| Case Report | ||
Open Vet. J.. 2024; 14(12): 3656-3664 Open Veterinary Journal, (2024), Vol. 14(12): 3656-3664 Case Report First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from BulgariaKrasimira Gospodinova1, Vladimir Petrov1, Iskren Stanilov2, Lyuba Miteva2, Ilia Tsachev1 and Magdalena Baymakova3*1Department of Veterinary Microbiology, Infectious and Parasitic Diseases, Faculty of Veterinary Medicine, Trakia University, Stara Zagora, Bulgaria 2Department of Molecular Biology, Immunology and Medical Genetics, Faculty of Medicine, Trakia University, Stara Zagora, Bulgaria 3Department of Infectious Diseases, Military Medical Academy, Sofia, Bulgaria *Corresponding Author: Magdalena Baymakova. Department of Infectious Diseases, Military Medical Academy, Sofia, Bulgaria. Email: dr.baymakova [at] gmail.com Submitted: 31/08/2024 Accepted: 06/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
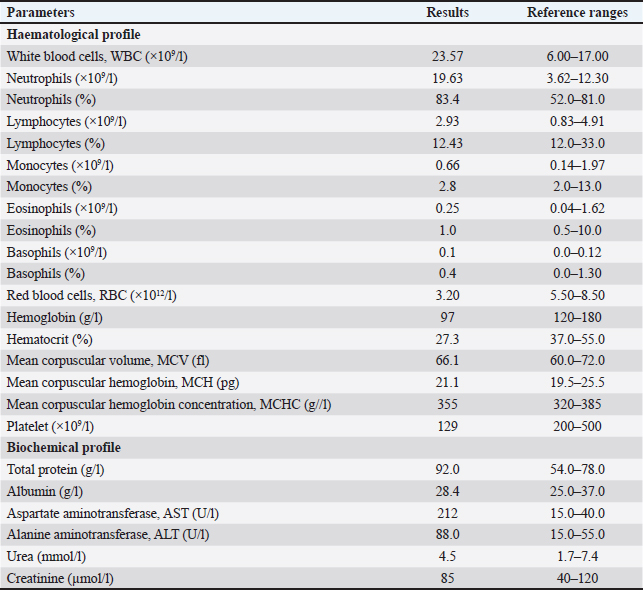
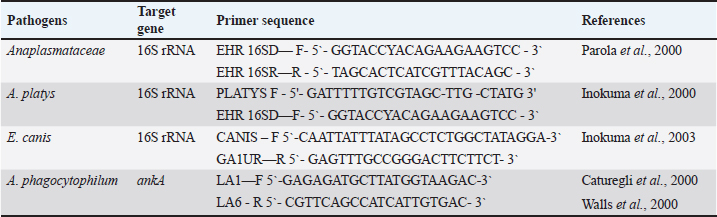
AbstractBackground: In recent years, the One Health approach and vector-borne diseases have become an increasingly topical problem around the world. In addition, climate change has a significant impact on zoonoses and public health. We present a case report of tick-borne disease in a dog. Case Description: A clinical case of Anaplasma platys infection in a 10-year-old female dog (Labrador retriever) is described. Clinical, hematological, biochemical, serological, cytological, and polymerase chain reaction tests supporting the diagnosis have been performed. Conclusion: To the best of our knowledge, this is the first report of A. platys from Bulgaria. This report adds to the overall knowledge of Anaplasma spp. in our country and the region of Southeastern Europe. Keywords: Anaplasma platys, Bulgaria, Dog, Molecular evidence. IntroductionAnaplasma platys (formerly Ehrlichia platys), responsible for canine infectious cyclic thrombocytopenia is an example of a tick-borne pathogen with zoonotic potential (Breitschwerdt et al., 2014). These microorganisms belong to the family Anaplasmataceae, order Rickettsiales, and it is gram-negative, obligate intracellular bacteria whose environmental cycle involves complex interactions between invertebrate vectors and vertebrate hosts (Granick et al., 2023). This bacterium is mainly transmitted by the members of the Rhipicephalus sanguineus group (Nava et al., 2018). Ticks serve as essential biological vectors in the spread and reproduction of A. platys during different stages of its life cycle (Snellgrove et al., 2020). Anaplasma platys infects canine platelets and is the causative agent of the infectious canine cyclic thrombocytopenia (Harvey et al., 1978). It is also important to highlight that this pathogen does not only infect dogs, it also infects other hosts—bactrian camel (Li et al., 2015), cats (Lima et al., 2010), cattle (Dahmani et al., 2015), collared peccary (Pecari tajacu) (Rojas-Jaimes and Del Valle-Mendoza, 2023), dromedary camel (Bastos et al., 2015), goats (Ben Said et al., 2017), red fox (Cardoso et al., 2015), roe deer (Remesar et al., 2022), sheep (Ben Said et al., 2017), striped field mouse (Apodemus agrarius) (Kim et al., 2006), and water buffalo (Nguyen et al., 2020). Although the role of A. platys as a zoonotic agent has not been conclusively proven, it is currently believed that A. platys can infect humans (CDC, 2024). There are reports suggesting potential zoonotic transmission of A. platys to humans (Maggi et al., 2013; Arraga-Alvarado et al., 2014; Breitschwerdt et al., 2014). This is supported by the fact that A. platys DNA was sequenced from two human cases and was identical to that found in their respective dogs (Breitschwerdt et al., 2014). The infection is typically asymptomatic or mild but can be fatal due to severe thrombocytopenia and subsequent hemorrhage (Diniz and Moura de Aguiar, 2022). Clinical symptoms vary but are mainly animal emaciation, lethargy, anorexia, respiratory distress, fever, increased mucous secretion, purulent discharge from the eyes, uveitis splenomegaly and hyperkeratosis of the muzzle (Sainz et al., 2015; Nimsuphan et al., 2020; Atif et al., 2021). Anaplasma platys was first discovered in 1978, in the United States (US) by Harvey et al. (1978) and his scientific team (Harvey et al., 1978). Since then, it has also been identified in Africa, Asia, Australia, Central America, and South America (Eiras et al., 2013; Soares et al., 2017; Atif et al., 2021). In Southern Europe, the presence of A. platys has been confirmed in several countries, particularly within the Mediterranean basin and the Balkan peninsula—Albania (Hamel et al., 2016), Bosnia and Herzegovina (Maksimovic et al., 2022), Croatia (Dyachenko et al., 2012), Cyprus (Bouzouraa et al., 2016), Greece (Kontos et al., 1991), Italy (de la Fuente et al., 2006), Portugal (Cardoso et al., 2010), Romania (Andersson et al., 2013), Serbia (Ilic Bozovic et al., 2021), Spain (Perez Perez et al., 2021), and Türkiye (Cetinkaya et al., 2016). In Bulgaria, several studies have investigated Anaplasma spp. in various hosts, including—ticks (Christova et al., 2003; Nader et al., 2018; Stanilov et al., 2023; Polsomboon Nelson et al., 2024), rodents (Christova and Gladnishka, 2005), dogs (Tsachev et al., 2008; Pantchev et al., 2015; Iliev et al., 2020; Manev, 2020; Gospodinova et al., 2024), and horses (Tsachev et al., 2018; Tsachev et al., 2019). While all these articles reported on Anaplasma phagocytophilum or Anaplasma spp., there has been no research from Bulgaria documenting the presence of A. platys. In this study, we present, for the first time, polymerase chain reaction (PCR)-based assays for detecting A. platys infection in dogs from Bulgaria. Case DetailsA 10-year-old female dog (Labrador retriever) was admitted for medical examination at the Small Animal Clinic, University Veterinary Hospital, Stara Zagora, Bulgaria, in August, 2022. Owners reported fever, fatigue, weight loss, skin lesions, sores on lower extremities, and tick bites about 2 weeks ago. We did not have access to these ticks. Written informed consent for participation in the current report was obtained from the dog owner. The present case description was approved by the Local Ethics Committee at Trakia University, Stara Zagora, Bulgaria (FVM-09 / 09 June 2020). A blood sample was obtained from the vena cephalica antebrachii externa using vacuum containers with EDTA. The blood was used for hematological and biochemical tests. The labrador‘s hematological examinations showed deviations in the values of indicators from the red and white blood count: thrombocytopenia, leukocytosis, erythropenia, neutrophilia, hemoglobinemia, and decreased hematocrit (Table 1). Furthermore, we also observed deviations in the biochemical indicators—increased levels of liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and increased levels of total protein and globulins, without changes in the levels of the albumin fraction. A blood smear from the buffy coat, stained with commercial kit Hemacolor Stain Set (Sigma-Aldrich, Merck, USA) was made for cytological investigation. On microscopic examination, A. platys morulae were not observed in platelets. The clinical signs and symptoms, along with deviations in hematological and biochemical data, provided justification to perform serological and subsequent molecular genetic assays to confirm infection with bacteria from the family Anaplasmataceae. The blood sample was initially tested for the presence of IgG-specific antibodies against A. phagocytophilum/A. platys, and Borrelia burgdorferi, as well as for the specific antigen of Dirofilaria immitis using a SNAP immunoassay (SNAP 4Dx Plus, IDEXX Laboratories, Inc. USA). The test was positive for antibodies against Anaplasma spp. Subsequently, a blood sample was used for molecular analysis for the presence of A. phagocytophilum, A. platys, and Ehrlichia canis DNA. DNA was extracted from blood samples by the column extraction method using the High Pure PCR Template Preparation Kit (Roche Diagnostic, Mannheim, Germany) following the manufacturer’s instructions. The extracted DNA was stored at −20.0°C until PCR analysis. To minimize the chances of contamination, DNA extraction was performed under a biosafety hood in a dedicated room, using equipment exclusively reserved for DNA extraction. The gradient PCR was conducted to optimize the annealing temperatures for any PCR (AERIS PCR system, Esco, Singapore). The DNA sample was then tested for the presence of the 16S rRNA gene specific to the family Anaplasmataceae, with a conventional PCR as previously described by Gospodinova et al. (2024). Further screening was performed, using genus-specific primers (Parola et al., 2000) (Table 2). A second PCR was performed using the A. platys species-specific primers targeting a 678 bp amplicon of the 16S rRNA gene. The A. platys specific forward primer (Table 2) was combined with the reverse primer from the first round of PCR (Inokuma et al., 2000). Each PCR run included both a positive and a negative control. DEPC-treated water was used as a template in the negative control, while positive A. platys genomic DNA was used for the positive control. The positive A. platys genomic DNA was kindly provided by Prof. Handan Cetinkaya from the Department of Parasitology, Faculty of Veterinary Medicine, Istanbul University, Türkiye. The PCR reaction mixture contained 10 µl ready for use buffer mix (KAPA2G Robust Hot Start ReadyMix with dye 2x; Roche Diagnostics, Mannheim, Germany) with 5U Taq DNA polymerase, 3 mM MgCl2, and 1 mM dNTPs, 10 pmol of each primer, 2 µl of DNA template and sterile distilled water to a final volume of 20 µl. The amplification was performed in an Aeris PCR Thermal Cycler (Esco Micro Pte Ltd, Singapore) at the appropriate thermal cycling parameters as follows: initial denaturation for 1 minute at 95.0°C, followed by 40 cycles of 30 seconds at 94.0°C, 30 seconds at 55.0°C, 30 seconds at 72.0°C, and a final extension for 5 minutes at 72.0°C. Table 1. Laboratory indicators in 10-year-old female dog (Labrador retriever) from Bulgaria.
Table 2. A family-specific primer set for Anaplasmataceae and species-specific primer sets for A. platys, E. canis, and A. phagocytophilum.
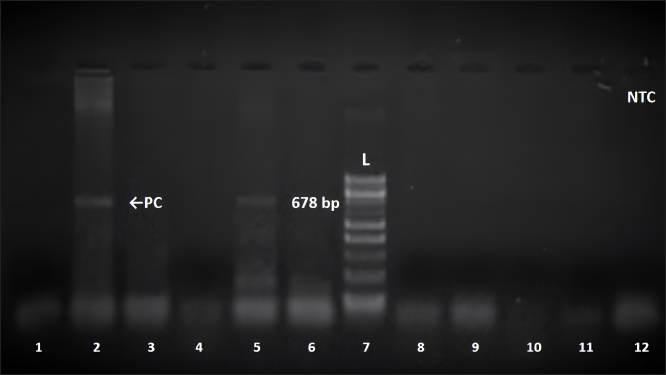
Subsequently, samples were tested with a third and fourth PCR assay using species-specific primer sets for E. canis and A. phagocytophilum, as presented in Table 2 and previously described by Inokuma et al. (2003), Caturegli et al. (2000), and Walls et al. (2000). The same procedure and conditions described by Gospodinova et al. (2024) were followed. Amplicons were detected by electrophoresis on a 1.5% agarose gel stained with ethidium bromide (0.5 mg/ml). A DNA Ladder (by 100 bp, ranging from 100 to 1,000 bp) was included to confirm the expected amplicon size. The results of the PCR amplification were visualised under UV light and documented using the Herolab documentation system and EasyWin32 software (Herolab GmbH Laborgeräte, Germany). To minimize the chances of contamination, all manipulations—DNA extraction, fragment amplification, and agarose gel electrophoresis were performed in separate rooms with equipment used exclusively for each step of molecular genetic investigation. A 678 bp fragment of 16S rRNA gene sequence characteristic of A. platys was only detected in the sample from the presented case report (Fig. 1). However, the differentiation of acute infections with A. platys from A. phagocytophilum was performed using conventional PCR-based methods with high sensitivity and specificity, the main limitation of our study should be pointed out. We cannot provide the sequence data and sequencing should be considered in future studies of A. platys. Additionally, the patient was tested for E. canis and A. phagocytophilum—the PCR tests were negative. On the basis of the performed examination, a diagnosis of A. platys infection was made. A doxycycline therapy (10 mg/kg) for 28 days was prescribed. In conclusion, we observed a good therapeutic outcome. DiscussionTick-borne diseases are of increasing importance in human and veterinary medicine (Vayssier-Taussat et al., 2015). The number of pathogens transmitted by ticks is higher than by any other arthropod. In Europe and other temperate zones, ticks are considered the most serious arthropod zoonotic vectors (Munderloh, 2017; Andersson et al., 2018; Kramer et al., 2020). This research presents the first molecular evidence of A. platys infection in а dog from Bulgaria. Anaplasma platys specific conventional PCR revealed a 678 bp fragment of the 16S rRNA gene of A. platys in the dog. The use of PCR allows the detection of acute A. platys infection earlier than by serology or microscopy (Lara et al., 2020). Molecular tests confirmed the current A. platys infection in the dog. From the hematological characteristics in the present case (increased WBC, neutrophils; decreased RBC, platelet, hemoglobin), only anemia and thrombocytopenia are common, major abnormal clinicopathological findings observed in A. platys infected dogs, but the severity of hematological abnormalities can be used to predict disease outcome (Piratae et al., 2019). Thrombocytopenia due to A. platys infection is thought to result from platelet destruction by the proliferating pathogen during the initial phase of infection (Dyachenko et al., 2012; Bouzouraa et al., 2016; Ilic Bozovic et al., 2021).
Fig. 1. Amplification of the 16S rRNA gene of A. platys with PLATYS F/EHR16SR primers with approximate size 678 bp. Position 2=positive control (PC); position 12=negative control (NTC); position 7=100 bp DNA ladder; position 5=positive sample; position 1,3,4,6,8,9,10,11=negative samples. Elevated concentration levels of liver transaminases were reported by Bouzouraa et al. (2016), who found an increase in the value of alkaline phosphatase (AP) or ALT in 6 of the 10 examined dogs mono- or co-infected with A. platys (Bouzouraa et al., 2016). The mechanisms of this phenomenon were not discussed by the authors (Bouzouraa et al., 2016). In our patient, we also found elevated AST and ALT levels. Commercially available point-of-care serological tests are commonly used in pet clinics as a diagnostic tool. They are used to detect antibodies against rickettsiae of the family Anaplasmataceae in clinically suspected dogs (Stillman et al., 2014; Liu et al., 2018). However, they only provide information about pathogen encounters but cannot detect active infection (Nair et al., 2016). Furthermore, SNAP 4Dx Plus Test (IDEXX Laboratories, Inc. USA) detects antibodies against the genus Anaplasma without differentiating A. phagocytophilum antibodies from those against A. platys (Evason et al., 2019). Nucleic acid-based methods allow for the detection and identification of species by using species-specific primers with high sensitivity and specificity (Silvestrini et al., 2023). Microscopy of stained blood smears, a method also suitable for in-clinic diagnosis, is often unreliable. Direct visualization of A. platys in blood smears can be difficult and time-consuming, with low sensitivity, as they are only detectable in the initial phase of the infection (Sykes, 2023). Outcomes are highly dependent on the amount of target cells (degree of thrombocytopenia), levels of bacteremia, and infectious status. For A. platys, light microscopic test shows low sensitivity also due to the cyclic nature of thrombocytopenia and the low percentage of infected cells (between 0.5% and 5.0%), recommending to examine between 2,000 and 20,000 platelets (Silaghi et al., 2017). In addition, Lara et al. (2020) reported that A. platys morula-like structures were occasionally observed in platelets of serologically negative, clinically suspected patients. The authors report that 13 samples showed structures resembling the morula of A. platys, with 61% confirmed as positive by PCR (Lara et al., 2020). In this regard, false-negative results from microscopic tests can be detrimental to the patient’s health, while false-positive results can lead to unnecessary antibiotic treatment (Lara et al., 2020). Тhe diagnosis of A. platys-related diseases in pet clinics can be challenging due to the complex pathogenesis, broad and non-specific clinical symptoms, as well as possible occurrence of co-infection with A. phagocytophilum (de Caprariis et al., 2011). In contrast to serological tests, PCR allows the differentiation of active infections with A. platys from those with A. phagocytophilum. The conventional PCR protocol, including amplification using a set of Anaplasmataceae family-specific primers, followed in a second step by primers specific for a 678 bp fragment of the 16S rRNA gene of A. platys should be used (Ferreira et al., 2007). Serological and DNA-based tests have complementary roles in the diagnosis of infectious diseases (Martinescu et al., 2023). Although no data have been published so far, the finding of A. platys in Bulgaria is not surprising. This pathogen was discovered in a number of Mediterranean countries (Rymaszewska and Grenda, 2008; Rene-Martellet et al., 2015). Infections of A. platys were also confirmed in several countries of the Balkan peninsula—Albania (Hamel et al., 2016), Bosnia and Herzegovina (Maksimovic et al., 2022), Croatia (Dyachenko et al., 2012; Huber et al., 2017), Greece (Kontos et al., 1991), Romania (Andersson et al., 2013), Serbia (Ilic Bozovic et al., 2021), and the European part of Türkiye (Cetinkaya et al., 2016). The vector of this infectious agent R. sanguineus is apparently the most prevalent tick species on dogs in Bulgaria (Panayotova-Pencheva et al., 2021). The present case report and the data by other authors from the Balkan peninsula suggest that A. platys is probably a more common pathogen in this part of the world than previously thought. This hypothesis can be confirmed or rejected by conducting large-scale future studies on A. platys infection among different animal species from Southeastern Europe. We identified an A. platys infection in a dog in Bulgaria for the first time using PCR assay. The reliable diagnosis provided by molecular methods is imperative for veterinarians in deciding whether to treat a seropositive or clinically suspected dog in disease-endemic areas. AcknowledgmentsThe authors thank Prof. Handan Cetinkaya (Istanbul University, Turkey) for their technical support and assistance. Furthermore, the authors thank their families for providing them with the time and support required to complete this report in a timely manner. Conflict of interestThe authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. FundingThis work was supported and funded by the Bulgarian Ministry of Education and Science in the frames of the Bulgarian National Recovery and Resilience Plan, Component “Innovative Bulgaria”, Project No. BG-RRP-2.004- 0006-C02 “Development of research and innovation at Trakia University in service of health and sustainable well-being”. Authors’ contributionsKG—conceptualization, methodology, data curation, data interpretation, writing (original draft preparation), and visualization. VP—conceptualization, data collection, and project administration. IS—methodology, data curation, and visualization. LM—methodology, data interpretation, writing (review and editing), and visualization. IT—conceptualization, data interpretation, writing (review and editing), supervision, and funding acquisition. MB—data interpretation, writing (review and editing), and supervision. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAndersson, M., Turcitu, M.A., Stefanache, M., Tamba, P., Barbuceanu, F. and Chitimia, L. 2013. First evidence of Anaplasma platys and Hepatozoon canis co-infection in a dog from Romania—a case report. Ticks Tick Borne Dis. 4, 317–319. Andersson, M.O., Marga, G., Banu, T., Dobler, G. and Chitimia-Dobler, L. 2018. Tick-borne pathogens in tick species infesting humans in Sibiu County, Central Romania. Parasitol. Res. 117, 1591–1597. Arraga-Alvarado, C.M., Qurollo, B.A., Parra, O.C., Berrueta, M.A., Hegarty, B.C. and Breitschwerdt, E.B. 2014. Case report: molecular evidence of Anaplasma platys infection in two women from Venezuela. Am. J. Trop. Med. Hyg. 91, 1161–1165. Atif, F.A., Mehnaz, S., Qamar, M.F., Roheen, T., Sajid, M.S., Ehtisham-Ul-Haque, S., Kashif, M. and Ben Said, M. 2021. Epidemiology, diagnosis, and control of canine infectious cyclic thrombocytopenia and granulocytic anaplasmosis: emerging diseases of veterinary and public health significance. Vet. Sci. 8, 312. Bastos, A.D., Mohammed, O.B., Bennett, N.C., Petevinos, C. and Alagaili, A.N. 2015. Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Vet. Microbiol. 179, 310–314. Ben Said, M., Belkahia, H., El Mabrouk, N., Saidani, M., Alberti, A., Zobba, R., Cherif, A., Mahjoub, T., Bouattour, A. and Messadi, L. 2017. Anaplasma platys-like strains in ruminants from Tunisia. Infect. Genet. Evol. 49, 226–233. Bouzouraa, T., Rene-Martellet, M., Chene, J., Attipa, C., Lebert, I., Chalvet-Monfray, K., Cadore, J.L., Halos, L. and Chabanne, L. 2016. Clinical and laboratory features of canine Anaplasma platys infection in 32 naturally infected dogs in the Mediterranean basin. Ticks Tick Borne Dis. 7, 1256–1264. Breitschwerdt, E.B., Hegarty, B.C., Qurollo, B.A., Saito, T.B., Maggi, R.G., Blanton, L.S. and Bouyer, D.H. 2014. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit. Vectors 7, 298. Cardoso, L., Gilad, M., Cortes, H.C., Nachum-Biala, Y., Lopes, A.P., Vila-Vicosa, M.J., Simoes, M., Rodrigues, P.A. and Baneth, G. 2015. First report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasit. Vectors 8, 144. Cardoso, L., Tuna, J., Vieira, L., Yisaschar-Mekuzas, Y. and Baneth, G. 2010. Molecular detection of Anaplasma platys and Ehrlichia canis in dogs from the North of Portugal. Vet. J. 183, 232–233. Caturegli, P., Asanovich, K.M., Walls, J.J., Bakken, J.S., Madigan, J.E., Popov, V.L. and Dumler, J.S. 2000. ankA: An Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect. Immun. 68, 5277–5283. CDC, 2024. Anaplasmosis: epidemiology and statistics (last reported year: 2021). Atlanta, Georgia: CDC. Available via https://www.cdc.gov (Accessed 30 August 2024). Cetinkaya, H., Matur, E., Akyazi, I., Ekiz, E.E., Aydin, L. and Toparlak, M. 2016. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace region of Turkey. Ticks Tick Borne Dis. 7, 706–714. Christova, I. and Gladnishka, T. 2005. Prevalence of infection with Francisella tularensis, Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in rodents from an endemic focus of tularemia in Bulgaria. Ann. Agric. Environ. Med. 12, 149–152. Christova, I., Van De Pol, J., Yazar, S., Velo, E. and Schouls, L. 2003. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group Rickettsiae in ticks from Southeastern Europe. Eur. J. Clin. Microbiol. Infect. Dis. 22, 535–542. Dahmani, M., Davoust, B., Benterki, M.S., Fenollar, F., Raoult, D. and Mediannikov, O. 2015. Development of a new PCR-based assay to detect Anaplasmataceae and the first report of Anaplasma phagocytophilum and Anaplasma platys in cattle from Algeria. Comp. Immunol. Microbiol. Infect. Dis. 39, 39–45. de Caprariis, D., Dantas-Torres, F., Capelli, G., Mencke, N., Stanneck, D., Breitschwerdt, E.B. and Otranto, D. 2011. Evolution of clinical, haematological and biochemical findings in young dogs naturally infected by vector-borne pathogens. Vet. Microbiol. 149, 206–212. de la Fuente, J., Torina, A., Naranjo, V., Nicosia, S., Alongi, A., La Mantia, F. and Kocan, K.M. 2006. Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet. Res. 2, 24. Diniz, P.P.V.P. and Moura de Aguiar, D. 2022. Ehrlichiosis and Anaplasmosis: an update. Vet. Clin. North Am. Small Anim. Pract. 52, 1225–1266. Dyachenko, V., Pantchev, N., Balzer, H.J., Meyersen, A. and Straubinger, R.K. 2012. First case of Anaplasma platys infection in a dog from Croatia. Parasit. Vectors 5, 49. Eiras, D.F., Craviotto, M.B., Vezzani, D., Eyal, O. and Baneth, G. 2013. First description of natural Ehrlichia canis and Anaplasma platys infections in dogs from Argentina. Comp. Immunol. Microbiol. Infect. Dis. 36, 169–173. Evason, M., Stull, J.W., Pearl, D.L., Peregrine, A.S., Jardine, C., Buch, J.S., Lailer, Z., O’Connor, T., Chandrashekar, R. and Weese, J.S. 2019. Prevalence of Borrelia burgdorferi, Anaplasma spp., Ehrlichia spp. and Dirofilaria immitis in Canadian dogs, 2008 to 2015: a repeat cross-sectional study. Parasit. Vectors 12, 64. Ferreira, R.F., Cerqueira, A.D.M.F., Pereira, A.M., Guimaraes, C.M., de Sa, A.G., Abreu, F.D.S., Massard, C.L. and Almosny, N.R.P. 2007. Anaplasma platys diagnosis in dogs: Comparison between morphological and molecular tests. Intern. J. Appl. Res. Vet. Med. 5, 113–119. Gospodinova, K., Stanilov, I., Miteva, L., Tsachev, I. and Petrov, V. 2024. Molecular detection of Ehrlichia canis and Anaplasma phagocytophilum in blood samples from dogs in Bulgaria. Bulg. J. Vet. Med. 27, 375–386. Granick, J., Lappin, M.R., Waner, T., Harrus, S. and Mylonakis, M.E. 2023. Anaplasmosis. 5th ed. In: Sykes JE (ed). Greene’s infectious diseases of the dog and cat. Saunders Elsevier, St. Louis, Missouri, USA, 542–554. Hamel, D., Shukullari, E., Rapti, D., Silaghi, C., Pfister, K. and Rehbein, S. 2016. Parasites and vector-borne pathogens in client-owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitol. Res. 115, 489–499. Harvey, J.W., Simpson, C.F. and Gaskin, J.M. 1978. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J. Infect. Dis. 137, 182–188. Huber, D., Reil, I., Duvnjak, S., Jurkovic, D., Lukacevic, D., Pilat, M., Beck, A., Mihaljevic, Z., Vojta, L., Polkinghorne, A. and Beck R. 2017. Molecular detection of Anaplasma platys, Anaplasma phagocytophilum and Wolbachia sp. but not Ehrlichia canis in Croatian dogs. Parasitol. Res. 116, 3019–3026. Ilic Bozovic, A., Radakovic, M., Spariosu, K., Tyrrell, P., Chandrashekar, R., Misic, D. and Kovacevic Filipovic, M. 2021. First confirmed clinical case of Anaplasma platys in a dog in Serbia. Acta Vet. 71, 107–112. Iliev, P.T., Kirkova, Z.T. and Tonev, A.S. 2020. Preliminary study on the prevalence of endoparasite infections and vector-borne diseases in outdoor dogs in Bulgaria. Helminthologia 57, 171–178. Inokuma, H., Beppu, T., Okuda, M., Shimada, Y. and Sakata, Y. 2003. Epidemiological survey of Anaplasma platys and Ehrlichia canis using ticks collected from dogs in Japan. Vet. Parasitol. 115, 343–348. Inokuma, H., Raoult, D. and Brouqui, P. 2000. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J. Clin. Microbiol. 38, 4219–4221. Kim, C.M., Yi, Y.H., Yu, D.H., Lee, M.J., Cho, M.R., Desai, A.R., Shringi, S., Klein, T.A., Kim, H.C., Song, J.W., Baek, L.J., Chong, S.T., O’guinn, M.L., Lee, J.S., Lee, I.Y., Park, J.H., Foley, J. and Chae, J.S. 2006. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl. Environ. Microbiol. 72, 5766–5776. Kontos, V.I., Papadopoulos, O. and French, T.W. 1991. Natural and experimental canine infections with a Greek strain of Ehrlichia platys. Vet. Clin. Pathol. 20, 101–105. Kramer, F., Husken, R., Krudewagen, E.M., Deuster, K., Blagburn, B., Straubinger, R.K., Butler, J., Fingerle, V., Charles, S., Settje, T., Schunack, B. and Stanneck, D. 2020. Prevention of transmission of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum by Ixodes spp. ticks to dogs treated with the Seresto® collar (imidacloprid 10% + flumethrin 4.5%). Parasitol. Res. 119, 299–315. Lara, B., Conan, A., Thrall, M.A., Ketzis, J.K., Branford, G.C. and Rajeev, S. 2020. Serologic and molecular diagnosis of Anaplasma platys and Ehrlichia canis infection in dogs in an endemic region. Pathogens 9, 488. Li, Y., Yang, J., Chen, Z., Qin, G., Li, Y., Li, Q., Liu, J., Liu, Z., Guan, G., Yin, H., Luo, J. and Zhang, L. 2015. Anaplasma infection of bactrian camels (Camelus bactrianus) and ticks in Xinjiang, China. Parasit. Vectors 8, 313. Lima, M.L., Soares, P.T., Ramos, C.A., Araujo, F.R., Ramos, R.A., Souza, I.I., Faustino, M.A. and Alves, L.C. 2010. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz. J. Microbiol. 41, 381–385. Liu, J., Eberts, M., Bewsey, H., O‘Connor, T.P., Chandrashekar, R. and Breitschwerdt, E.B. 2018. Sensitivity and specificity levels of two rapid assays for antibodies to Anaplasma spp. in dogs. J. Vet. Diagn. Invest. 30, 290–293. Maggi, R.G., Mascarelli, P.E., Havenga, L.N., Naidoo, V. and Breitschwerdt, E.B. 2013. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasit. Vectors 6, 103. Maksimovic, Z., Dervisevic, M., Zahirovic, A. and Rifatbegovic, M. 2022. Seroprevalence of Anaplasma spp. and Ehrlichia spp. and molecular detection of Anaplasma phagocytophilum and Anaplasma platys in stray dogs in Bosnia and Herzegovina. Ticks Tick Borne Dis. 13, 101875. Manev, I. 2020. Serological survey of vector-borne pathogens in stray dogs from Sofia area, Bulgaria. Vet. Parasitol. Reg. Stud. Reports 21, 100441. Martinescu, G.V., Ivanescu, L., Stefanescu, R., Andronic, L., Matiut, S., Mindru, R., Solcan, G. and Miron, L. 2023. Strategies for the diagnosis of granulocytic anaplasmosis in two naturally infected dogs. Animals 14, 49. Munderloh, U. 2017. Comparative studies in tick-borne diseases in animals and humans. Vet. Sci. 4, 32. Nader, J., Krol, N., Pfeffer, M., Ohlendorf, V., Marklewitz, M., Drosten, C., Junglen, S. and Obiegala, A. 2018. The diversity of tick-borne bacteria and parasites in ticks collected from the Strandja Nature Park in south-eastern Bulgaria. Parasit. Vectors 11, 165. Nair, A.D., Cheng, C., Ganta, C.K., Sanderson, M.W., Alleman, A.R., Munderloh, U.G. and Ganta, R.R. 2016. Comparative experimental infection study in dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum. PLoS One 11, e0148239. Nava, S., Beati, L., Venzal, J.M., Labruna, M.B., Szabo, M.P.J., Petney, T., Saracho-Bottero, M.N., Tarragona, E.L., Dantas-Torres, F., Silva, M.M.S., Mangold, A.J., Guglielmone, A.A. and Estrada-Pena, A. 2018. Rhipicephalus sanguineus (Latreille, 1806): neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick Borne Dis. 9, 1573–1585. Nguyen, A.H.L., Tiawsirisup, S. and Kaewthamasorn, M. 2020. Molecular detection and genetic characterization of Anaplasma marginale and Anaplasma platys-like (Rickettsiales: Anaplasmataceae) in water buffalo from eight provinces of Thailand. BMC Vet. Res. 16, 380. Nimsuphan, B., Prasroedsang, S., Kengradomkij, C., Thayananuphat, A. and Kromkhun, P. 2020. Characterization of serum protein fractions of dogs naturally infected with Ehrlichia canis or Anaplasma platys associated with uveitis. Trop. Biomed. 37, 551–559. Panayotova-Pencheva, M.S., Vichova, B., Dakova, V.I. and Salkova, D.S. 2021. Ticks and associated tick-borne pathogens from dogs and red foxes from Bulgaria. Bulg. J. Vet. Med. 24, 608–613. Pantchev, N., Schnyder, M., Vrhovec, M.G., Schaper, R, and Tsachev, I. 2015. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol. Res. 114, S117–S130. Parola, P., Roux, V., Camicas, J.L., Baradji, I., Brouqui, P. and Raoult, D. 2000. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 94, 707–708. Perez Perez, P., Rodriguez-Escolar, I., Carreton, E., Sanchez Agudo, J.A., Lorenzo-Morales, J., Montoya-Alonso, J.A. and Morchon, R. 2021. Serological survey of canine vector-borne infections in North-Center Spain. Front. Vet. Sci. 8, 784331. Piratae, S., Senawong, P., Chalermchat, P., Harnarsa, W. and Sae-Chue, B. 2019. Molecular evidence of Ehrlichia canis and Anaplasma platys and the association of infections with hematological responses in naturally infected dogs in Kalasin, Thailand. Vet. World 12, 131–135. Polsomboon Nelson, S., Ergunay, K., Bourke, B.P., Reinbold-Wasson, D.D., Caicedo-Quiroga, L., Kirkitadze, G., Chunashvili, T., Tucker, C.L. and Linton, Y.M. 2024. Nanopore-based metagenomics reveal a new Rickettsia in Europe. Ticks Tick Borne Dis. 15, 102305. Remesar, S., Prieto, A., García-Dios, D., Lopez-Lorenzo, G., Martinez-Calabuig, N., Díaz-Cao, J.M., Panadero, R., Lopez, C.M., Fernandez, G., Diez-Banos, P., Morrondo, P. and Díaz, P. 2022. Diversity of Anaplasma species and importance of mixed infections in roe deer from Spain. Transbound. Emerg. Dis. 69, e374–e385. Rene-Martellet, M., Lebert, I., Chene, J., Massot, R., Leon, M., Leal, A., Badavelli, S., Chalvet-Monfray, K., Ducrot, C., Abrial, D., Chabanne, L. and Halos, L. 2015. Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in Southern Europe. Parasit. Vectors 8, 3. Rojas-Jaimes, J. and Del Valle-Mendoza, J. 2023. Detection of Bartonella vinsonii, Anaplasma platys and Bartonella sp. in didelphis marsupialis, Pecari tajacu and Chelonoidis denticulate: Peru. BMC Res. Notes 16, 150. Rymaszewska, A. and Grenda, S. 2008. Bacteria of the genus Anaplasma—Characteristics of Anaplasma and their vectors: a review. Vet. Med. 53, 573–584. Sainz, A., Roura, X., Miro, G., Estrada-Pena, A., Kohn, B., Harrus, S. and Solano-Gallego, L. 2015. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 8, 75. Silaghi, C., Santos, A.S., Gomes, J., Christova, I., Matei, I.A., Walder, G., Domingos, A., Bell-Sakyi, L., Sprong, H., von Loewenich, F.D., Oteo, J.A., de la Fuente, J. and Dumler, J.S. 2017. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector Borne Zoonotic Dis. 17, 12–22. Silvestrini, P., Lloyd-Bradley, B., Glanemann, B., Barker, E.N., Badham, H., Tappin, S., Pascual, M., Haines, A., Mas, A., Roura, X. and Piviani, M. 2023. Clinical presentation, diagnostic investigations, treatment protocols and outcomes of dogs diagnosed with tick-borne diseases living in the United Kingdom: 76 cases (2005-2019). J. Small Anim. Pract. 64, 392–400. Snellgrove, A.N., Krapiunaya, I., Ford, S.L., Stanley, H.M., Wickson, A.G., Hartzer, K.L. and Levin, M.L. 2020. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick Borne Dis. 11, 101517. Soares, R., Ramos, C.A., Pedroso, T., Babo-Terra, V., Cleveland, H. and Araujo F. 2017. Molecular survey of Anaplasma platys and Ehrlichia canis in dogs from Campo Grande, Mato Grosso do Sul, Brazil. An. Acad. Bras. Cienc. 89, 301–306. Stanilov, I., Blazhev, A. and Miteva, L. 2023. Anaplasma and Ehrlichia species in Ixodidae ticks collected from two regions of Bulgaria. Microorganisms 11, 594. Stillman, B.A., Monn, M., Liu, J., Thatcher, B., Foster, P., Andrews, B., Little, S., Eberts, M., Breitschwerdt, E.B., Beall, M.J. and Chandrashekar, R. 2014. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J. Am. Vet. Med. Assoc. 245, 80–86. Sykes, J.E. 2023. Tick-borne diseases. Vet. Clin. North Am. Small Anim. Pract. 53, 141–154. Tsachev, I., Baymakova, M. and Pantchev, N. 2019. Seroprevalence of Anaplasma phagocytophilum, Ehrlichia spp. and Borrelia burgdorferi infections in horses: first report from Northern Bulgaria—Short communication. Acta Vet. Hung. 67, 197–203. Tsachev, I., Pantchev, N., Marutsov, P., Petrov, V., Gundasheva, D. and Baymakova, M. 2018. Serological evidence of Borrelia burgdorferi, Anaplasma phagocytophilum and Ehrlichia spp. infections in horses from Southeastern Bulgaria. Vector Borne Zoonotic Dis. 18, 588–594. Tsachev, I., Petrov, V., Flaming, K. and Brown, C. 2008. First detected case of Anaplasma phagocytophilum in a dog in Bulgaria. Revue Med. Vet. 159, 562–564. Vayssier-Taussat, M., Kazimirova, M., Hubalek, Z., Hornok, S., Farkas, R., Cosson, J.F., Bonnet, S., Vourch, G., Gasqui, P., Mihalca, A.D., Plantard, O., Silaghi, C., Cutler, S. and Rizzoli, A. 2015. Emerging horizons for tick-borne pathogens: from the ‘one pathogen-one disease’ vision to the pathobiome paradigm. Future Microbiol. 10, 2033–2043. Walls, J.J., Caturegli, P., Bakken, J.S., Asanovich, K.M. and Dumler, J.S. 2000. Improved sensitivity of PCR for diagnosis of human granulocytic ehrlichiosis using epank1 genes of Ehrlichia phagocytophila-group Ehrlichiae. J. Clin. Microbiol. 38, 354–356. | ||
| How to Cite this Article |
| Pubmed Style Gospodinova K, Petrov V, Stanilov I, Miteva L, Tsachev I, Baymakova M. First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. Open Vet. J.. 2024; 14(12): 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 Web Style Gospodinova K, Petrov V, Stanilov I, Miteva L, Tsachev I, Baymakova M. First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. https://www.openveterinaryjournal.com/?mno=218134 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.47 AMA (American Medical Association) Style Gospodinova K, Petrov V, Stanilov I, Miteva L, Tsachev I, Baymakova M. First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. Open Vet. J.. 2024; 14(12): 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 Vancouver/ICMJE Style Gospodinova K, Petrov V, Stanilov I, Miteva L, Tsachev I, Baymakova M. First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 Harvard Style Gospodinova, K., Petrov, . V., Stanilov, . I., Miteva, . L., Tsachev, . I. & Baymakova, . M. (2024) First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. Open Vet. J., 14 (12), 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 Turabian Style Gospodinova, Krasimira, Vladimir Petrov, Iskren Stanilov, Lyuba Miteva, Ilia Tsachev, and Magdalena Baymakova. 2024. First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. Open Veterinary Journal, 14 (12), 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 Chicago Style Gospodinova, Krasimira, Vladimir Petrov, Iskren Stanilov, Lyuba Miteva, Ilia Tsachev, and Magdalena Baymakova. "First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria." Open Veterinary Journal 14 (2024), 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 MLA (The Modern Language Association) Style Gospodinova, Krasimira, Vladimir Petrov, Iskren Stanilov, Lyuba Miteva, Ilia Tsachev, and Magdalena Baymakova. "First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria." Open Veterinary Journal 14.12 (2024), 3656-3664. Print. doi:10.5455/OVJ.2024.v14.i12.47 APA (American Psychological Association) Style Gospodinova, K., Petrov, . V., Stanilov, . I., Miteva, . L., Tsachev, . I. & Baymakova, . M. (2024) First molecular evidence of Anaplasma platys infection in a dog (Labrador retriever) from Bulgaria. Open Veterinary Journal, 14 (12), 3656-3664. doi:10.5455/OVJ.2024.v14.i12.47 |