| Research Article | ||
Open Vet. J.. 2025; 15(1): 98-107 Open Veterinary Journal, (2025), Vol. 15(1): 98-107 Research Article Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica)Arni Diana Fitri1,2*, Deni Noviana3, Gunanti Gunanti3, Anita Esfandiari4, Mawar Subangkit51Graduate Student of Animal Biomedical Science, School of Veterinary and Biomedical Sciences, Bogor, Indonesia 2Veterinary Teaching Hospital, School of Veterinary Medicine and Biomedical Sciences, IPBUniversity, Bogor, Indonesia 3Division of Surgery and Radiology, School of Veterinary Medicine and BiomedicalSciences, IPB University, Bogor, Indonesia 4Division of Internal Medicine, School of Veterinary Medicine and Biomedical Sciences, IPBUniversity, Bogor, Indonesia 5Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPBUniversity, Bogor, Indonesia *Corresponding Author: Arni Diana Fitri. School of Veterinary Medicine and Biomedical Sciences (SVMBS) IPB University, Bogor, Indonesia. Email: arnidianafitri [at] apps.ipb.ac.id Submitted: 18/09/2024 Accepted: 03/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
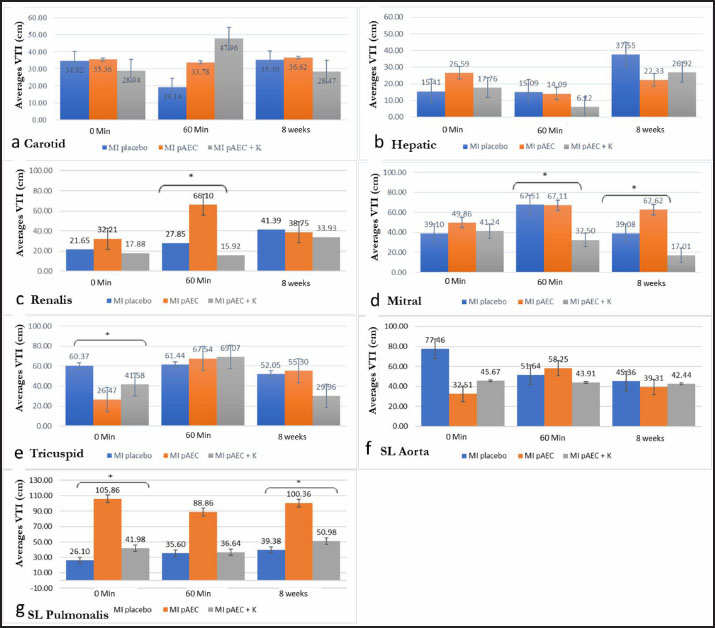
AbstractBackground: Myocardial infarction (MI) is the most common heart disorder in patients with cardiovascular disease. Obstruction of blood flow to the heart causes ischemia and results in heart pump failure. Aim: This study aims to evaluate the effects of porcine amniotic epithelial cell (pAEC)-based stem cell therapy alone and in combination with cardiomyocytes to understand its potential benefits in MI treatment. Methods: In this study, we conducted MI therapy using pluripotent stem cells derived from the amnion (AEC) implanted into the infarcted heart. Nine domestic pigs (Sus scrofa domestica), aged 3-4 months and weighing 35-40 kg, were used as models. The animal models were divided into three groups: the placebo MI group, the MI group treated with pAEC stem cells, and the MI group treated with amniotic epithelial stem cells combined with cardiomyocyte cells. Electrocardiography (ECG), pulsed wave (PW) Doppler, and biomarker examinations were performed to evaluate the effects of stem cell intervention on MI. Results: Single pAEC stem cell therapy and its combination with cardiomyocytes in MI lesions demonstrated improvements in VTI, particularly renal artery, mitral, and tricuspid valve compared to untreated infarction (placebo). Notably, single pAEC therapy significantly improved more than combining them with cardiomyocytes. Conclusion: Treatment with AEC stem cells alone resulted in more significant improvements in heart valve function and large blood vessels compared to combining AEC with cardiomyocytes. This study allowed us to understand better the effects of stem cell therapy on blood hemodynamics and biomarker parameters of heart injury. Keywords: Biomarker, Echocardiography, Pigs, Stem cell. IntroductionMyocardial infarction (MI) is a cardiac condition with high morbidity and mortality rates. It occurs when the blood flow to a specific area of the myocardium is completely or partially blocked, leading to ischemia and subsequent heart failure (Cui et al ., 2022). The post-infarction evaluation of cardiac function and hemodynamics facilitated by echocardiography is essential for determining appropriate treatment strategies and predicting patient prognosis (Robinson et al., 2024). Echocardiography, a pivotal noninvasive imaging technique in cardiology, offers real-time visualization of the structure and function of the heart through ultrasonic waves. Its widespread application in animal models of MI, as demonstrated in a study by Abe et al. (2024), underscores its value. Pulsed wave (PW) Doppler, an essential modality in echocardiography, is instrumental in measuring the velocity and direction of blood flow, enabling the assessment of cardiac hemodynamics without the need for complex procedures. Further research is warranted to explore its application and efficacy in specific animal models, such as pigs (Bont et al., 2020). PW Doppler is one of the most commonly used types of Doppler echocardiography for assessing blood flow in the heart. It works by transmitting short pulses of ultrasonic waves into the tissue and detecting reflected waves (Noviana and Alham, 2012). The blood flow velocity is determined by analyzing the frequency shifts (Doppler shifts) of these reflected waves (Villemain et al., 2020). Biomarkers are crucial in providing critical information regarding normal or pathological processes and the body’s response to therapy. Their particular value lies in the early detection of cardiac abnormalities before clinical symptoms appear, offering more precise diagnostic assessments and instilling confidence in the diagnostic process. Biomarkers also serve as vital tools for monitoring disease progression and treatment efficacy and predicting patient prognosis. Various treatment methods for MI have been developed and continue to evolve. One such treatment is colchicine, which has been shown to improve hemodynamic parameters and reduce cardiac fibrosis (Akodad et al., 2017). Recent advancements include exploring stem cell therapy, which has demonstrated regenerative potential (Bargehr et al., 2019; Park et al., 2019). Stem cell therapy offers a promising alternative for treating MI by replacing lost cardiomyocytes and stimulating neovascularization (Chepeleva, 2023). This promising approach instils hope and optimism for future MI treatment. Stem cells possess two fundamental properties: the ability to differentiate into various cell types and self-renewal (Yamanaka, 2020; Hoang et al ., 2022). For successful therapy, stem cell differentiation must be aligned with the target tissue or organ. Stem cells can be derived from multipotent or pluripotent sources, with multipotent stem cells obtained from the bone marrow, peripheral blood, umbilical cord, placenta, adipose tissue, muscle, and skin (Hade, Suire and Suo, 2021). Compared to pluripotent stem cells, multipotent cells are more accessible to obtain and isolate, have more significant potential for proliferation, genetic stability, and angiogenesis, and are compatible with tissue engineering principles, making them valuable for repairing critical tissues (Hoang et al., 2022). Human amniotic membrane-derived stem cells are a potent source for stem cell therapy. These cells contain a rich matrix of extracellular proteins, growth factors, cytokines, and other beneficial proteins (Fénelon et al ., 2021). Stem cells derived from the amniotic membrane are highly recommended due to their low cost, versatile storage options, availability, and lack of ethical concerns (Andrysiak et al., 2021). Amniotic membranes are biocompatible, permeable, and elastic and can be used as biodegradable scaffolds (Zakrzewski et al., 2019; Fénelon et al., 2021). Furthermore, these stem cells exhibit low immunogenicity and anti-inflammatory, antifibrotic, and antimicrobial properties. They also contribute to pain reduction, re-epithelialization, and faster wound healing (Fénelon et al., 2021; Calabrese, 2022). Stem cell therapy has gained widespread use beyond cosmetic applications and is now employed in disease treatment, such as heart disease and diabetes. Pluripotent stem cells are effective as experimental models and therapeutic agents for progressive cancers (Alexander et al., 2019). Stem cells derived from the endometrium and menstrual blood also promise to regenerate endometrial tissue and treat reproductive disorders, such as infertility and recurrent miscarriage (Valatkaitė et al., 2021). Stem cell therapy has also been proven effective in treating heart diseases (Müller et al., 2018; Han et al., 2019). Stem cells derived from pAECs offer significant advantages due to the abundance of tissue available compared to other stem cell sources. As an extra-fetal tissue, the amniotic membrane has the potential as a primitive mesenchymal stem cell (MSC). In vitro studies have shown that pAECs proliferate rapidly, reaching the lag phase within hours and entering the log phase within 12 days (Lange-Consiglio et al., 2015). Although numerous studies have investigated the effects of stem cell therapy on myocardial tissue healing, research on the impact of such therapies on blood flow measurements using echocardiography and ultrasonography is limited. Additionally, few studies have explored the effects of stem cell therapy on hemodynamic parameters and NT-ProBNP biomarkers in animal models of MI. Therefore, this study aims to evaluate further the effects of pAEC-based stem cell therapy alone and in combination with cardiomyocytes to understand its potential benefits in MI treatment. Material and MethodsAll procedures in this study were approved by the Animal Ethics Review Committee of the IPB Faculty of Veterinary Medicine (certificate number: 152/KEH/SKE/X/2021). Animal modelNine domestic pigs (Sus scrofa domestica), aged 3–4 months and weighing 35–40 kg, were used as a MI model. Gender was not considered a differentiating factor. MI was induced and confirmed by observing changes in the ST segment through ECG. The animal models were divided into three groups: the placebo MI group, the MI group treated with pAEC stem cells, and the third group, the MI group treated with amniotic epithelial stem cells combined with cardiomyocyte cells. These cardiomyocyte cells were obtained from each experimental animal and then extracted and mixed with AEC cells in the form of scaffolds. Velocity–time integral (VTI) parameters were measured using PW Doppler echocardiography, and serum NT-proBNP levels were assessed as a biomarker of myocardial damage. Ethical approvalAll procedures were conducted in accordance with institutional and international guidelines for the care and use of animals, demonstrating our unwavering commitment to ethical research practices. Pig amniotic epithelial stem cell culture/pAECSingle-cell pAECs were isolated following a modified version of established methods (Putra et al., 2022). After performing a bioburden test, the fetal membranes were placed in a sterile Petri dish, and the chorion was separated. The amniotic membranes were thoroughly washed twice with 50 ml of Hank’s balanced salt solution and twice with Versene solution to remove all blood residues. The membranes were then spread with the fetal side up and incubated in 50 ml of TripleSelect for 1 hour at 37°C. After gently removing the cell layer, the membrane was neutralized with DTI solution, and the cell suspension was filtered through a 100 µm cell strainer and centrifuged at 150 g for 10 minutes. The amniotic membrane was then inverted, and the maternal side was incubated with collagenase and hyaluronidase for 1 hour at 37°C. After filtering the cell suspension, the cells were centrifuged, and digestion was stopped using a complete cardiomyocyte differentiation medium. Isolated cells were collected as previously described. Cardiomyocyte single-cell isolationThe protocol for isolating cardiomyocytes from cardiac tissue involves enzymatic, thermal, and mechanical methods (Putra et al., 2022; Sandora et al., 2022). Upon arrival at the laboratory, the tissue was weighed, and a 500–1000 mg sample was selected. The tissue was washed twice with calcium- and magnesium-free Dulbecco’s phosphate-buffered saline (DPBS, Sigma-Aldrich, USA), minced into 2 × 2 × 2 mm³ pieces, and placed into gentleMACS™ C- Tubes (Miltenyi Biotec, Germany) containing collagenase type V (250 U/ml, Gibco™) and proteinase type XXIV (Sigma-Aldrich, USA). The tubes were attached to a heated MACS Octo Dissociator (Miltenyi Biotec, Germany) and incubated for 1 hour at 37°C. Digestion was neutralized by adding two volumes of AscleStem complete medium supplemented with supplements (Nacalai Tesque, Japan). The cell suspension was filtered through a 70 µm strainer (Biologix, China) and centrifuged at 600 × g for 5 minutes at room temperature. Temperature: Viability was assessed using Trypan Blue staining, and the cells were counted using a Neubauer hemocytometer. Cardiomyocyte morphology was visualized using a Brightfield AxioVert.A1 microscope (Carl Zeiss, Germany). Further confirmation of cardiomyocytes was performed using a live/dead assay (Invitrogen, USA) and visualized using an LSM 900 microscope (Carl Zeiss, Germany). 174 Sinistra circumflex artery ligationProcedure Sequence: Sinistra circumflex artery ligation began with a thoracotomy on the animal’s left side while positioned in right recumbency. Coronary artery ligation was performed using a 6.0 polypropylene monofilament thread. Ischemia was confirmed by visible ST-segment elevation on the electrocardiography (ECG) monitor after 60 minutes. In the control group (placebo), the pericardium was closed. In treatment groups 2 and 3, after waiting 60 minutes for the ischemic reaction, animals in group 2 received pAEC injections into the ischemic area. In contrast, those in group 3 were treated with a combination of pAEC and a cardiomyocyte scaffold before closing the pericardium. ECG examination procedureThe ECG examination was conducted under anesthesia, with the animal carefully positioned laterally on a table. Electrodes were placed after shaving and cleaning the area to minimize the resistance and enhance signal quality. Five electrodes were applied on both front limbs, hind limbs, and at the tip of the sternum slightly to the left. Observations focused on recording changes in the ST segment. PW Doppler ultrasonography examinationPW Doppler echocardiography was performed at three intervals: immediately (0 min), 60 minutes, and 8 weeks after left circumflex artery ligation. The animals were carefully positioned in right parasternal recumbency on a specialized cardiac examination table, with utmost care to ensure their comfort and safety. The ultrasound machine used was a Chison™ Ebit 60 Vet portable system. Different probes were employed for specific examinations: the Chison P3-E 1.5–5.3 MHz Phased Array Transducer for heart valve assessment, the L7S 5–13.2 MHz Linear Transducer for carotid artery evaluation, and the MC6 6.0 MHz Micro Convex Transducer for hepatic vein and renal artery imaging. The probe was placed on the right thorax for heart valve examination, the left lateral neck for carotid artery evaluation, the caudal sternum for hepatic vein assessment, and the left caudal ribs for renal artery examination. Biomarker examination processBlood samples were collected from the jugular or femoral vein using a 10 ml syringe, drawing 5–10 ml of blood. The blood samples were transported to the laboratory, and they were centrifuged to separate plasma from blood cells. The plasma was stored in Eppendorf tubes at −80°C until further analysis. NT-proBNP levels were measured in the plasma using enzyme-linked immunosorbent assay at three time points: 0 min, 60 min, and 8 weeks post- MI across the three treatment groups. These biomarker levels were evaluated to assess the extent of cardiac stress, damage, and physiological response after infarction. Statistical analysis was performed to determine the significance of the treatment effects, and the results were correlated with electrocardiographic (ECG) findings and VTI measurements from PW Doppler echocardiography. Statistical analysisStatistical analyses were performed using SPSS and Excel. Data distribution was assessed using the Shapiro–Wilk normality test. Parametric data were analyzed using one-way ANOVA followed by Duncan’s post hoc test for multiple comparisons. Nonparametric data were evaluated using the Kruskal–Wallis test and Duncan’s post hoc test. Correlation tests were performed using Pearson’s correlation for parametric data and Spearman’s correlation for nonparametric data. The significance level was set at a p-value of less than 0.05, underscoring the importance of the results. Comparisons were made between groups for electrocardiographic ST segment values, VTI values, and NT-ProBNP biomarker levels. ResultsThe effect of stem cell treatment on blood vesselsIn this in vivo study, we compared stem cell interventions with a group that did not receive therapy (placebo). We measured the VTI parameters of extracardiac blood flow, specifically in the carotid artery, hepatic vein, and renal artery. We also measured VTI in the mitral, tricuspid, aortic, and pulmonary semilunar valves (Fig. 1). Blood flow in vessels outside the heart typically increases during the acute phase of infarction and decreases after 8 weeks, as shown in Figures 1a, 1b, and 1c. Carotid Artery (Fig. 1a)At 0 minutes, the average VTI values were similar between the MI placebo and MI pAEC groups, with the MI pAEC + K group showing slightly lower values. However, after 60 min, the MI pAEC + K group showed a significant increase in VTI, which was the highest among all groups. The MI placebo group experienced a sharp decline to 19.14, whereas the MI pAEC group remained stable. At the 8-week mark, the MI pAEC group exhibited the highest VTI, whereas the MI pAEC + K group showed a decrease but remained above its baseline value.
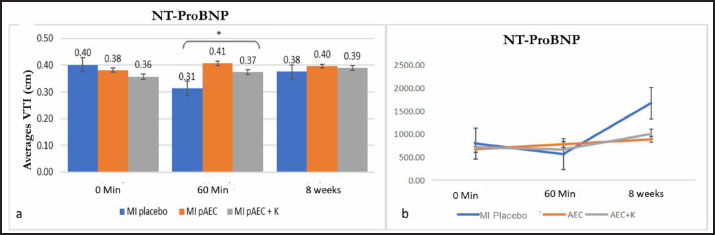
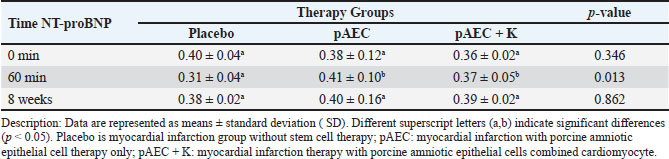
Figure. 1. The figure illustrates the temporal changes in vascular and heart valve parameters in response to therapeutic interventions. (a) shows the average carotid VTI, (b) the average heptic VTI, and (c) the average renal VTI. (d) depicts the average mitral VTI, while (e) presents the average tricuspid VTI. (f) highlights the mean pulmonary VTI, followed by (g) which shows th average aortic VTI. All comparisons are based on time-dependent therapeutic effects. Data are presented as means ± standard deviations (SD). Different superscript letters (a,b) indicate significant differences (p < 0.05) marking with a star (*). Hepatic Vein (Fig. 1b)At 0 min, the MI pAEC group had the highest VTI, while the MI placebo and MI pAEC + K groups showed lower values. After 60 min, there was a notable decrease in VTI across all groups, with the MI pAEC + K group showing the most significant decrease. At 8 weeks, the MI placebo group showed the most significant increase, reaching the highest VTI among all the groups. These results suggest that the MI pAEC group had the highest VTI at onset, but by 60 min, all groups showed a decrease, with the MI placebo group showing solid recovery at 8 weeks, as seen in studies by Cuadra et al. (2024) and Zhang et al. (2024). Renal Artery (Fig. 1c)At 0 min, the MI pAEC group exhibited the highest VTI value. After 60 min, the MI pAEC group showed a significant increase in VTI, whereas the MI pAEC + K group showed a decrease. At 8 weeks, the VTI values among all groups converged, with the MI placebo group having a slightly higher VTI than the other groups. This finding correlates with the observations made Wang et al. (2022) and Maksimczuk et al. (2022) regarding the influence of infarction on renal artery function and kidney disruption due to reduced blood flow. The effect of stem cell treatment on heart valvesThe study measured VTI in the heart valves (mitral, tricuspid, pulmonary semilunar, and aortic) of pigs experiencing MI (Figs. 1d, 1e, 1f, and 1g). Mitral Valve (Fig. 1d)The MI pAEC group initially demonstrated the highest VTI and was maintained throughout the study. The MI placebo group returned to baseline, while the MI pAEC + K group declined significantly, as supported by the studies by Kobe et al. (2019). Tricuspid valve (Fig. 1e)The MI placebo group initially had the highest VTI, whereas the MI pAEC group showed the lowest VTI. At 60 min, all the groups showed increased VTI, with the MI pAEC + K group reaching the highest level. After 8 weeks, the MI pAEC + K group significantly declined, while the other groups remained stable. These results are consistent with previous findings on stem cell therapy by Hachimi-Idrissi (2023). Pulmonary Semilunar Valve (Fig. 1f)The MI placebo group initially had the highest VTI; however, the MI pAEC group surpassed it at 60 min. By week 8, all groups declined in VTI, with the MI placebo group retaining a slight advantage. This observation was consistent with the findings of Zanoli et al. (2020). Aortic Valve (Fig. 1g)The MI pAEC group showed a significant increase in VTI at 0 min and maintained the highest VTI throughout the study. The MI pAEC + K group showed some recovery but remained lower than that of the MI pAEC group. These findings are supported by the research by Liu et al. (2023) and Nyvad et al. (2022), who discussed the role of stem cells in improving vascular function. The effect of stem cell therapy on NT-ProBNP biomarker valueNT-proBNP levels: biomarker response over time Figure 2 presents the mean NT-proBNP levels across different time points (0 minutes, 60 minutes, and 8 weeks) for three treatment groups: MI placebo, MI pAEC, and MI pAEC + K. This biomarker reflects cardiac stress and is commonly used to evaluate cardiovascular function, particularly in the context of MI and related interventions. 0 minutes At baseline, NT-proBNP levels were relatively similar among the groups. The MI placebo group exhibited the highest baseline value at 0.40 cm, while the MI pAEC and MI pAEC + K groups had slightly lower values at 0.38 and 0.36 cm, respectively. These differences were not statistically significant, indicating comparable baseline characteristics regarding NT- proBNP levels across the three treatment groups. 60 minutes After 60 minutes, a significant divergence in NT-proBNP levels was observed between the groups. The MI placebo group showed a modest increase to 0.41 cm, while the MI pAEC group also experienced a rise to 0.37 cm. However, the most notable change occurred in the MI pAEC 297 + K group, which significantly declined to 0.31 cm (p < 0.05 compared to the MI placebo and MI pAEC). This statistically significant reduction in NT-proBNP in the MI pAEC + K group suggests that this intervention may have led to an early decrease in cardiac stress or injury markers compared to the other groups, sparking further interest in the potential of this intervention. 8 weeks At the 8-week mark, the NT-proBNP levels across all groups appeared to stabilize, with minimal variation. The MI placebo group maintained a level of 0.38 cm, while the MI pAEC group slightly increased to 0.40 cm. The MI pAEC + K group also increased slightly to 0.39 cm. The lack of statistically significant differences between the groups suggests that by eight weeks, the effects of the different interventions on NT-proBNP levels had converged, reassuring us about the potential long-term normalization of biomarker levels across treatments. NT-proBNP levels across therapy groupsTable 1 summarizes the NT-proBNP levels (mean ± standard deviation) in pigs with myocardial infarction (MI) receiving different stem cell therapy interventions: the MI placebo group (without therapy), the MI pAEC group (pAEC therapy), and the MI pAEC + K group (combined pAEC and cardiomyocyte therapy). The p-values reflect statistical comparisons across the groups at 0 minutes, 60 minutes, and 8 weeks.
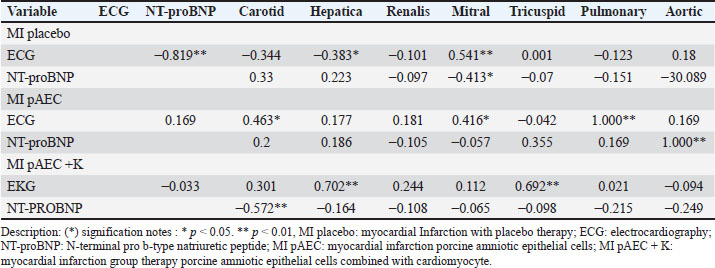
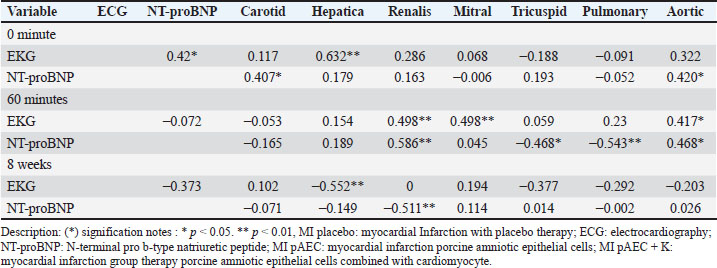
Figure. 2. a) Illustrates the mean NT-proBNP levels, reflecting the long-term biomrker response. b) Interction plot between stem cell therapy group and time for NT-proBMP biomarker concentration in myocardial infarcted Pigs. 0 minutes At baseline (0 minutes), NT-proBNP levels were comparable across the therapy groups, with no statistically significant differences (p=0.346). The placebo group showed an NT-proBNP level of 0.40 ± 0.04 cm, while the MI pAEC and MI pAEC + K groups exhibited values of 0.38 ± 0.12 cm and 0.36 ± 0.02 cm, respectively. These baseline levels indicate that the groups were evenly matched before the interventions began, with no significant pre-treatment differences in NT-proBNP concentrations. 60 minutes At the 60-minute mark, notable changes in NT-proBNP levels were observed between the groups. The MI placebo group decreased NT-proBNP to 0.31 ± 0.04 cm, while the MI pAEC group significantly increased to 0.41 ± 0.10 cm. In contrast, the MI pAEC + K group exhibited a more modest rise to 0.37 ± 0.05 cm. The statistical analysis revealed a significant difference between the groups (p=0.013), suggesting that the MI pAEC intervention may induce an acute increase in NT-proBNP levels compared to placebo. At the same time, the combined therapy (MI pAEC + K) showed a more attenuated response. 8 weeks NT-proBNP levels had stabilized across all groups by 8 weeks, and the differences were no longer statistically significant (p=0.862). The MI placebo group maintained a 0.38 ± 0.02 cm level, while the MI pAEC and MI pAEC + K groups showed levels of 0.40 ± 0.16 cm and 0.39 ± 0.02 cm, respectively. These results suggest that despite the initial divergence at 60 minutes, the long-term effects of the different therapies on NT-proBNP levels converged by the 8-week time point. The data presented in Table 1 highlight an initial, statistically significant difference in NT- proBNP levels between the therapy groups at 60 minutes, followed by a normalization of these levels by 8 weeks. The MI pAEC group demonstrated a sharp early increase in NT-proBNP levels, suggesting an acute cardiac response to the therapy. In contrast, the combined MI pAEC 342 + K therapy gradually changed. Over the long term, however, NT-proBNP levels were similar across all groups, indicating that both therapies may contribute to the eventual normalization of this biomarker in MI settings. At baseline, NT-proBNP levels were comparable across all groups. At 60 min, the MI pAEC group showed the highest increase in NT-proBNP levels, while the MI pAEC + K group showed a less pronounced increase. After 8 weeks, NT-proBNP levels returned to near-baseline values in all the groups. These findings are consistent with those of Song et al. (2015) and Tajabadi et al. (2022), indicating a transient increase in cardiac biomarkers after stem cell therapy. Correlation analysisA significant negative correlation (−0.819**) was observed between ECG and NT-proBNP levels in the MI placebo group. Positive correlations were found between ECG and carotid artery function (0.463*) in the MI pAEC group. In the MI pAEC + K group, significant positive correlations were found between ECG values and carotid artery (0.702**) and tricuspid valve (0.692**) functions (Tables 2 and 3). These results are consistent with those of Castiglione et al. (2022) and Rajagopal et al. (2023) regarding the relationship between cardiac function and extracardiac vascular dynamics. DiscussionThese results indicate that stem cell therapy, particularly pAEC therapy, had varied effects on blood vessels and heart valves over time. In the carotid artery, the combination of pAEC and cardiomyocytes (MI pAEC + K) led to a significant short-term increase in VTI at 60 minutes, suggesting an immediate positive impact on carotid artery function. However, this effect diminished by 8 weeks, indicating that while combination therapy may offer substantial short-term benefits, the long-term sustainability of these effects requires further investigation, as suggested by Cuadra et al. (2024). These findings reiterate the potential of stem cell therapy and inspire a sense of possibility and potential in the audience, encouraging further exploration and research in this field. Table 1. N-terminal pro-b-type natriuretic peptide (NT-proBNP) variables in pigs with myocardial infarction stem cells therapy groups.
Table 2. Correlation between VTI pulsed wave Doppler echocardiography measurement variables in pigs with myocardial infarction based on stem cell therapy treatment groups.
Table 3. Correlation between VTI pulsed wave Doppler echocardiography measurement variables in pigs with myocardial infarction based on observation time.
The MI pAEC group showed the highest initial VTI in the hepatic vein, suggesting a positive early response to the therapy. However, by 60 minutes, all groups exhibited a general decline in VTI, with the MI pAEC + K group showing the most significant reduction. By 8 weeks, the MI placebo group showed the most substantial recovery, which raises questions about natural compensatory mechanisms in untreated MI models, a concept discussed by Zhang et al. (2024). This suggests that while stem cell therapies may offer immediate benefits, their long-term impact may be limited compared to natural healing processes, particularly in the hepatic vein. In the renal artery, pAEC therapy resulted in a significant short-term improvement in VTI. However, after 8 weeks, the VTI values converged across all groups, with the MI placebo group showing a slight advantage. This finding suggests that although pAEC therapy can induce robust short-term improvements, these effects may not persist over time, a phenomenon observed by Wang et al. (2022) and Maksimczuk et al. (2022) in their studies on MI and kidney function. Regarding heart valves, pAEC therapy demonstrated consistent benefits, particularly in the mitral and aortic valves, where the MI pAEC group maintained the highest VTI over time. In contrast, the combination therapy (MI pAEC + K) showed a significant decline in VTI, particularly in the mitral valve, suggesting that combining pAEC with cardiomyocytes might reduce the long-term effectiveness of the therapy, as indicated by Kobe et al. (2019). The tricuspid valve also showed a short-term benefit from combination therapy, but this effect was diminished by 8 weeks, similar to the carotid artery findings noted by Hachimi-Idrissi 390 (2023). The NT-proBNP biomarker results revealed an initial increase in cardiac stress, particularly in the MI pAEC group. However, this elevation was transient, with NT-proBNP levels returning to baseline by 8 weeks. This suggests that while pAEC therapy induces an acute response, this does not translate into long-term cardiac stress. The combination therapy did not significantly enhance or prolong the effect on NT-proBNP levels, further indicating that the addition of cardiomyocytes does not provide significant synergistic benefits, which is consistent with the findings by Song et al. (2015) and Tajabadi et al. (2022). In conclusion, these findings highlight the potential for pAEC therapy to provide short-term hemodynamic improvements, particularly in the carotid artery and heart valves. However, the long-term effects may be less sustained, particularly when cardiomyocytes are added to the treatment. Further research is needed to explore the mechanisms behind these differential responses and to optimize stem cell therapies for long-term benefits in MI recovery (Castiglione et al. 2022; Nyvad et al. 2022; Liu et al. 2023). The complex interaction between stem cell types and the body’s natural compensatory mechanisms, as described by Rajagopal et al. (2023) and Zanoli et al. (2020), warrants further exploration to improve therapeutic outcomes. ConclusionThe findings indicate that AEC stem cell therapy seems to be more effective in mitigating cardiac stress and safeguarding the function of associated organs, particularly over extended periods. The combined treatment of AEC with cardiomyocytes did not demonstrate significant benefits compared to AEC therapy alone, suggesting the need for additional research to elucidate the underlying mechanisms of this stem cell intervention. In the absence of treatment, as evidenced by the placebo MI group, cardiac damage and stress tended to worsen considerably over time. This observation underscores the necessity of prompt intervention for patients with MI. In summary, the results support the potential of AEC stem cell therapy as an efficient method for managing and alleviating cardiac stress in MI cases. Additionally, the study highlights the importance of extended follow-up to evaluate the long-term effects of treatment and disease progression in individuals with MI. List of AbbreviationsAEC, Amniotic epithelial cells; ECG, Electrocardiogram; K, Cardiomyocyte; MI, Myocardial infarction; NT-ProBNP, N-terminal pro B-type natriuretic peptide; pAEC, Porcine amniotic epithelial cells; pAEC + K, combination of porcine amniotic epithelial cells with cardiomyocytes collected from animal models that were implanted autologously; PW Doppler, Pulsed wave Doppler; VTI, Velocity time integrals AcknowledgmentThe authors thank the Ministry of Research and Technology/National Research and Innovation Agency for funding the superior applied research for higher education (PTUPT) with number 2048/IT3.L1/PN/2021 which provided funding for preliminary in vivo research. Conflict of interestsThe authors declare no conflicts of interest with the parties involved in this research. FundingThis work was supported by the Fundamental Research Scheme Lecturer Research Program (RI-FUND) for the 20235–2024 budget year with number 419/IT3.D10/PT.01.03/P/B/2023), which provided financial assistance for biomarker data testing. Author’s contributionsADF: general study design, data collection and interpretation, manuscript drafting. DN: cardiologist expertise and interpretation, manuscript evaluating. G: design of infarction induction in animal models. AE: interpretation of clinical pathology data. MS: statistical data analysis. Data availabilityAll data supporting the findings of this study are available within the manuscript. There are no additional data sources required. 453 References Akodad, M., Fauconnier, J., Sicard, P., Huet, F., Blandel, F., Bourret, A., de Santa Barbara, P., Aguilhon, S., LeGall, M., Hugon, G., Lacampagne, A. and Roubille, F. 2017. Interest of colchicine in thetreatment of acute myocardial infarct responsible for heart failure in a mouse model. Int. J. Cardiol. 240, 347–353. Alexander, A., Saraf, Shailendra, Saraf, Swarnlata, Agrawal, M., Patel, R.J., Agrawal, P., Khan, J. and Ajazuddin. 2019. Amalgamation of stem cells with nanotechnology: a unique therapeutic approach. Curr. Stem Cell Res. Ther. 14(2), 83–92. Andrysiak, K., Stępniewski, J. and Dulak, J. 2021. Human-induced pluripotent stem cell-derivedcardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research. Pflugers Arch. 473(7), 1061–1085. Bargehr, J., Ong, L.P., Colzani, M., Davaapil, H., Hofsteen, P., Bhandari, S., Gambardella, L., Le Novère, N., Iyer, D., Sampaziotis, F., Weinberger, F., Bertero, A., Leonard, A., Bernard, W.G., Martinson, A., Figg, N., Regnier, M., Bennett, M.R., Murry, C.E. and Sinha, S. 2019. Epicardial cells derived from humanembryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat. Biotechnol. 37(8), 895–906. Bont, C., Malik, J. and Beach, K. 2020. Comment on doppler ultrasound in vascular access care: the pearls and pitfalls of flow volume measurements. Port. J. Nephrol. Hypert. 34(2). Calabrese, E.J. 2022. Hormesis and embryonic stem cells. Chem. Biol. Interac. 352, 475, 109783. Castiglione, V., Aimo, A., Vergaro, G., Saccaro, L., Passino, C. and Emdin, M. 2022. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 27(2), 625–643. Chepeleva, E.V. 2023. Cell therapy in the treatment of coronary heart disease. Int. J. Mol. Sci. 24(23), 630055. Cuadra, B., Silva, V., Huang, Y.-L., Diaz, Y., Rivas, C., Molina, C., Simon, V., Bono, M.R., Morales, B., Rosemblatt, M., Silva, S., Acuña, R., Ezquer, F. and Ezquer, M. 2024. The immunoregulatory and regenerative potential of activated human stem cell secretome mitigates acute-on-chronic liver failure in a rat model. Int. J. Mol. Sci. 25(4), 2073. Cui, J.-N., Zhao, Y.-N., Wang, W. and Li, T. 2022. Associations of infarct size and regional myocardial function examined by cardiac magnetic resonance feature tracking strain analysis with the infarct location in patients with acute st-segment elevation myocardial infarction. Chin. Med. Sci J. 37(4), 309–319. Fénelon, M., Catros, S., Meyer, C., Fricain, J.C., Obert, L., Auber, F., Louvrier, A. and Gindraux, F. 2021. Applications of human amniotic membrane for tissue engineering. Membranes (Basel). MDPI AG. 11(6), 387. Hachimi-Idrissi, S. 2023. Stem cell therapy in neurological disorders: promises and concerns. Explor. Neuroprot. Ther. 3, 346–362. Hade, M.D., Suire, C.N. and Suo, Z. 2021. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells 10(8). Han, Yu, Li, X., Zhang, Y., Han, Yuping, Chang, F. and Ding, J. 2019. Mesenchymal stem cells for regenerative medicine. Cells 8(8), 866. Hoang, D.M., Pham, P.T., Bach, T.Q., Ngo, A.T.L., Nguyen, Q.T., Phan, T.T.K., Nguyen, G.H., Le, P.T.T., Hoang, V.T., Forsyth, N.R., Heke, M. and Nguyen, L.T. 2022. Stem cell-based therapy for human diseases. Signal Transduct. Target Ther. 7(1), 272. Kobe, J., Mishra, N., Arya, V., Al-Moustadi, W., Nates, W. and Kumar, B. 2019. Cardiac output monitoring: technology and choice. Ann. Card. Anaesth. 22(1), 6. Lange-Consiglio, A., Corradetti, B., Bertani, S., Notarstefano, V., Perrini, C., Marini, M.G., Arrighi, S., Bosi, G., Belloli, A., Pravettoni, D., Locatelli, V., Cremonesi, F. and Bizzaro, D. 2015. Peculiarity of porcine amniotic membrane and its derived cells: a contribution to the study of cell therapy from a large animal model. Cell Reprogram. 17(6), 472–483. Liu, Z.-L.L., Chen, H.-H.H., Zheng, L.-L.L., Sun, L.-P.P. and Shi, L. 2023. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 8(1), 198. Maksimczuk, J., Galas, A. and Krzesiński, P. 2022. What promotes acute kidney injury in patients with myocardial infarction and multivessel coronary artery disease—contrast media, hydration status or something else? Nutrients 15(1), 21. Müller, P., Lemcke, H. and David, R. 2018. Stem cell therapy in heart diseases-cell types, mechanisms and improvement strategies. Cell Physiol. Biochem. S. Karger AG, 48(6):2607–2655. Noviana, D. and Alham, F. 2012. Characteristics of blood flow in semilunar aorta valve of mongrel dog assessed by pulsed wave doppler echocardiography. Jurnal Veteriner 13(1), 1–8. Nyvad, J., Lerman, A. and Lerman, L.O. 2022. With a little help from my friends: the role of the renal collateral circulation in atherosclerotic renovascular disease. Hypertension (Dallas, Tex. : 1979). 516 79(4), 717. Park, S.-J., Kim, R.Y., Park, B.-W., Lee, S., Choi, S.W., Park, J.-H., Choi, J.J., Kim, S.-W., Jang, J., Cho, D.- W., Chung, H.-M., Moon, S.-H., Ban, K. and Park, H.-J. 2019. Dual stem cell therapy synergisticallyimproves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 10(1), 3123. Putra, M.A, Sandora, N., Kusuma, T.R., Fitria, N.A., Soetisna, T.W., Busro, P.W., Ardiansyah, Muttaqin, C., Makdinata, W. and Alwi, I. 2022. Co-culture of human cardiomyocyte and human amnion epithelial stem cells in amnion bilayer matrix for cardiomyogenesis. Cell Ther. Trans. 11(2), 72–83. Putra, M.A., Sandora, N., Suwarti, Nurhayati, R.W., Nauli, R., Kusuma, T.R., Fitria, N.A., Ardiansyah, Muttaqin, C., Makdinata, W. and Alwi, I. 2022. Transport viable heart tissue at physiological temperature yielded higher human cardiomyocytes compared to the conventional temperature. Cell Tissue Bank 23(4), 717–727. Rajagopal, S., Ruetzler, K., Ghadimi, K., Horn, E.M., Kelava, M., Kudelko, K.T., Moreno-Duarte, I., Preston, I., Rose Bovino, L.L., Smilowitz, N.R. and Vaidya, A. 2023. evaluation and management of pulmonary hypertension in noncardiac surgery: a scientific statement from the American Heart Association. Circulation 147(17), 1317–1343. Robinson, S., Ring, L., Oxborough, D., Harkness, A., Bennett, S., Rana, B., Sutaria, N., Lo Giudice, F., Shun-Shin, M., Paton, M., Duncan, R., Willis, J., Colebourn, C., Bassindale, G., Gatenby, K., Belham, M., Cole, G., Augustine, D. and Smiseth, O.A. 2024. The assessment of left ventricular diastolic function: guidance and recommendations from the British Society of Echocardiography. Echo. Res. Pract. 11(1),16. Sandora, N., Putra, M.A., Nurhayati, R.W., Suwarti, Nauli, R., Kusuma, T.R., Fitria, N.A., Ardiansyah, Muttaqin, C., Makdinata, W. and Alwi, I. 2022. Characterisation of the single-cell human cardiomyocytes taken from the excess heart tissue of the right ventricular outlet in congenital heart disease. Cell Tissue Bank. 23(3), 489–497. Song, Y.-S., Joo, H.-W., Park, I.-H., Shen, G.-Y., Lee, Y., Shin, J.H., Kim, H., Shin, I.-S. and Kim, K.-S. 2015. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplant. 24(10), 2055–2064. Tajabadi, M., Goran Orimi, H., Ramzgouyan, M.R., Nemati, A., Deravi, N., Beheshtizadeh, N. and Azami, M. 2022. Regenerative strategies for the consequences of myocardial infarction: chronological indication and upcoming visions. Biomed. Pharmacother. 146, 112584. Valatkaitė, E., Baušytė, R., Vitkevičienė, A., Ramašauskaitė, D. and Navakauskienė, R. 2021. Decidualization potency and epigenetic changes in human endometrial origin stem cells during propagation. Front. Cell Dev. Biol. 9, 765265. Villemain, O., Baranger, J., Friedberg, M.K., Papadacci, C., Dizeux, A., Messas, E., Tanter, M., Pernot, M. and Mertens, L. 2020. Ultrafast ultrasound imaging in pediatric and adult cardiology. JACC Cardiovasc. Imaging. 13(8), 1771–1791. Wang, Y., Qi, Z., Yan, Z., Ji, N., Yang, X., Gao, D., Hu, L., Lv, H., Zhang, J. and Li, M. 2022. Mesenchymal stem cell immunomodulation: a novel intervention mechanism in cardiovascular disease. Front. Cell Dev. Biol. 9, 742088. Yamanaka, S. 2020. Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell. 55827(4), 523–531. Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. and Rybak, Z. 2019. Stem cells: past, present, and future. Stem Cell Res. Ther. 10(1), 68. Zanoli, L., Briet, M., Empana, J.P., Cunha, P.G., Mäki-Petäjä, K.M., Protogerou, A.D., Tedgui, A., Touyz, R.M., Schiffrin, E.L., Spronck, B., Bouchard, P., Vlachopoulos, C., Bruno, R.M. and Boutouyrie, P. 2020. Vascular consequences of inflammation: a position statement from the ESH working group on vascular structure and function and the ARTERY Society. J. Hypert. 38(9), 1682–1698. Zhang, M., Liu, Q., Meng, H., Duan, H., Liu, X., Wu, J., Gao, F., Wang, S., Tan, R. and Yuan, J. 2024. Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct. Target Ther. 9(1), 12. 568. | ||
| How to Cite this Article |
| Pubmed Style Fitri AD, Noviana D, Gunanti G, Esfandiari A, Subangkit M. Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). Open Vet. J.. 2025; 15(1): 98-107. doi:10.5455/OVJ.2025.v15.i1.9 Web Style Fitri AD, Noviana D, Gunanti G, Esfandiari A, Subangkit M. Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). https://www.openveterinaryjournal.com/?mno=221027 [Access: January 08, 2026]. doi:10.5455/OVJ.2025.v15.i1.9 AMA (American Medical Association) Style Fitri AD, Noviana D, Gunanti G, Esfandiari A, Subangkit M. Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). Open Vet. J.. 2025; 15(1): 98-107. doi:10.5455/OVJ.2025.v15.i1.9 Vancouver/ICMJE Style Fitri AD, Noviana D, Gunanti G, Esfandiari A, Subangkit M. Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). Open Vet. J.. (2025), [cited January 08, 2026]; 15(1): 98-107. doi:10.5455/OVJ.2025.v15.i1.9 Harvard Style Fitri, A. D., Noviana, . D., Gunanti, . G., Esfandiari, . A. & Subangkit, . M. (2025) Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). Open Vet. J., 15 (1), 98-107. doi:10.5455/OVJ.2025.v15.i1.9 Turabian Style Fitri, Arni Diana, Deni Noviana, Gunanti Gunanti, Anita Esfandiari, and Mawar Subangkit. 2025. Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). Open Veterinary Journal, 15 (1), 98-107. doi:10.5455/OVJ.2025.v15.i1.9 Chicago Style Fitri, Arni Diana, Deni Noviana, Gunanti Gunanti, Anita Esfandiari, and Mawar Subangkit. "Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica)." Open Veterinary Journal 15 (2025), 98-107. doi:10.5455/OVJ.2025.v15.i1.9 MLA (The Modern Language Association) Style Fitri, Arni Diana, Deni Noviana, Gunanti Gunanti, Anita Esfandiari, and Mawar Subangkit. "Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica)." Open Veterinary Journal 15.1 (2025), 98-107. Print. doi:10.5455/OVJ.2025.v15.i1.9 APA (American Psychological Association) Style Fitri, A. D., Noviana, . D., Gunanti, . G., Esfandiari, . A. & Subangkit, . M. (2025) Implantation of porcine amniotic epithelial cells model for myocardial infarction combined with cardiomyocyte therapy of pigs (Sus scrofa domestica). Open Veterinary Journal, 15 (1), 98-107. doi:10.5455/OVJ.2025.v15.i1.9 |