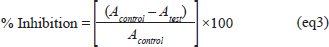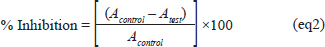| Research Article | ||
Open Vet. J.. 2024; 14(12): 3257-3268 Open Veterinary Journal, (2024), Vol. 14(12): 3257-3268 Research Article Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing toolJihen Taleb1,2, Saida Ncibi3, Sabah Dhibi2, Khaled Athmouni4, Najet Ghouaidia1, Aida Dhibi5, Abdurraouf Zaet6,7 and Lazhar Zourgui1*1Research Lab. BMA “Biodiversities, Molecules, Applications”, Higher Institute of Applied Biology of Medenine, University of Gabes, Medenine, Tunisia 2Laboratory of Environmental Biotechnologies and Oasis Ecosystem (BEEO), Gafsa, Tunisia 3Department of Biology, College of Science, Jazan University, P.O. Box 114, Jazan 45142, Kingdom of Saudi Arabia 4Department of Life Sciences, Laboratory of Biodiversity and Aquatic Ecosystems, Faculty of Sciences, University of Sfax, Sfax, Tunisia 5Computer Science Department, Science College, Northern Border University, Arar, Kingdom of Saudi Arabia 6Faculty of Medical Technology, University of Zawia, Zawia, Libya 7Biomedical Research Team, University of Zawia, Zawia, Libya *Corresponding Author: Lazhar Zourgui. Research Lab. BMA “Biodiversities, Molecules, Applications”, Higher Institute of Applied Biology of Medenine, University of Gabes, Medenine, Tunisia. Email: Lazhar.zourgui [at] gmail.com Submitted: 16/08/2024 Accepted: 11/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
AbstractBackground: Several studies reported the health benefits of Opuntia ficus indica extracts. Aim: This study aimed to (1) optimize extraction conditions. (2) Point out its antioxidant and cytoprotective potential against cadmium toxicity in rat liver homogenate cells and HepG2. Methods: This experiment used the response surface methodology. It helped to determine the optimum conditions necessary to get the highest phenolic compound yield from Opuntia ficus indica cladodes aqueous extract. Then, a chromatography analysis was conducted to determine the phenolic compounds present in this extract. To explore the antioxidant abilities of this extract, this study used superoxide radical and hydroxyl radicals scavenging assays. Finally, to explore its biological benefits and its protective effect against cadmium toxicity, these research investigators carried out (1) an in vitro protein glycation test, (2) MTT and inverted microscope imaging in HepG2 and HEK293 cell line, and (3) a lipid peroxidation test using rat erythrocytes. Results: The best extraction conditions were 10 minutes for the extraction time, 80°C for the temperature, and 30 ml/g for the liquid-solid ratio. The in vitro tests showed that Opuntia ficus indica aqueous extract has a high capacity to disable the free radical effect and prevent HepG2 and erythrocytes. Besides, the HPLC method identified 5 phenolic compounds within this extract. Conclusion: This study confirmed the health benefits of O. ficus indica. Thus, more attention should be given to investigating this plant’s capacities and isolating and identifying its bioactive molecules. Keywords: Antioxidant, Cytoprotective, Extraction, Optimization, Opuntia ficus indica cladode. IntroductionCadmium (Cd) is among the most poisonous heavy metals. Due to its chemical properties, Cd has several industrial uses (Belghith et al., 2016). In addition, it is a widely spread environmental pollutant (Athmouni et al., 2018a). Its residues are present in several food products; water, vegetables, meat, grains, cigarettes, and so on (Ruoyu et al., 2023). Extended cadmium exposure can induce various health damages, including liver and kidney injuries, and cardiovascular diseases (Maria et al., 2014; Gabr et al., 2017; Xiaoli et al., 2022). Many scientific reports associated health damage with cadmium-induced oxidative stress (Bojarski et al., 2022). Alternatively, natural phenolic compounds exhibit strong antioxidant potential that can help to go beyond oxidative stress damage. Several researchers confirm the ability of natural phenolic compounds to treat several acute and chronic damages in animals (Athmouni et al., 2018a; Waqas et al. 2023). Generally, free radicals and oxygen ions enhance oxidative damage when their production exceeds the antioxidant system (Wang et al., 2014; Harrabi et al., 2017). While phenolic compounds decrease ROS production and thus attenuate their harmful effects (da Silva et al., 2013; Hanganu et al., 2016; Wang et al., 2018). Cactus species are widely distributed in Latin America, South Africa, and the Mediterranean area. It is cultivated in arid and semi arid zones as a fruit and forage crop (Shirazinia et al., 2021). In Tunisia, this indigenous plant has several ecological (anti-erosive) and economic (fodder) roles. Furthermore, Tunisian folk medicine uses this plant to treat many diseases (Harrabi et al., 2017; Athmouni et al., 2018a). This plant showed a lot of benefits averse to free radical damage in various experimental models (Ben Saad et al., 2017; Smida et al., 2017). This study aimed to determine (1) the adequate extraction parameters; duration, water heat, liquid–solid ratio, to get the highest phenolic compounds yield from cactus cladodes aqueous extract, (2) to investigate the chemical characterization of this extract, and (3) to explore its possible antioxidant and cytoprotective potentials against cadmium-toxicity in HepG2 and HEK293 cells and rat erythrocyte cells. Material and MethodsPlant materialThe plant is from Elguettar, Gafsa, south of Tunisia. Cladodes were collected in the summer. Then they were washed using distilled water, cut, dehydrated at 37°C, and powdered. Then, distilled water was used to do the extraction from the mass of 400 g of the powder was macerated using as solvent. After extraction, the rotary evaporator was used to concentrate the aqueous extract at 40°C. The aqueous extract was kept at −20°C until use. Optimization of phenolic contentThe full factorial design (23) was used to determine the impact of time of extraction, temperature, and liquid-solid ratio on the phenolic extraction yield. The different variables were listed as follows: A=extraction time, B=decoction temperature, and C=liquid-solid ratio. This factorial design uses the following equation: xi=((Xi-Xz))/ΔXi;i=1,2,3,…,k (eq1) where, Xi is the real value of an independent variable, Xzis the real value of an independent variable at the center point, and ΔXi is the step change of the real value of the variable i. HPLC analysisThe phenolic compounds of this aqueous extract were identified high-performance liquid chromatography coupled with a UV diode array detector (Abdu Hussen et al., 2022). This system consisted of a vacuum degasser, an autosampler, and a binary pump with a maximum pressure of 400 bar; Agilent 1260, Agilent Technologies, Germany equipped with a reversed-phase C18 analytical column of 4.6 × 100 mm and 3.5 μm particle size (Zorbax Eclipse XDB C18). The DAD detector was set to scan from 200 to 400 nm. Column temperature was maintained at 25°C. The injected sample volume was 2 μl and the flow rate of the mobile phase was 0.4 ml/minute. Mobile phase B was milli-Q water consisting of 0.1% formic acid and mobile phase A was Methanol. The optimized gradient elution is illustrated as follows: 0–5 minutes, 10%–20% A; 5–10 minutes, 20%–30% A; 10–15 minutes, 30%–50% A; 15–20 minutes, 50%–70% A; 20–25 minutes, 70%–90% A; 25–30 minutes, 90%–50% A; 30–35 minutes, return to initial conditions. Standards were used for phenolic compound identification and quantification and a standard curve is provided. Scavenging of superoxide radical testThe ability of OFAE extract at different concentrations to scavenge the superoxide radical (O2•) in the reaction mixture (Beauchamp and Fridovich, 1971). Briefly, the superoxide anion was generated in a non-enzymatic system by reducing nitroblue tetrazolium. Briefly, 100 μl of the extract at different concentrations (0–1 mg/l) was mixed with 1 ml of phosphate buffer (50 mM). Then, 85 µl and 22µl of NBT (1 mM) and riboflavin were added to the reaction mixture, respectively. The absorbance was determined at 580 nm after illumination under a UV lamp for 10 minutes. The control tube contains all reagents except the sample volume, which is replaced by distilled water. The percentage of inhibition was expressed as the following equation:
Hydroxyl radicals (OH) scavenging assayThe method mentioned by Mathew and Abraham (2006) is used to study the ability of OFAE to inhibit the hydroxyl radical. Briefly, 1 ml of the OFAE (varying concentrations) was mixed with 1 ml phosphate buffer (20 mM, pH 7.4) containing ferric chloride (1 mM), EDTA (1 mM), deoxyribose (2.8 mM), 0.1 ml ascorbic acid (1 mM), and 0.5 ml hydrogen peroxide (20 mM). After, the solution mixture was incubated at 37°C for 1 hour. After incubation, TBA (1 ml 1%, w/v) and TCA (1 ml 2.8%, w/v) were added to the reaction mixture. After incubation at 100°C for 20 minutes the absorbance was readed at 532 nm. The inhibition rate was determined as follows:
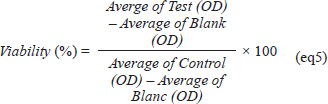
Acont is the absorbance of the control reaction and Atest is the absorbance of the extract reaction. The standard of this test is butylated hydroxytoluene BHT. Protein glycation assayThe capacity of OFAE extract to inhibit the protein glycation was determined according to the method developed by (Joe et al., 1996). Briefly, the solution reaction contains bovine serum albumin (BSA, 7 mg.ml-1) dissolved in phosphate buffer (50 mM, pH 7.4) and 0.02% (w/v) sodium azide. Then the reaction was mixed with the OFAE extract at different concentrations of 10, 20, 50, and 100 μg/ml for 30 minutes at room temperature. After incubation, 25 mM of glucose and fructose, were added to the reaction mixture, respectively. The fluorescence reader was used to determine the protein glycation rate and quercetin was used as standard (20 μg/ml). In vitro cytotoxicity assayCell cultureThe HepG2 and HEK293 cells were cultivated in MEM and DMEM-F12 medium, respectively containing RCS (10%), L-glutamine (1%), and gentamycin sulfate (50 µg/ml). The cells were incubated at 37°C in a CO2 incubator in an atmosphere of humidified 5% CO2 in 95% air. Determination of cytotoxicity testThe impact of OFAE extract and cadmium (Cd) on HepG2 and HEK293 cells line was determined according to the MTT test (Carmichael et al., 1987). After 24 hours of cell seeding, HepG2 and HEK293 cells were exposed to different concentrations of OFAE (50, 100, 150, 200, 300, and 400 µg ml-1) and CdCl2 (25 to 60 µM). MEM medium was used to prepare OFAE and CdCl2 concentrations. After incubation for 24 hours, the absorbance read at 570 nm against control cells. The cell viability was determining according the following formula:
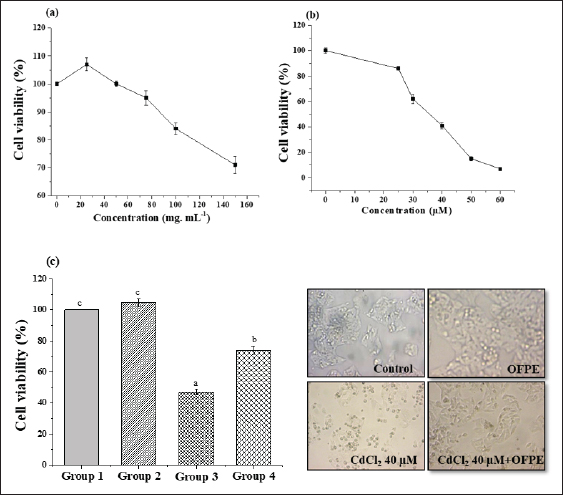
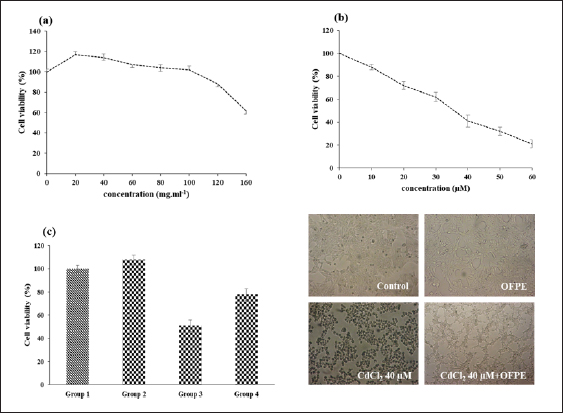
Cytoprotective potential of OFAEThe capacity of OFAE extract to protect exposed cells against oxidative damage enhanced by Cd exposure was evaluated by the method found by (Athmouni et al. 2018b). Briefly, the cells (1×105 cells/ml) seeded in a flask of 75 cm2. Then, HepG2 and HEK 293 cells pre-treated with OFAE (100 µg/ ml) diluted in MEM medium at 37°C for 24 hours. After incubation, 30 µM of CdCl2 was added to each flask. The cells without cadmium were considered as control cultures. The different groups are: -Group 1: Considered as normal cells treated with 100 µl of culture medium for 24 hours; -Group 2: The cells treated with OFAE (100 µl, 100 µg/ml) dissolved in culture medium. -Group 3: Considered as a stressed group treated with CdCl2 (30 µM) dissolved in medium culture. -Group 4: The cells pre-treated with OFAE (100µg/m) for 24 hours followed by exposure to CdCl2 (30 µM). After incubation, cells were fixed and stained with Giemsa solution as previously reported (Bovio et al., 2021). Then, the cell morphology was examined using a Zeiss microscope. In vitro protective effects of OFPE phenolic extractPreparation of erythrocytes suspensionThe blood was collected from normal rats. Briefly, erythrocytes were obtained from the plasma by centrifuging at 1200 × g for 10 minutes at 4°C. The erythrocytes were washed three times with PBS (0.1 µM NaCl, 2.7 KCl, 1.7 Kh2PO4, 10 mM Na2HPO4 pH 7.4). The 10% suspension of erythrocytes was obtained by resuspending the erythrocytes in PBS (pH 7.4) (Zhang et al. 2011). Protective effects against lipid peroxidationThe ability effect of the phenolic extract was determined against ferrous sulfate-induced oxidative stress in blood cells (Gutteridge and Halliwell 1990). In this study, the mean level of ferrous sulfate was about 50 µM. Briefly, 1 ml of phenolic extract at different concentrations was mixed with a test tube containing 200 µl of 10% (v/v) suspension of erythrocyte. To each tube, 50 µl of 0.07 M ferrous sulfate (in 0.1 M PBS) was added. The tube containing cells without phenolic extract served as a control. The BHT was used as a standard. After 30 minutes of incubation, each tube was centrifuged at 1200 × g for 10 minutes at 4°C. The pellet of each tube was used to evaluate the protective effect of phenolic extract against ferrous sulphate. Briefly, the erythrocytes pellet was ground in 1 ml TBS pH 7.4 using an Ultra-turax homogenizer. The homogenates contained in the tubes were centrifuged at 9,000 rpm at 4°C for 15 minutes, to recover the supernatant, which was rapidly stored at −20°C and used for TBARS assays. A volume of 375 µl of each tube was mixed with 375 µl of TCA (5%). The tubes were shaken and then centrifuged at 1000 rpm for 10 minutes. A volume of 200 µl of supernatant was taken, then 40 µl of HCl (0.6 M) and 160 µl of thiobarbituric acid were added. The contents were stirred again and incubated at 80°C for 10 minutes. The results were measured at 532 nm, and BHT was used as a standard. The % of inhibition was expressed as the following formula:
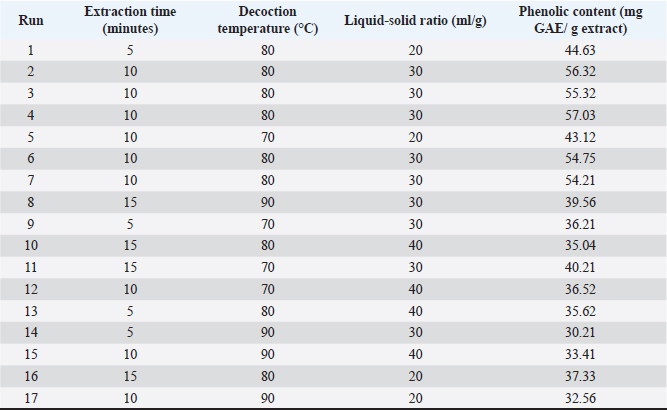
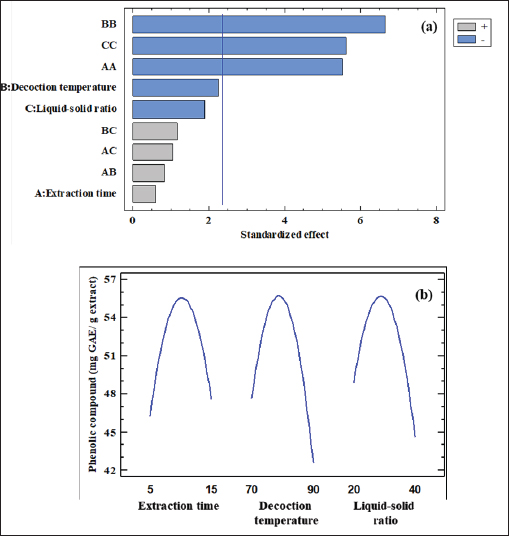
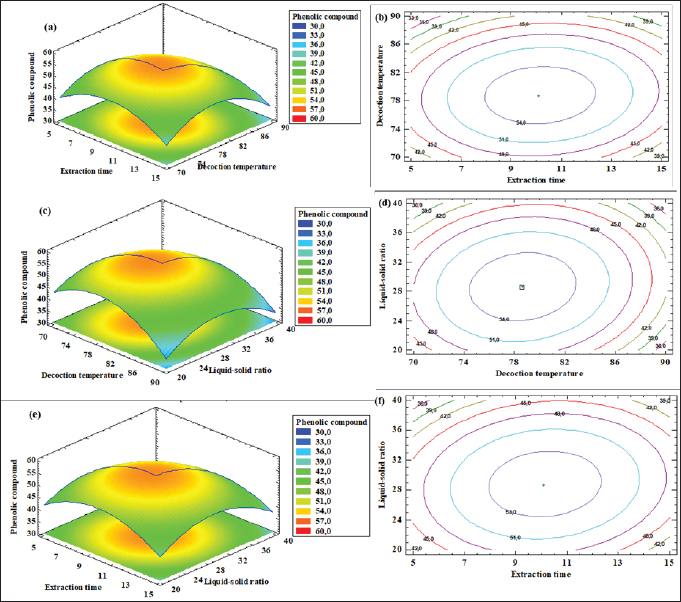
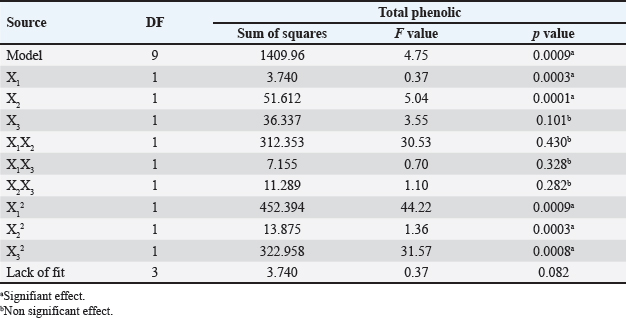
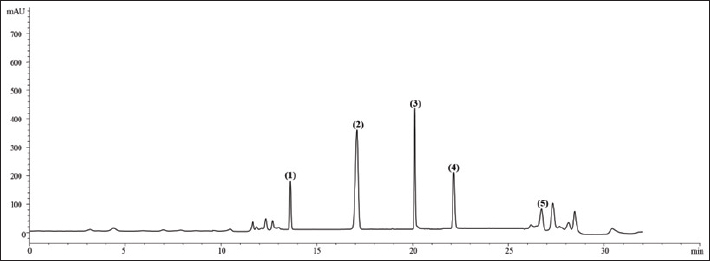
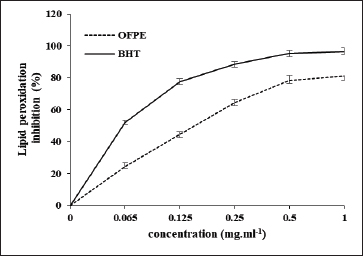
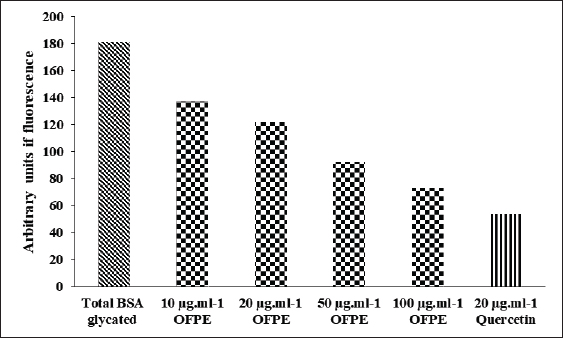
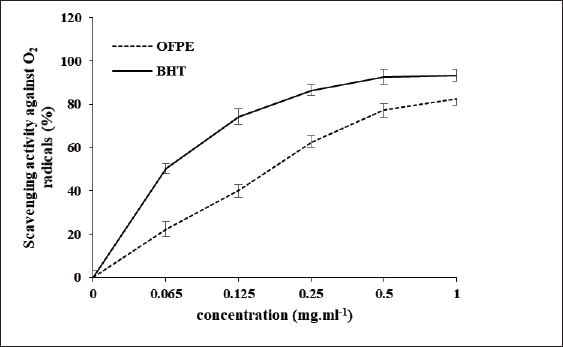
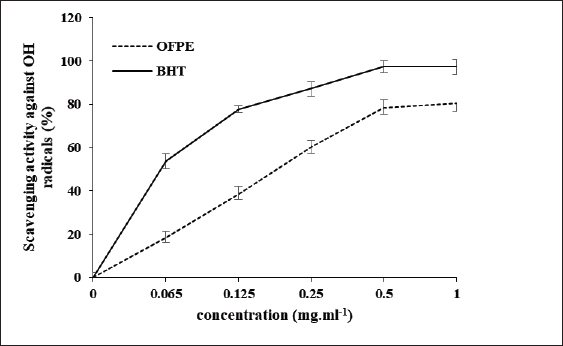
where Acont is the absorbance of the control reaction and Atest is the absorbance of the extract reaction. Statistical analysisData were expressed as the mean ± standard deviation and analyzed by one-way ANOVA according to SPSS software (version 20). The significant difference (p < 0.05) was separated using the Turkey post-hoc test. Ethical approvalThe experimental procedure (erythrocytes’ rat extraction) was carried out according to the Tunisian Code of Practice for the Care and Use of Animals for Scientific Purposes to get her with the European Convention for the Protection of Vertebrate animals used for experimental and other scientific purposes (Council of Europe No 123, Strasbourg, 1985). ResultsOptimization of OFAE extractionIn this experiment, the extraction yield is about 8.45%. Table 1 presents the total phenolic amount of this extract. It varied between 30.21 mg GAE/ g extract and 57.03 mg GAE/ g extract. Figures 1a and 1b illustrate the outcome of each parameter on the total phenolic amount and their interactions. Figure 1b stipulates the action of the extraction duration. This analysis demonstrates that the increment in the extraction time over 10 minutes lowers the phenolic content. In addition, temperatures up to 80°C, these elements level rise significantly. Higher than 80°C, the same levels decreased. Concerning liquid-solid ratio parameter, the highest phenolic content was at 30 ml/g. Figure 2 summarizes the influence of each parameter on phenolic compound extraction. Assessing the relationship of the studied factors, the best circumstances for obtaining the maximum concentration of phenolic compounds are 30 ml/g at 80°C for 10 minutes. The model is sufficient, as proven by the p-value of 0.0009 for the phenolic extraction yield progress. The actual interaction between the independent variables and response was satisfactorily validated by the model, as evidenced by its coefficient of determination (R2) of 0.912. In this study, the decoction temperature influences the phenolic concentration ( p < 0.0003). One important factor influencing the amount of phenolic compound was the liquid-solid ratio ( p < 0.0009) (Table 2). HPLC analysis of OFAE extractFigures 3a and 3b summarize the OFAE HPLC analysis result. 5 phenolic compounds were identified by the present analysis; Catechin, Caffeic acid, Ferulic acid, Rosmarinic acid, and Amentoflavone. These compounds were identified using relative pure standards at the same extract chromatography conditions. Inhibition of lipid peroxidation by OFAE The ability of OFAE extract to inhibit lipid peroxidation was evaluated. The lipid peroxidation inhibition varied between 45.9 to 86.2% (Figure 4). The control in this assay is BHT. Antioxidant capacity of OFAEFigures 6 and 7 represent the antioxidant capacity of OFAE againstO2•- and OH• free radicals. The antioxidant capacity against O2•-radicals varied between 45.23% and 86.15%. The OH• free radical inhibition rate varied between 37.21% and 82.45%. Inhibition of protein glycation by OFAEThe ability of OFAE extract to inhibit protein glycation was evaluated using a glycation system. The OFAE extract showed a strong ability to protect protein against glycation reactions at 10, 20, 50, and 100 µg/ml (Fig. 5). The control in this assay is Quercetin. Cytoprotective effect of OFAE against CdCl2Figures 8 and 9 show the ability of OFAE extract to protect HepG2 cells and HEK293. OFAE extract at different concentrations for 24 hours does not affect cell viability. Meanwhile, the Cd-exposure significantly decreased (0.01) viability when compared to control cells (Fig. 8b, 9b). Cells pre-treatment by OFAE at 100 µg/ml decreased significantly (p < 0.01) cell death compared with cadmium-treated cells (Figs. 8c and 9c). Besides, this result extract was proved by morphological structure images (Figs. 8d and 9d). The Cd-treated cells displayed signs of apoptosis, including shape irregularities and cell shrinkage. Table 1. Quadratic orthogonal rotation combination design and results.
Fig. 1. Standardized Pareto chart for efficiency (a), effects of four parameters variation (b), and surface response. DiscussionDue to their potential effects against environmental toxic substances and diverse pathologies, medicinal plants’ use has increased worldwide (Waisberg et al., 2003). Their beneficial effects investigation needs important quantities of flora and organic solvents. This can lead to economic and environmental problems. Therefore, research should focus on optimizing extraction conditions to guarantee the best extraction yield. This study aims to determine (1) the adequate extraction parameters; time of extraction, temperature, and liquid-solid ratio, to get the highest phenolic compounds to yield from Opuntia ficus indica cladodes aqueous extract (2) to characterize some compounds in this extract and (3) to explore its possible antioxidant and cytoprotective potentials against cadmium-toxicity in HepG2 and rat liver homogenate cells. Extraction conditions were manipulated using the surface methodology tool. By assessing the inter-relationship of the studied factors (Extraction time, temperature, and liquid-solid ratio) in the response of total phenolic content, the best conditions for extraction were 30 ml/g for 10 minutes at 80°C. Results showed an increase in the phenolic content with a temperature increase to 80°C. Further increases in temperature beyond 80°C resulted in a significant decrease. This result can be due to the induction of higher solubility of phenolic compound and lower density and viscosity of solvent at elevated temperature during extraction time (Xu et al., 2017). The presented results follow earlier studies. Indeed, (Chupin et al., 2013) proved that hot extraction water is better than cold water for phenolic compound extraction. Furthermore, they confirmed that these components’ extraction yields may be associated with various variables such as extraction time, temperature, and liquid-solid ratio (Chupin et al., 2013, Diwani et al., 2020).
Fig. 2. (a) The response surface plots of the effect of temperature, extraction time, and liquid-solid ratio on phenolic yield (%). Table 2. Statistical analysis of model.
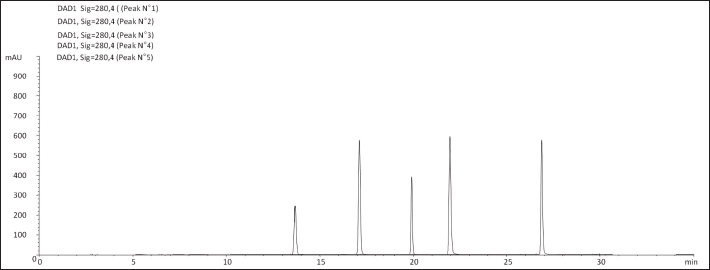
Fig. 3a. Chromatographic profiles acquired at 280 nm of O. ficus indica aqueous extract (OFAE). The identified compounds are 1: Catechin (Rt: 13.71 minutes); 2: Caffeic acid (Rt:17.424 minutes); 3: Ferulic acid (Rt:20.230 minutes); 4:Rosmarinic acid (Rt:21.753 minutes); 5: Amentoflavone (Rt:26.875 minutes). (Zorbax Eclipse XDB C18).
Fig. 3b. Chromatographic profiles acquired at 280 nm Standard of O. ficus indica aqueous extract (OFAE). The identified compounds are 1: Catechin (Rt: 13.71 minutes); 2: Caffeic acid (Rt:17.424 minutes); 3: Ferulic acid (Rt:20.230 minutes); 4:Rosmarinic acid (Rt:21.753 minutes); 5: Amentoflavone (Rt:26.875 minutes).
Fig. 4. Effect of different OFAE concentrations on lipid peroxidation. BHT was used as standard.
Fig. 5. Effect of different OFAE concentrations on protein glycation. Quercetin was used as standard.
Fig. 6. Scavenging effects on O2•-radical assay of OFAE extract at different concentrations compared to respective standards BHT. Values are presented as mean ± SD (n=3). HPLC analysis demonstrated that OFAE contains Catechin, Caffeic acid, Ferulic acid, Rosmarinic acid, and Amentoflavone. This result matches earlier research (Kolniak-Ostek et al., 2020). As presented this study revealed amentoflavone in the extract. This molecule is very important and it has variable bioactivities. In particular, amentoflavone mediates multiple signaling pathways, including apoptosis induction. Also, it exhibits anti-SARS-CoV-2 activity. Thus, this molecule is regarded as a medicinal drug with further clinical promise (Xiong et al., 2020). This study’s results demonstrated the free radical scavenging ability of cactus aqueous extract. This result is following previous studies (Athmouni et al., 2018a). Our previous studies, (Taleb et al., 2020) revealed that cactus extract exhibited a strong ability to scavenge,2-diphenyl-1-picrylhydrazyl and 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid. Also, previous investigations observed this cactus property (Chen et al., 2012).
Fig. 7. Scavenging effects on OH• radical assay of OFAE extract at different concentrations compared to respective standards BHT. Values are presented as mean ± SD (n=3).
Fig. 8. Cytoprotective activity of OFAE against Cd-induced toxicity in HepG2 cells line.
Fig. 9. Cytoprotective activity of OFAE against Cd-induced toxicity in HEK293 cells line. Furthermore, OFAE attenuates protein glycation. Previous research reported this result (Ben khedher et al., 2020; Abdnim et al., 2024). This attenuation is due to O. ficus indica fatty acids (Abdnim et al., 2024). After analyzing the OFAE composition and testing its antioxidant and anti-protein glycation capacities, this study investigated its ability to attenuate Cd-induced damage in HepG2, HEK293, and rat erythrocytes. Agency for Toxic Substances and Disease Registry classifies cadmium as a highly toxic molecule. Many health problems such as osteoporosis, immune dysfunction, and hepatic injuries are related to cadmium exposure (Xiaoli et al, 2022). This harm is induced via oxidative stress (Athmouni et al., 2018a). Unfortunately, cadmium exposure harms cannot be treated. However, prevention can help to manage this toxicity. Exposure to cadmium occurs through alimentation. Also, dietary and nutrition can either increase or decrease cadmium toxicity risk. This study emphasizes the role of O. ficus indica extract in alleviating Cd damages. In rat erythrocytes, this extract inhibits the lipid peroxidation. Lipid peroxidation frequently precedes permanent cell damage (Shirazinia et al., 2021). Cd-induced apoptosis in HEK293 is caspase-dependent and is due to mitochondrial dysfunction (Mao et al., 2007). This study confirms that Cd induces lipid peroxidation within erythrocytes. A previous study reports that this metal induces erythrocytes and nucleus erythrocytes width and length increase (Bojarski et al., 2022). Besides, chronic exposure to cadmium is correlated to increased risks of all-cause, cardiovascular, and Alzheimer’s disease mortality in patients with hypertension (Shuaijie et al., 2023). Thus, this metal becomes a health issue. Prevention methods need more research. Mainly prevention can be through a phenols and flavonoids rich alimentation. As presented, cactus is an important source of these components. This plant is widely present in North Africa. It is a future drug resource. It provides active biomolecules that can prevent several diseases, especially those induced by oxidative stress. ConclusionIn conclusion, this report has proven that the present work, the O. ficus indica phenolic was found to possess excellent antioxidant activities based on various in vitro assays. The different in vitro antioxidant tests proved that our plant is rich in phenolic compounds and was ameliorated using response surface methodology as an optimization tool. Therefore, OFAE protected HepG2 and HEK293 cells against Cd-induced injuries in cells. Also, Future research is needed to carry out further biochemical investigations to isolate and clarify the mechanism behind the activity of this extract. AcknowledgmentsThis research was funded by the Tunisian Ministry of Higher Education and Scientific Research through the Laboratory of Active Biomolecule Valorisation, the Higher Institute of Applied Biology of Medenine, Mednine, University of Gabes, Tunisia. FundingThis research received no specific grant. Authors’ contributionsThe concept and design of the study: J.T., S.N., L.Z. Collection and processing of material: L.Z., J.T., S.D. Statistical processing: J.T., K.A., L.Z., Text writing: J.T., S.D., L.Z. Editing: J.T., S.N., N.G., S.D., A.Z., L.Z. Supervision: L.Z. Conflict of interestThe authors declare that they have no competing interests and non-financial competing interests. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbdnim, R., Lafdil, F.Z., Elrherabi, A., El fadili, M., Kandsi, F., Benayad, W., Legssyer, A., Ziyyat, A., Mekhfi, H. and Bnouham, B. 2024. Fatty acids characterisation by GC-MS, antiglycation effect at multiple stages and protection of erythrocytes cells from oxidative damage induced by glycation of albumin of Opuntia ficus-indica (L.) Mill seed oil cultivated in Eastern Morocco: experimental and computational approaches. J. Ethnopharmacol. 329, 118106. Abdu Hussen, A. 2022. High-performance liquid chromatography (HPLC): a review. Ann. Adv. Chem. 6, 10–20. Athmouni, K., Belhaj, D., Mkadmini Hammi, K., El Feki, A. and Ayadi, H. 2018a. Phenolic compounds analysis, antioxidant, and hepatoprotective effects of Periploca angustifolia extract on cadmium-induced oxidative damage in HepG2 cell line and rats. Arch. Physiol. Biochem. 124, 261–274. Athmouni, K., Belhaj, D., El Feki, A. and Ayadi, H. 2018b. Optimization, antioxidant properties and GC–MS analysis of Periploca angustifolia polysaccharides and chelation therapy on cadmium-induced toxicity in human HepG2 cells line and rat liver. Int. J. Biol. Macromol. 108, 853–862. Beauchamp, C. and Fridovich, I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287. Belghith, T., Athmouni, K., Belhaj, D., El Feki, A. and Ayadi, H. 2016. Physiological and biochemical response of Dunaliella salina to cadmium pollution. J. Appl. Phycol. 28, 991–999. Ben Khedher, M.R., Hafsa, J., Haddad, M. and Hammami, M. 2020. Inhibition of protein glycation by combined antioxidant and antiglycation constituents from a phenolic fraction of sage (Salvia officinalis L.). Plant Foods Hum. Nutr.75, 505–511. Ben Saad, A., Dalel, B., Rjeibi, I., Smida, A., Ncibi, S., Zouari, N. and Zourgui, L. 2017. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium induced liver injury in rats. Pharm. Biol. 55, 516–525. Bojarski, B., Chmurska-Gąsowska, M., Gałuszka, A., Kotula-Balak, A., Trela, M., Kirpaniova, A., Kustra, K., Stonawski, B., Łapiński, S., Arent, Z. and Lis, M. 2022. Effects of embryonic cadmium exposure on erythrocyte indices and morphology in newly hatched Gallus gallus domesticus chicks. Poult. Sci. 101, 101862. Bovio, F., Melchioretto, P., Forcella, M., Fusi, P. and Urani, C. 2021. Cadmium promotes glycolysis upregulation and glutamine dependency in human neuronal cells. Neurochem. Int. 149, 105144. Carmichael, J., DeGraff, W.G., Gazdar, A.F., Minna, J.D. and Mitchell, J.B. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47, 936–942. Chen, Y.H., Yanm, Z.S., Wen, C.C., Chang, Y.S., Wang, B.C., Hsiao, C.A. and Shih, T.L. 2012. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 134, 717–724. Chupin, L., Motillon, C., Bouhtoury, F.C., Pizzi, A. and Charrier, B. 2013. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 49, 897–903. da Silva Morrone, M., de Assis, A.M., da Rocha, R.F., Gasparotto, J., Gazola, A.C. and Costa, G.M. 2013. Passiflora manicata (Juss.) aqueous leaf extract protects against reactive oxygen species and protein glycation in vitro and ex vivo models. Food Chem. Toxicol. 60, 45–51. Diwani, N., Fakhfakh, J., Athmouni, K., Belhaj, D., El Feki, A., Allouche, N., Ayadi, H. and Ketata, H. B. 2020. Optimization, extraction, structure analysis and antioxidant properties of flavan-3-ol polymers: proanthocyanidins isolated from Periploca angustifolia using surface response methodology. Ind. Crops Prod. 144, 112040. Gabr, S.A., Alghadir, A.H. and Ghoniem, G.A. 2017. Biological activities of ginger against cadmium-induced renal toxicity. Saudi J. Biol. Sci. 26, 382–389. Gutteridge, J.M.C. and Halliwell, B. 1990. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 15, 129–135. Hanganu, D., Olah inga, N.K., Benedec, D., Mocan, A., Crisan, G., Vlase, L., Julia, P. and Ilioara, O. 2016. Comparative polyphenolic content and antioxidant activities of Genista tinctoria L. and Genistella sagittalis (L.) Gams (Fabaceae). Pak J. Pharm. Sci. 29, 301–307. Harrabi, B., Athmouni, K., Hamdaoui, L., Ben Mahmoud, L., Hakim, A., El Feki, A., Zeghal, K. and Ghozzi, H. 2017. Polysaccharides extraction from Opuntia stricta and their protective effect against HepG2 cell death and hypolipidaemic effects on hyperlipidaemia rats induced by high-fat diet. Arch. Physiol. Biochem. 123, 225–237. Joe, A., Vinson and Thomas, B. and Howard, T.B.1996. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 7, 659–663. Kolniak-Ostek, J., Kita, A., Miedzianka, J., Andreu-Coll, L., Legua, P. and Hernandez, F. 2020. Characterization of bioactive compounds of Opuntia ficus-indica (L.) Mill. Seeds from Spanish Cultivars. Molecules 25(23), 5734. Mao, P., Ye, J.L., Guan, Z.P., Zhao, J.M., Zhang, C., Zhang, N.N., Jiang, P. and Tian, T. 2007. Cadmium induces apoptosis in human embryonic kidney (HEK) 293 cells by caspase-dependent and -independent pathways acting on mitochondria. Toxicol. Vitro 21(3), 343–354. Maria, T.P., Miranda, R. J., Alejandro D.L., Eliseo, G. and Ana, N.A. 2014. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr. Atheroscler. Rep. 15(10), 356. Mathew, S. and Abraham, T.E. 2006. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 94, 520–528. Ruoyu, W., Panting, S., Yahui, G., Ping, J., Yuliang, C., Hang, Y., Yunfei, X., Weirong, Y. and He, Q. 2023. Cadmium in food: source, distribution and removal. Food Chem. 405, 134666. Shirazinia, R., Golabchifar, A.A., Rahimi, V.B., Jamshidian, A., Samzadeh-Kermani, A., Hasanein, P., Hajinezhad, M. and Askari, V.R. 2021. Protective effect of Opuntia dillenii haw fruit against lead acetate-induced hepatotoxicity: in vitro and in vivo studies. Evid. Based Complement Alternat. Med. 2021, 6698345. Shuai, Z., Xinyun, D., Rongwei, M., Chunying, H., Fangchao, L., Long, S., Ying, Y. and Xu. M. 2023. A study of the association between husband′s smoking and wife′s risk of hypertension. Chin. J. Dis. Control Prev. 27(10), 1182–1187. Smida, A., Ncibi, S., Taleb, J., Saad, A.B., Ncibi, S. and Zourgui, L. 2017. Immunoprotective activity and antioxidant properties of cactus (Opuntia ficus indica) extract against chlorpyrifos toxicity in rats. Biomed. Pharmacother. 88, 844–851. Taleb, J., Ncibi, S., Smida, A., Mabrouki, L. and Zourgui, L. 2020. Evaluation of biological activities of Opuntia Ficus Indica cladodes extract against cadmium-induced osteoporosis in male Wistar rats. JAPST. 2, 26–39. Waisberg, M., Joseph, P., Hale, B. and Beyersmann, D. 2003. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 192, 95–117. Waqas, M., Salman, M. and Sharif, M.S. 2023. Application of polyphenolic compounds in animal nutrition and their promising effects. JAFS. 3, 233–256. Wang, T., Yang Li, Q. and Bi shun, K. 2018. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 13(1), 12–23. Wang, Y.F., Wang, J., Wu, J., Xu, P., Wang, Y.Q., Gao, J.J and Hochstetter, D. 2014. In vitro antioxidant activity and potential inhibitory action against α-glucosidase of polysaccharides from fruit peel of tea (Camellia sinensis L.). J. Zhejiang Univ. Sci. B. 15(2), 173–180. Xiaoli, Z. and Khaled, A. 2022. HPLC Analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules 27(15), 4972. Xiong, J., Lipsitz, O., Nasri, F., Jiaqi Xiong, A., Orly Lipsitz, C., Flora Nasri, C., Leanna, M.W., Gill, H., Phan, L., Chen-Li, D., Iacobucci, M., Ho, R., Majeed, A. and McIntyre, R.S. 2020. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 277, 55–64. Xu, D.P., Li, Y., Meng, X., Zhou, T., Zhou, Y., Zheng, J., Zhang, J.J. and Li, H.B. 2017. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 18(1), 96. Zhang, S. and Kuhn, J. R. 2011. Cell isolation and culture. In: WormBook: The online Review of C. elegans Biology [Internet]. Pasadena, CA: WormBook, pp: 2005–2018. | ||
| How to Cite this Article |
| Pubmed Style Taleb J, Ncibi S, Dhibi S, Athmouni K, Ghouaidia N, Dhibi A, Zaet A, Zourgui L. Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. Open Vet. J.. 2024; 14(12): 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 Web Style Taleb J, Ncibi S, Dhibi S, Athmouni K, Ghouaidia N, Dhibi A, Zaet A, Zourgui L. Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. https://www.openveterinaryjournal.com/?mno=215882 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.11 AMA (American Medical Association) Style Taleb J, Ncibi S, Dhibi S, Athmouni K, Ghouaidia N, Dhibi A, Zaet A, Zourgui L. Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. Open Vet. J.. 2024; 14(12): 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 Vancouver/ICMJE Style Taleb J, Ncibi S, Dhibi S, Athmouni K, Ghouaidia N, Dhibi A, Zaet A, Zourgui L. Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 Harvard Style Taleb, J., Ncibi, . S., Dhibi, . S., Athmouni, . K., Ghouaidia, . N., Dhibi, . A., Zaet, . A. & Zourgui, . L. (2024) Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. Open Vet. J., 14 (12), 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 Turabian Style Taleb, Jihen, Saida Ncibi, Sabah Dhibi, Khaled Athmouni, Najet Ghouaidia, Aida Dhibi, Abdurraouf Zaet, and Lazhar Zourgui. 2024. Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. Open Veterinary Journal, 14 (12), 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 Chicago Style Taleb, Jihen, Saida Ncibi, Sabah Dhibi, Khaled Athmouni, Najet Ghouaidia, Aida Dhibi, Abdurraouf Zaet, and Lazhar Zourgui. "Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool." Open Veterinary Journal 14 (2024), 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 MLA (The Modern Language Association) Style Taleb, Jihen, Saida Ncibi, Sabah Dhibi, Khaled Athmouni, Najet Ghouaidia, Aida Dhibi, Abdurraouf Zaet, and Lazhar Zourgui. "Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool." Open Veterinary Journal 14.12 (2024), 3257-3268. Print. doi:10.5455/OVJ.2024.v14.i12.11 APA (American Psychological Association) Style Taleb, J., Ncibi, . S., Dhibi, . S., Athmouni, . K., Ghouaidia, . N., Dhibi, . A., Zaet, . A. & Zourgui, . L. (2024) Cytoprotective potential of Opuntia ficus indica cladode extract against cadmium toxicity in HepG2 and HEK293 cell lines using surface methodology as an optimizing tool. Open Veterinary Journal, 14 (12), 3257-3268. doi:10.5455/OVJ.2024.v14.i12.11 |