| Research Article | ||
Open Vet. J.. 2025; 15(1): 244-251 Open Veterinary Journal, (2025), Vol. 15(1): 244-251 Reserach Article Frozen semen quality of Pasundan bulls with different individual variationsAbdullah Baharun1*, Pirda Parida Permadani Pertiwi1, Annisa Rahmi1, Natashya Chandra Rachmadanti1, Ristika Handarini1, Hikmayani Iskandar2, Daud Samsudewa3, Tulus Maulana4, Syahruddin Said4, Imam Darussalam5, Nurcholis Nurcholis6 and Raden Iis Arifiantini71Department of Animal Science, Faculty of Agriculture, Universitas Djuanda, Bogor, Indonesia 2Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Department of Animal Science, Faculty of Animal and Agricultural Sciences, Diponegoro University, Semarang, Indonesia 4Research Center for Applied Zoology, National Research and Innovation Agency (BRIN), Bogor, Indonesia 5Technical Unit of Bulls Breeding and Artificial Insemination Development Ciamis, Department of Food Security and Livestock, West Java, Indonesia 6Department of Animal Husbandry, Faculty of Animal Agriculture, Musamus University, Papua, Indonesia 7Division of Veterinary Reproduction and Obstetrics, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia *Corresponding Author: Abdullah Baharun. Department of Animal Science, Faculty of Agriculture, Universitas Djuanda, Bogor, Indonesia. Email: abdullah.baharun [at] unida.ac.id Submitted: 11/09/2024 Accepted: 05/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractBackground: Pasundan bulls are one of Indonesia’s indigenous cattle breeds. The quality of frozen semen is essential for the success of artificial insemination (AI), as individual variations in parameters such as sperm motility, viability, and membrane integrity greatly influence reproductive outcomes. Aim: The objective of this study was to evaluate the quality of frozen semen from Pasundan bulls with different individual variations. Methods: Frozen semen from eight Pasundan bulls was obtained from the West Java Regional Artificial Insemination Center. Sperm motility was assessed using computer-assisted sperm analysis (CASA; SpermVision®, Germany). Sperm viability and abnormalities were determined by eosin-nigrosine staining. Plasma membrane integrity was evaluated using the hypoosmotic swelling test, acrosome integrity was assessed using fluorescein isothiocyanate–propidium iodide, and protamine deficiency was measured using chromomycin A3 fluorescence staining. Results: Significant individual variations (p < 0.05) were found across all parameters of frozen semen quality, except for sperm abnormalities, which showed no significant differences (p > 0.05). Conclusion: The results showed that individual variations significantly influence all quality parameters of frozen semen from Pasundan bulls, except for sperm abnormalities. Therefore, a thorough assessment of each bull is crucial to ensure the production of quality frozen semen. Keywords: Fluorescent, Local bulls, Comprehensive evaluation, Pasundan bull. IntroductionTo meet the increasing demand for meat in Indonesia, it is crucial to enhance the populations of bulls, particularly local breeds. One notable local breed is the Pasundan bull, which has been formally recognized as the genetic resource (germplasm) for West Java, as stated in the Decree of the Minister of Agriculture of the Republic of Indonesia Number 1051/KPTS/SR.120/10/2014. Phenotypically, Pasundan bulls share similarities with Bos indicus and Bos javanicus (Widyastuti et al., 2021). This breed offers several advantages, including resistance to tropical diseases, high adaptability to various environmental conditions, and a carcass yield of 53.05% (Said et al., 2017). The Pasundan bull population is distributed across various regions of West Java, including Sukabumi, Cianjur, Tasikmalaya, Majalengka, Pangandaran, Garut, Ciamis, Kuningan, Sumedang, Indramayu, and Purwakarta (Baharun et al., 2017). However, the population has declined from 31,000 head in 2017 to 25,000 head in 2021 (Widyastuti et al., 2021). This reduction can be attributed to factors such as the deterioration of the bulls’ genetic quality and the impact of foot-and-mouth disease. Artificial insemination (AI) represents a widely adopted strategy for enhancing the Pasundan bull population. The primary goal of AI is to enhance the genetic quality of the herd by using superior bulls to produce high-quality offspring (Iskandar et al., 2023). The success of AI is largely dependent on the quality of frozen semen, which plays a critical role in fertilization, embryonic development, and pregnancy outcomes (Hasbi et al., 2024). Semen quality is influenced by various factors, including diet, season, temperature, and genetic predispositions (Indriastuti et al., 2020). Individual breeding factors, which refer to genetic elements, can affect semen quality across different livestock species, including sheep (Hodge et al., 2022), ducks (Zhang et al., 2023), horses (Wilson et al., 2019), and bulls (Indriastuti et al., 2020). One effect of individual variation is that bulls older than 10 years can continue to be used for breeding, as long as their semen quality and reproductive performance remain at a satisfactory level and meet established standards (Heraini et al., 2023). Conventional semen evaluation techniques, including the assessments of sperm motility, viability, and morphological abnormalities, do not provide a complete assessment of semen quality in relation to sperm fertility. A decline in semen quality, encompassing parameters such as sperm motility, capacitation, and DNA integrity, is often associated with the sperm membrane’s capacity to withstand the freezing process (Baharun et al., 2023). The resistance of sperm membranes to temperature fluctuations during cryopreservation is a critical determinant in the selection of bulls for artificial insemination (AI) programs. Therefore, a comprehensive evaluation of semen quality is important for ensuring reproductive efficiency. The aim of the present study was to analyze the frozen semen of Pasundan bull that could be used as indicators of potential fertility at the Regional Artificial Insemination Center (RAIC) in Indonesia. Materials and MethodsSemen and ethical clearanceCommercial straws of frozen semen from eight Pasundan bulls, aged 4–13 years with a body weight (BW) range of 450–550 kg, were obtained from the RAIC in West Java. Semen from these Pasundan bulls was frozen in the Andromed® extender. Semen was collected twice a week every morning using an artificial vagina, with a female cow serving as the treasure (Fig. 1). In this study, the breeding soundness examination applied to the Pasundan bulls involved measuring their scrotal circumference and assessing their general health status, which are important indicators of fertility. The bulls were maintained under ethical standards for animal care by the AI Centre. Each bull was housed individually in pens equipped with designated feed and water. The dietary regimen consisted of 10% fresh forage and 1% concentrate based on BW, administered twice daily, in the morning and evening, with continuous access to water (ad libitum). Ethical approval for the study was granted by the Animal Ethics Commission of the School of Veterinary Medicine and Biomedical Science, IPB University (certificate number 067/KEH/SKE/VII/2023). Semen evaluationThe frozen semen from RAIC West Java was analyzed at the Genomic Laboratory, National Research and Innovation Agency (BRIN), in Bogor, and was transported to liquid nitrogen (N2) containers. The study analyzed several parameters, including sperm motility, viability, abnormalities, plasma membrane integrity (PMI), acrosome integrity, and protamine deficiency. For analysis, the frozen semen was thawed in a water bath at 37°C for 30 seconds. After thawing, the straw was dried, and the sperm was transferred to an Eppendorf® tube by cutting off the two straw plugs. The semen tube was subsequently immersed in a water bath maintained at 37°C for further assessment. Computer-assisted semen analysis (CASA), viability, and abnormality in frozen-thawed semenSperm motility was evaluated by placing a 10 µL aliquot of thawed semen onto a glass slide, covered with a cover slip. Motility parameters were assessed using CASA with the SpermVision® 3.7 software (Minitub®, Tiefenbach, Germany) according to standard procedures by Iskandar et al. (2022). Analyzed parameters included progressive motility (PM: %), distance average path (DAP: µm/s), distance curvilinear (DCL: µm/s), distance straight line (DSL: µm/s), average path velocity (VAP: µm/s), velocity curve linear (VCL: µm/s), velocity straight line (VSL: µm/s), straightness (STR: VSL/VAP), linearity of curve linear (LIN: VSL/VCL), wobble (WOB: VAP/VCL), and amplitude of lateral head displacement (ALH: µm). Additionally, beat cross frequency (BCF: Hz), indicating the frequency at which the sperm head crosses the central path, was measured. The observations were conducted under a microscope at 200× magnification across up to seven fields, with motility values recorded as percentages ranging from 0% to 100%. Sperm viability and morphological abnormalities were assessed using the eosin-nigrosine staining technique according to standard procedures by Maulana et al. (2024). Sperms were classified by their morphologies into (i) sperms with normal morphologies, (ii) sperm with abnormal heads, (iii) sperms with abnormal midpieces, and (iv) sperms with abnormal principles piece tail (Ntemka et al., (2016). For viability analysis, 10 μl of semen was dispensed onto a slide and mixed with 40 μl of eosin-nigrosine stain (1:4 ratio). The mixture was thoroughly homogenized, smeared across the slide, and dried at 37°C. The observations were conducted under a microscope at 400× magnification. Viable sperm cells remained unstained (transparent), while non-viable cells absorbed the dye. A total of 200 sperm cells were analyzed to determine the viability percentage. The morphological assessment was performed by examining an additional 200 sperm cells under 400× magnification, differentiating between morphologically normal and abnormal cells. Evaluation of sperm PMI, acrosome status, and sperm protamine deficiency in frozen-thawed semenTo evaluate PMI, the hypoosmotic swelling (HOS) test was conducted. A 20-µl semen sample was combined with 300 µl of HOS solution (0.735 g Na citrate, 1.351 g fructose, and 100 ml distilled water). This mixture was incubated in a water bath at 37°C for 30 minutes (Arifiantini, 2012). Post-incubation, a small aliquot was placed on a glass slide, covered with a cover slip, and examined under a light microscope at 40× magnification. Sperm with intact plasma membranes exhibited a characteristic bulging or circular tail morphology. The proportion of reactive versus non-reactive sperm was determined by assessing a minimum of 200 sperm cells. The acrosome status of sperm was determined using fluorescein isothiocyanate-peanut agglutinin (FITC-PNA, St. Louis, MO) staining, following a modified protocol from Rajabi-Toustani et al. (2019). Protamine deficiency, indicative of chromatin packaging abnormalities, was assessed using chromomycin A3 (CMA3), a DNA-specific fluorochrome that binds to the minor groove of DNA (Baharun et al., 2023). Semen samples from eight bulls were thawed at 37°C for 30 seconds, washed in calcium- and magnesium-free phosphate-buffered saline (PBS), and subsequently fixed in Carnoy’s solution (3:1 methanol: glacial acetic acid; Merck, Darmstadt, Germany) at 4°C for 5 minutes. Smears were prepared by incubating each slide with 100 µl of sperm suspension for 20 minutes and then rinsed in PBS. Fluorescence microscopy was performed at 400× magnification, with a minimum of 500 cells analyzed per slide (Fig. 2A). Protamine deficiency was assessed using the CMA3 fluorescence staining technique, following the protocol described by Baharun et al. (2023). Semen samples were fixed in 200 ml of Carnoy’s solution for 10 minutes at 4°C, followed by thorough washing with PBS and air drying. A 30 µl aliquot of CMA3 solution was then applied to the prepared slide and incubated for 20 minutes at 37°C. After incubation, the slides were washed with McIlvaine’s buffer (17 ml of 0.1 mol/l citric acid, 83 ml of 0.2 mol/l Na2HPO4, and 10 mmol/l MgCl2, adjusted to pH 7.0), air-dried, and sealed with a clear nail polish coating. The observations were conducted under a fluorescence microscope (AxioPhot Zeiss, Oberkochen, Germany; 490/530 nm excitation filter) equipped with a 380/420 nm excitation/barrier filter (Fig. 2B).
Fig. 1. A graphical abstract of the experimental design.
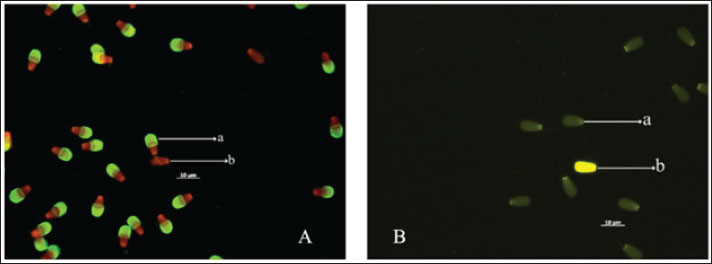
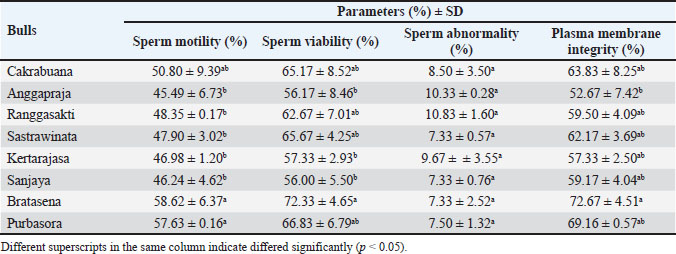
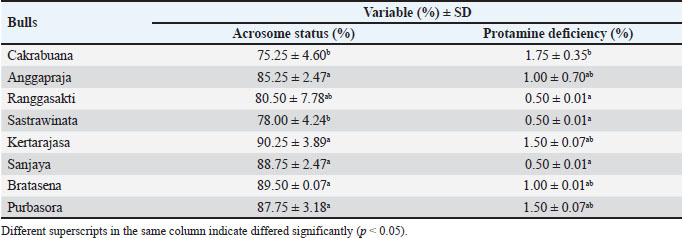
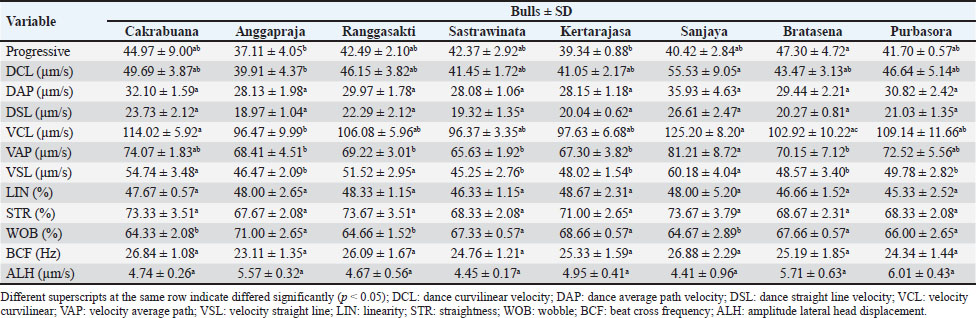
Fig. 2. The sperm evaluation used fluorescence microscopy: (A) Acrosome integrity FITC-PNA with PI staining of the Pasundan bulls sperm show acrosome-intact bright green (a) over the acrosomal cup, and acrosome-loss sperm (b) show no FITC-PNA staining; (B) Sperm deficiency protamine using chromomycin A3 (CMA3) staining. Yellowish green (a) round-headed sperm cells show protamine deficiency, and bright yellow (b) round-headed sperm cells show average protamine content. Statistical analysisThe quality of frozen semen of Pasundan bulls was analyzed descriptively. Statistical differences among sperm parameters were determined using a one-way analysis of variance with Duncan’s multiple range test using SPSS version 26.0 (IBM® Corp., Armonk, NY), and p < 0.05 was considered statistically significant. ResultsThe analysis of frozen semen quality in Pasundan bulls revealed significant individual variations in sperm motility parameters (p < 0.05). Notably, Bratasena (58.62% ± 6.37%) and Purbasora (57.63% ± 0.16%) exhibited higher sperm motility compared to the other bulls, including Anggapraja, Ranggasakti, Sastrawinata, Kartarajasa, and Sanjaya, which ranged from 45.49% to 48.35% (Table 1). Furthermore, Bratasena showed significantly greater sperm viability and PMI, with values of 72.33% ± 4.65% and 72.67% ± 4.51%, respectively (p < 0.05), compared to the other bulls (56.00%-57.33%). However, sperm abnormalities did not differ significantly across the bulls (p > 0.05) within the standard range of 7.33% to 10.83%. The ability of sperm to reach the fertilization site is critically influenced by their motility patterns. In the present study, the DCL exhibited the highest value in Sanjaya and the lowest in Anggapraja (Table 2). The analysis showed that the percentage of acrosome-intact sperm was significantly higher in Kartarajasa (90.25% ± 3.89%), Sanjaya (88.75% ± 2.47%), Bratasena (89.50% ± 0.00%), and Purbasora (87.75% ± 3.18%) compared to the other bulls, such as Cakrabuana (75.25%) and Sastrawinata (78%). Anggapraja and Ranggasakti showed moderate acrosomal integrity, with values ranging from 80.50% to 85.25% (Table 3). Cakrabuana exhibited the highest protamine deficiency (1.76% ± 0.35%) among the bulls, while Ranggasakti, Sastrawinata, and Sanjaya showed a deficiency of 0.50%. Anggapraja, Bratasena, Kertarajasa, and Purbasora had protamine deficiencies ranging from 1.00% to 1.50%. Table 1. Post-thawing semen quality of Pasundan bulls.
Table 2. The sperm acrosome status and protamine deficiency of Pasundan bulls.
Table 3. The sperm motility of post-thawed sperm in Pasundan bulls.
DiscussionSperm motility is a crucial determinant of frozen semen quality (Santoso et al., 2021). The ability of spermatozoa to endure the cryopreservation process, referred to as their ‟cryotolerance,” is a fundamental criterion in the selection of bulls in RAIC (Maulana et al., 2024). Motility serves as a key indicator of sperm quality and its potential successful fertilization (Baharun et al., 2023). The semen motility in this study still meets the Indonesian National Standard (SNI) for frozen bovine semen (SNI 4869-2:2021), which requires a minimum motility of 40%. However, the motility observed in the Pasundan cattle semen was lower than that reported for Bali cattle semen by Iskandar et al. (2022), which exhibited an average motility of 72.88%. This discrepancy can be attributed to the sperm’s capacity to maintain cellular integrity and effectively respond to osmotic stress during cryopreservation. The plasma membrane of sperm plays a crucial role in regulating the influx and efflux of potassium, sodium, and calcium ions, which are essential for mitochondrial function and motility. Cryopreservation-induced alterations in mitochondrial structure can disrupt mitochondrial function, leading to a reduction in ATP production, which is necessary for sperm motility, by approximately 15% (Khalil et al., 2018). The reduced sperm motility observed in Anggapraja, Ranggasakti, Sastrawinata, Kartajasa, and Sanjaya may be associated with impaired ATP production via glycolysis and oxidative phosphorylation (OXPHOS) within the mitochondrial axonemes, both of which are essential for sustaining sperm motility (Magdanz et al., 2019). In this study, the DCL parameter was highest in Sanjaya and lowest in Anggapraja. These results were higher than those reported by Raafi et al. (2021), which were Bali bull sperm (31.70 µm/s), Brahman (38.60 µm/s), and Brangus (36.50 µm/s). Sperm movement distance/DCL refers to the ability of sperm to move from one point to another, ultimately reaching the egg cell during the fertilization process (Hasbi et al., 2024). The parameters of VCL and VAP in this study were higher than those reported by Bahmid et al. (2023), with a range of 39–75 µm/s. Additionally, Diansyah et al. (2022) reported that a VCL/VAP value greater than 25 µm/s, with a progressive movement velocity exceeding 20 µm/s, could significantly influence fertilization success. O’Meara et al. (2022) reported that VCL, VAP, and VSL with values of 121.10–146.14 µm/s, 64.01–76.04 µm/s, and 33.77–44.31 µm/s, respectively, and a WOB of 48%–61% could be associated with potential hyperactivation and penetration into the zona pellucida and correlated with high fertilization success (Kowalczyk and Piatkowska, 2021). Sperm viability and integrity of the plasma membrane are critical determinants of frozen semen quality and its potential for successful fertilization. The high sperm viability and PMI are linked to the reduced generation of reactive oxygen species (ROS) during the cryopreservation process (Morrel et al., 2018). This reduction in ROS activity can be attributed to the inclusion of antioxidants in the diluents used during cryopreservation, which help maintain sperm membrane fluidity. Furthermore, the decreased ROS production in frozen sperm indicates a lower metabolic rate, which may contribute to the preservation of sperm function. The elevated ROS levels during freezing can adversely affect sperm motility and viability and increase the incidence of sperm abnormalities. The variability in individual sperm quality in this study may also be influenced by the lipid and cholesterol composition of the plasma membrane, which modulates membrane stability and affects structural integrity, particularly in the sperm head, following cryopreservation (Indriastuti et al., 2020). Khalil et al. (2018) reported approximately 18% damage to the sperm head membrane and alterations in osmotic pressure during cryopreservation. Damage to the sperm plasma membrane during cryopreservation can impair motility and viability, potentially leading to cell death (Maulana et al., 2024). Post-thaw damage to the plasma membrane disrupts the homeostasis of crucial intracellular ions such as potassium, sodium, and calcium, thereby reducing motility and impairing mitochondrial activity (Khalil et al., 2018). The individual variation in sperm abnormalities observed in this study was higher than that reported by Indriastuti et al. (2020) in Bali cattle and by Baharun et al. (2021) in Simmental bulls. An increase in sperm abnormalities was related to the age of the bulls (Baharun et al., 2021). The differences in sperm abnormalities may be attributed to varying survival rates of sperm during the cryopreservation process (Indriastuti et al., 2020). Major morphological sperm abnormalities are strongly associated with reduced fertility (Chenoweth, 2005), and their detection in the spermiogram is critical for assessing the fertility potential of the ejaculate. Ghirardosi et al. (2017) reported that abnormalities in sperm morphology may arise from the effects of freezing and thawing. Therefore, it is essential to optimize the evaluation of semen quality prior to freezing in order to assess the impact of semen processing on sperm morphology. The acrosomal status of individual Pasundan bulls showed variability. Bull fertility is closely related to acrosome integrity (Oliveira et al., 2014), which is influenced by the fluidity of the sperm cell membrane. Damage to the cell membrane can trigger the production of reactive oxygen species (ROS), such as hydrogen peroxide, and induce osmotic stress, leading to a reduction in acrosomal integrity during the freezing process (Lusignan et al., 2018). The ability of sperm to withstand the freezing process varies between individuals and is determined by the biochemical composition and metabolic characteristics of the sperm cells (Loomis and Graham, 2008), which can contribute to differences in semen quality among individuals. Semen quality can also differ between breeds due to variations in specific proteins, ranging from histones to protamines. Protamine plays a crucial role in chromatin structure formation, stabilizing sperm DNA. A deficiency in protamine can lead to DNA damage (Fortes et al., 2012). Protamine optimizes DNA packaging, facilitating chromatin condensation and protecting genomic integrity from potential DNA damage caused by nuclease enzymes, mutagens, and other factors (Pardede et al., 2021). Therefore, a comprehensive evaluation encompassing both conventional semen parameters (sperm motility, viability, and membrane integrity) and molecular aspects, such as acrosomal status and protamine density, is essential to ensure the production of high-quality frozen semen. The limitations of this study are the aged-related variability among the Pasundan bulls, and the research only focuses on bulls from a single artificial insemination center in West Java. This narrow scope may not capture the full spectrum of semen quality variations influenced by different environmental and management factors. Expanding the study to include bulls from multiple regions or additional breeding centers would enable a more comprehensive assessment of how these external factors contribute to variations in semen quality. ConclusionIn frozen semen, individual variations significantly impact all quality parameters except sperm abnormalities. Therefore, conducting a comprehensive evaluation of each bull is essential to ensure the production of high-quality frozen semen from Pasundan bulls. List of abbreviationsAI, artificial insemination; ALH, amplitude of lateral head displacement; BCF, beat cross frequency; BSE, breeding soundness examination; BS, phosphate-buffered saline; BW, body weight; CASA, computer-assisted sperm analysis; CMA3, chromomycin A3; DAP, distance average path; DCL, distance curve linear; DSL, distance straight line; LIN, linearity of curve linear; HOS, hypoosmotic swelling; PBS, phosphate buffered saline; PMI, plasma membrane integrity; PM, progressive motility; ROS, reactive oxygen species RAIC, Regional Artificial Insemination Center; STR, straightness; VAP, average path velocity; VCL, velocity curve linear; VSL, velocity straight line; WOB, wobble. AcknowledgementsThe authors thank the Center for Artificial Insemination (BBIB) Ciamis, West Java, and the National Research and Innovation Agency (BRIN) for providing semen samples and supporting research facilities. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research was supported by the Directorate of Research, Technology and Community Service, Directorate General of Research and Development Strengthening, Ministry of Education, Culture, Research and Technology of the Republic of Indonesia (work agreement letter number: 106/E5/PG.02.00.PL/2024, 014/SP2H/RT-MONO/LL4/2024, 801/01/K-X/VI/2024). Author’s contributionsConceptualization: AB, PPPP, and AR. Data curation: NCR, FAP, RH, ID, and N. Investigation: AB, HI, and TM. Methodology: AB and S; Resources: AB, PPPP, and TM. Supervision: AB, TM, SS, and RIA. Writing: original draft: AB, PPPP, and AR. Writing: review and editing: TM, HI, SS, and RIA. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesArifiantini, R.I. 2012. Semen collection and evaluation techniques in animals. Bogor, Indonesia: IPB Press. Baharun, A., Arifiantini, R.I. and Yusuf, T.L. 2017. Freezing capability of Pasundan bull sperm using Tris-egg yolk, Tris-soy, and Andromed® diluents. J Ked Hewan. 11(1), 45–49. Baharun, A., Said, S., Arifiantini, R.I. and Karja, N.W.K. 2021. Correlation between age, testosterone and adiponectin concentration, and sperm abnormalities in Simmental bulls. Vet World. 14(8), 2124–2130. Baharun, A., Setiawan, A.B., Rahmi, A., Iskandar, H., Gunawan, M., Anwar, S., Maulana, T., Kaiin, E.M. and Said, S. 2023. Frozen semen characteristics of Limousin bull at different ages. Trop Anim Sci J. 46(3), 306–312. Bahmid, N.A., Karja, N.W.K. and Arifiantini, R.I. 2023. The quality of frozen semen Friesian Holstein semen after long-term storage. Trop Anim Sci J. 46(1), 13–19. BSN (Badan Standarisasi Nasional). 2021. SNI Semen Beku-Bagian 2: Sapi. SNI Nomor 4869-2. Jakarta. Chenoweth, PJ. 2005. Genetic sperm defects. Theriogenology 64, 457–468. Diansyah, A.M., Yusuf, M., Toleng, A.L. and Dagong, M.I.A. 2022. Characteristic and kinematics of Bali-Polled bull sperms. Adv Anim Vet Sci. 10(8), 1787–1798. Fortes, M.R.S., Holroyd, R.G., Reverter, A., Venus, B.K., Satake, N. and Boe-Hansen., G.B. 2012. The integrity of sperm chromatin in young tropical composite bulls. Theriogenology 78, 326–333. Ghirardosi, M.S., Fischman, M.L., Jorge, A.E., Chan, D. and Cisale, H. 2017. Relationship between morphological abnormalities in commercial bull frozen semen doses and conception rate. Andrologia 50, e12884. Hasbi, H., Iskandar, H., Sonjaya, H., Purwantara, B., Arifiantini, R.I., Agil, M., Pardede, B.P., Suyadi., Septian, W.A., Samsudewa, D., Damayanti, E., Maulana, T. and Said, S. 2024. Comparative developmental competence of in vitro embryos recovered from Bali cattle with normal and poor sperm motility. Vet World. 17(3), 593–601. Heraini, D., Fatmila, D.T., Pardede, B.P., Yudi, Y. and Purwantara, B. 2023. Age and individual variation affect semen quality and field fertility of Bali bulls (Bos sondaicus): A preliminary study. Dev Mod Liv Prod Trop Coun. 1st Edition, Boca Raton, FL: CRC Press. Hodge, M.J., Rindfleish, S.J., de las Heras-Saldana, S., Stephen, C.P. and Pant, S.D. 2022. Heritability and genetic parameters for semen traits in Australian sheep. Animals. 12(21), 2946. Indriastuti, R., Ulum, M.F., Arifiantini, R.I. and Purwantara, B. 2020. Individual variation in fresh and frozen semen of Bali bulls (Bos sondaicus). Vet World. 13(5), 840–846. Iskandar, H., Andersson, G., Sonjaya, H., Arifiantini, R.I., Said, S., Hasbi, H., Maulana, T. and Baharun, A. 2023. Protein identification of seminal plasma in Bali bull (Bos javanicus). Animals. 13:514. Iskandar, H., Sonjaya, H., Arifiantini, R.I. and Hasbi, H. 2022. The quality of fresh and frozen semen and its correlation with molecular weight of seminal plasma protein in Bali cattle. Trop Anim Sci J. 45(4), 405–412. Kementan [Kementerian Pertanian Republik Indonesia] Ministry of Agriculture; Jakarta: 2014. Decree of the Minister of Agriculture No. 1051/Kpts/SR.120/10/2014 concerning the determination of Pasundan cattle clumps. Khalil, W.A., El-harairy, M.A., Zeidan, A.B. and Hassan, M.A.E. 2018. Evaluation of bull spermatozoa during and after cryopreservation: structural and ultrastructural insights. Int J Vet Sci Med. 6 (Supp 1), S49–S56. Kowalczyk, A. and Piatkowska, E.C. 2021. Antioxidant effect of Elamipretide on bull’s sperm cells during freezing/thawing process. Andrology. 9(4), 1275–1281. Loomis, P.R. and Graham, J.K. 2008. Commercial semen freezing: Individual male variation in cryosurvival and the response of stallion sperm to customized freezing protocols. Anim. Reprod Sci. 105(1-2), 119–128. Lusignan, M.F., Li, X., Herrero, B., Delbes, G. and Chan, P.T.K. 2018. Effect of different crypreservation methods on DNA integrity and sperm chromatin quality in men. Andrology. 6(6), 829–835. Magdanz, V., Boryshpolets, S., Ridzewski, C., Eckel, B. and Reinhardt, K. 2019. The motility-based swim-up technique separates bull sperm based on differences in metabolic rates and tail length. PLoS One. 14(10), e0223576. Maulana, T., Hasbi, H., Iskandar, H., Said, S., Arifiantini, R.I., Jakaria. And Gunawan, A. 2024. Electrophoretic protein profiles of seminal plasma and their correlation with fresh semen quality in Indonesia toraya buffalo (Bubalus bubalis carabanesis) bulls. Int J Vet Sci. 13(6), 819–826. Morrell, J.M., Valeanu, A.S., Lundeheim, N. and Johannisson, A. 2018. Sperm quality in frozen beef and dairy bull semen. Acta Vet Scand. 60(1), 41. Ntemka, A., Tsousis, G., Brozos, C., Kiossis, E., Boscos, C.M. and Tsakmakidis, I.A. 2016. Breed differences of bull frozen-thawed semen. Reprod Domest Anim. 51, 945–952. O’Meara, C., Henrotte, E., Kupisiewicz, K., Latour, C., Broekhuijse, M. and Camus, A. 2022. The effect of adjusting setting within a computer-assisted sperm analysis (CASA) system on bovine sperm motility and marphology results. Anim Reprod. 19(1), e20210077. Oliveira, B.M., Arruda, R.P., Thome, H.E., Filho, M.M., Oliveira, G., Guimaraes, C., Nichi, M., Silva, L.A. and Celeghini, E.C.C. 2014. Fertility and uterine hemodynamic in cows after artificial insemination with semen assessed by fluorescent probes. Theriogenology 82(5), 767–772. Pardede, B.P., Maulana, T., Kaiin, E.M., Agil, M., Karja, N.W.K., Sumantri, C. and Supriatna, I. 2021. The potential of sperm bovine protamine as a protein marker of semen production and quality at the National Artificial Insemination Center of Indonesia. Vet World. 14(9), 2473–2481. Raafi, M., Yusuf, M., Toleng, A.L., Diansyah, A.M., Surahman. and Sahiruddin. 2021. Movement patterns of sperm at different bull breeds using computer-assisted sperm analysis (CASA). IOP Conf. Ser.: Earth Environ. Science. 788, 012137. Rajabi-Toustani, R., Akter, Q.S., Almadaly, E.A., Hoshino, Y., Adachi, H., Mukoujima, K. and Murase, T. 2019. Methodogical improvement of fluorescein isothiocyanate peanut agglutinin (FITC-PNA) acrosomal integrity staining for frozen-thawed Japanese Balack bull spermatozoa. J Vet Med Scie. 81, 649–702. Said, S., Putra, W.P.B., Anwar, S., Agung, P.P. and Yuhani, H. 2017. Phenotypic, morphometric characterization and population structure of Pasundan cattle at West Java, Indonesia. Biodiversitas 18(4), 1638–1645. Santoso, S., Herdis, S., Arifiantini, R.I., Gunawan, A. and Sumantri, C. 2021. Characteristics and potential production of frozen semen of Pasundan bull. Trop Anim Sci J. 44(1), 24–31. Smith, L.B. and Walker W.H. 2014. The regulation of spermatogenesis by androgens. Semin. Cell Dev Biol. 30, 2–13. Widyastuti, R., Pristihadi, D.N., Prastowo, S., Maheshwari, H., Sumantri, C. and Boediono, A. 2021. Assisted reproductive technology in tropical animals: case in pasundan cattle genetic conservation and utilization. IOP Conf. Ser.: Earth Environ. Science. 902, 012036. Wilson, M., Williams, J., Mentrose, V.T. and Williams, J. 2019. Variance in stallion semen quality among equestrian sporting disciplines and competition levels. Animals 9(8), 485. Zhang, Z., Yang, Y., Huang, L., Chen, L., Zhang, G., Gong, P., Ye, S. and Feng, Y. 2023. Identification of potential candidate genes and regulatory pathways related to reproductive capacity in hypothalamus and pituitarium of male duck (Anas platyrynchopus) by differential transcriptome analysis. J Anim Sci. 101, 363. | ||
| How to Cite this Article |
| Pubmed Style Baharun A, Pertiwi PPP, Rahmi A, Rachmadanti NC, Handarini R, Iskandar H, Samsudewa D, Maulana T, Said S, Darussalam I, Nurcholis N, Arifiantini RI. Frozen semen quality of Pasundan bulls with different individual variations. Open Vet. J.. 2025; 15(1): 244-251. doi:10.5455/OVJ.2025.v15.i1.22 Web Style Baharun A, Pertiwi PPP, Rahmi A, Rachmadanti NC, Handarini R, Iskandar H, Samsudewa D, Maulana T, Said S, Darussalam I, Nurcholis N, Arifiantini RI. Frozen semen quality of Pasundan bulls with different individual variations. https://www.openveterinaryjournal.com/?mno=219914 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.22 AMA (American Medical Association) Style Baharun A, Pertiwi PPP, Rahmi A, Rachmadanti NC, Handarini R, Iskandar H, Samsudewa D, Maulana T, Said S, Darussalam I, Nurcholis N, Arifiantini RI. Frozen semen quality of Pasundan bulls with different individual variations. Open Vet. J.. 2025; 15(1): 244-251. doi:10.5455/OVJ.2025.v15.i1.22 Vancouver/ICMJE Style Baharun A, Pertiwi PPP, Rahmi A, Rachmadanti NC, Handarini R, Iskandar H, Samsudewa D, Maulana T, Said S, Darussalam I, Nurcholis N, Arifiantini RI. Frozen semen quality of Pasundan bulls with different individual variations. Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 244-251. doi:10.5455/OVJ.2025.v15.i1.22 Harvard Style Baharun, A., Pertiwi, . P. P. P., Rahmi, . A., Rachmadanti, . N. C., Handarini, . R., Iskandar, . H., Samsudewa, . D., Maulana, . T., Said, . S., Darussalam, . I., Nurcholis, . N. & Arifiantini, . R. I. (2025) Frozen semen quality of Pasundan bulls with different individual variations. Open Vet. J., 15 (1), 244-251. doi:10.5455/OVJ.2025.v15.i1.22 Turabian Style Baharun, Abdullah, Pirda Parida Permadani Pertiwi, Annisa Rahmi, Natashya Chandra Rachmadanti, Ristika Handarini, Hikmayani Iskandar, Daud Samsudewa, Tulus Maulana, Syahruddin Said, Imam Darussalam, Nurcholis Nurcholis, and Raden Iis Arifiantini. 2025. Frozen semen quality of Pasundan bulls with different individual variations. Open Veterinary Journal, 15 (1), 244-251. doi:10.5455/OVJ.2025.v15.i1.22 Chicago Style Baharun, Abdullah, Pirda Parida Permadani Pertiwi, Annisa Rahmi, Natashya Chandra Rachmadanti, Ristika Handarini, Hikmayani Iskandar, Daud Samsudewa, Tulus Maulana, Syahruddin Said, Imam Darussalam, Nurcholis Nurcholis, and Raden Iis Arifiantini. "Frozen semen quality of Pasundan bulls with different individual variations." Open Veterinary Journal 15 (2025), 244-251. doi:10.5455/OVJ.2025.v15.i1.22 MLA (The Modern Language Association) Style Baharun, Abdullah, Pirda Parida Permadani Pertiwi, Annisa Rahmi, Natashya Chandra Rachmadanti, Ristika Handarini, Hikmayani Iskandar, Daud Samsudewa, Tulus Maulana, Syahruddin Said, Imam Darussalam, Nurcholis Nurcholis, and Raden Iis Arifiantini. "Frozen semen quality of Pasundan bulls with different individual variations." Open Veterinary Journal 15.1 (2025), 244-251. Print. doi:10.5455/OVJ.2025.v15.i1.22 APA (American Psychological Association) Style Baharun, A., Pertiwi, . P. P. P., Rahmi, . A., Rachmadanti, . N. C., Handarini, . R., Iskandar, . H., Samsudewa, . D., Maulana, . T., Said, . S., Darussalam, . I., Nurcholis, . N. & Arifiantini, . R. I. (2025) Frozen semen quality of Pasundan bulls with different individual variations. Open Veterinary Journal, 15 (1), 244-251. doi:10.5455/OVJ.2025.v15.i1.22 |