, K. 2016. Effect of thyroid volume on radioiodine therapy outcome in hyperthyroid cats. J. Feline Med. Surg. 18, 144–149. Wakeling, J., Hall, T. and Williams, T.L. 2020. Correlation of thyroid hormone measurements with thyroid stimulating hormone stimulation test results in radioiodine-treated cats. J. Vet. Intern. Med. 34, 2265–2275. Williams, T.L., Elliott, J. and Syme, H.M. 2010. Association of Iatrogenic Hypothyroidism with Azotemia and Reduced Survival Time in Cats Treated for Hyperthyroidism. J. Vet. Intern. Med. 24, 1086–1092. Yu, L., Lacorcia, L., Finch, S. and Johnstone, T. 2020. Assessment of treatment outcomes in hyperthyroid cats treated with an orally administered fixed dose of radioiodine. J. Feline Med. Surg. 22, 744–752. | ||
| How to Cite this Article |
| Pubmed Style Matos J, Lutz B, Grandt L, Meneses F, Schweizer D, Francey T, Campos M. Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. Open Vet. J.. 2022; 12(2): 231-241. doi:10.5455/OVJ.2022.v12.i2.11 Web Style Matos J, Lutz B, Grandt L, Meneses F, Schweizer D, Francey T, Campos M. Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. https://www.openveterinaryjournal.com/?mno=21277 [Access: January 13, 2026]. doi:10.5455/OVJ.2022.v12.i2.11 AMA (American Medical Association) Style Matos J, Lutz B, Grandt L, Meneses F, Schweizer D, Francey T, Campos M. Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. Open Vet. J.. 2022; 12(2): 231-241. doi:10.5455/OVJ.2022.v12.i2.11 Vancouver/ICMJE Style Matos J, Lutz B, Grandt L, Meneses F, Schweizer D, Francey T, Campos M. Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. Open Vet. J.. (2022), [cited January 13, 2026]; 12(2): 231-241. doi:10.5455/OVJ.2022.v12.i2.11 Harvard Style Matos, J., Lutz, . B., Grandt, . L., Meneses, . F., Schweizer, . D., Francey, . T. & Campos, . M. (2022) Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. Open Vet. J., 12 (2), 231-241. doi:10.5455/OVJ.2022.v12.i2.11 Turabian Style Matos, Joana, Berenice Lutz, Lisa-maria Grandt, Felix Meneses, Daniela Schweizer, Thierry Francey, and Miguel Campos. 2022. Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. Open Veterinary Journal, 12 (2), 231-241. doi:10.5455/OVJ.2022.v12.i2.11 Chicago Style Matos, Joana, Berenice Lutz, Lisa-maria Grandt, Felix Meneses, Daniela Schweizer, Thierry Francey, and Miguel Campos. "Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system." Open Veterinary Journal 12 (2022), 231-241. doi:10.5455/OVJ.2022.v12.i2.11 MLA (The Modern Language Association) Style Matos, Joana, Berenice Lutz, Lisa-maria Grandt, Felix Meneses, Daniela Schweizer, Thierry Francey, and Miguel Campos. "Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system." Open Veterinary Journal 12.2 (2022), 231-241. Print. doi:10.5455/OVJ.2022.v12.i2.11 APA (American Psychological Association) Style Matos, J., Lutz, . B., Grandt, . L., Meneses, . F., Schweizer, . D., Francey, . T. & Campos, . M. (2022) Outcome of radioiodine therapy for feline hyperthyroidism: fixed dose versus individualized dose based on a clinical scoring system. Open Veterinary Journal, 12 (2), 231-241. doi:10.5455/OVJ.2022.v12.i2.11 |