| Research Article | ||
Open Vet. J.. 2025; 15(1): 108-117 Open Veterinary Journal, (2025), Vol. 15(1): 108-117 Research Article Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitataMaged Fouda1, Amira Negm2, Mousa Germoush1 and Shaymaa Mahmoud3*1Biology Department, College of Science, Jouf University, Sakaka, Saudi Arabia 2Horticulture Pests Department, Plant Protection Research Institute, Agriculture Research Center, Giza, Egypt 3Zoology Department, Faculty of Science, Menoufia University, Shebeen Elkom, Egypt *Corresponding Author:Shaymaa Mahmoud. Zoology Department, Faculty of Science, Menoufia University, Shebeen Elkom, Egypt. Email: dr.shaymaahussein [at] science.menofia.edu.eg Submitted: 20/09/2024 Accepted: 10/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
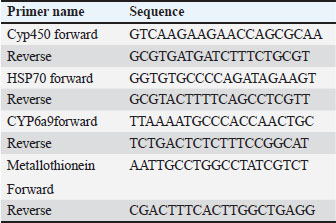
AbstractBackground: Environmental changes and rising temperatures have intensified the emergence of insect species as significant agricultural pests. Understanding the physiological and molecular responses of these pests to heat stress is essential for developing effective pest management strategies. Aim: To investigate the antioxidant enzyme activities and gene expression profiles of Ceratitis capitata under heat stress, spinosad exposure, and their combination to understand adaptive mechanisms and potential pathways for pest control. Methods: In this study, adult C. capitata were collected from grapes (Vitis mustangensis), mangoes (Mangifera indica), and yellow guava (Psidium guajava) cultivated in Egypt during June, July, and August 2023. Laboratory experiments assessed antioxidant enzyme activities (superoxide dismutase, catalase, peroxidase, malondialdehyde, and glutathione-S-transferases) and gene expression levels of heat-shock protein 70, cytochrome P450, CYP6a9, and metallothionein. Adults were exposed to three conditions; high temperature (40°C); spinosad at its LC50 (3.2 µg/ml) at 25°C, and combined spinosad exposure and high temperature. Results: Heat stress significantly increased the activity of antioxidant enzymes and the expression of stress-response genes in C. capitata. Spinosad exposure induced moderate increases in these activities, suggesting a detoxification response. The combined treatment of spinosad and heat stress amplified these effects, indicating a synergistic stress response. Conclusion: These findings provide insight into the molecular mechanisms underlying C. capitata’s heat tolerance and suggest potential pathways for pest control interventions under climate change. Keywords: Fruit fly, Ceratitis capitata, Heat-shock proteins, Antioxidant enzymes, Spinosad. IntroductionClimate change significantly influences the quantity and distribution of species. The range and quantity of terrestrial arthropods will be influenced by rising temperatures (Bale et al. 2002; Deutsch et al. 2008). Temperature is a crucial factor that affects the growth and distribution of ectothermic insects (Dixon et al. 2008). It has a significant role in determining several aspects of insect life, such as development, reproduction, behavior, physiology, and community composition (Zhang et al. 2015). The climatic variability hypothesis posits that animals subjected to greater fluctuations in their thermal environment have increased resilience to severe temperatures (Käfer et al. 2020). Insect life cycles rely on their ability to tolerate heat stress, which may lead to changes in life history, morphology, behavior, and physiological traits. The primary mechanisms employed by insects to develop resistance to temperature-induced stress are generally recognized as the enhancement of antioxidant enzyme activities and the synthesis of heat shock proteins (HSPs) (King and MacRae 2015; Kang et al. 2017). HSPs are a group of proteins that are produced in response to many stressors, such as excessive temperatures, diseases, poisons, lack of oxygen, and exposure to ionizing and UV radiation (Ali et al., 2017). These proteins are highly conserved, meaning they are quite similar across different species. HSPs serve as an initial defensive mechanism against harmful stress and have crucial activities in several molecular and physiological processes, such as repairing DNA, facilitating oogenesis, promoting embryo development, and enabling diapause (Dubrez et al. 2020). HSPs may be categorized into five groups based on their molecular weights and sequence similarities. These families include tiny Hsps, Hsp60s, Hsp70s, Hsp90s, and Hsp110s (Zhao and Jones 2012). Hsp70s is a highly heat-responsive and evolutionarily conserved protein family among the several families of HSPs, both in terms of its structure and function (Hu et al. 2022). Hsp70 may inhibit apoptosis by disrupting the signaling processes that initiate the apoptotic process (Lanneau et al. 2008; Liu et al. 2019). Thermal stress may cause oxidative damage and oxidative stress, characterized by increased amounts of reactive oxygen species (ROS) inside cells, which can harm lipids, proteins, and DNA (Lopez-Martinez et al. 2008). Antioxidant enzymes have a role in the response to oxidative damage, hence preventing damage caused by ROS (Yang et al. 2010; Chen et al. 2018). There exists an equilibrium between the generation of ROS and the mechanisms that counteract their harmful effects via antioxidant activities. Thermal stress may disturb the equilibrium and cause the production of more ROS, which in turn can lead to lipid peroxidation by damaging cell lipids (Lalouette et al. 2011; Schieber and Chandel, 2014). According to (Wang et al. 2001), insects are equipped with three primary antioxidant enzymes: catalase (CAT), peroxidase (PODs), and superoxide dismutase (SOD). An increase in antioxidant enzyme activities is indicative of the presence of oxidative stress and exhibits a robust ability to reduce oxidative stress by removing ROS from cells (Jia et al. 2011; Zhang et al. 2015). Ceratitis capitata is a significant agricultural pest affecting a variety of fruit crops globally, leading to substantial economic losses. Its adaptability to different climates and the potential for range expansion under warming temperatures make it an ideal model for studying heat stress responses in insects. Understanding the molecular mechanisms that enable C. capitata to withstand temperature fluctuations can enhance pest management strategies and mitigate the impact on agriculture (Rao et al., 2024). No research has been conducted to date to investigate the collective impact of temperatures on the activity of antioxidant enzymes and the expression of genes in C. capitata, to the best of our knowledge. As a result, the objective of this study was to examine the impact of temperature fluctuations on the response of antioxidant enzymes and HSPs genes to brief episodes of heat stress. In addition to providing new opportunities for the successful management of this parasite through the use of heat interventions, this study will provide insights into the molecular processes that give C. capitata its heat tolerance. Materials and MethodsField experimentsCeratitis capitata was originally collected from grapes (Vitis mustangensis ), mangoes (Mangifera indica), and yellow guava (Psidium guajava) cultivated in Giza and Ismailia, Egypt during June, July, and August 2023 and then reared in the laboratory as previously described by (Chen and Kang, 2005). To investigate the effect of variable temperatures among summer season on C. capitata adults, HSP, metallothionine and hydrocarbons proteins expressions level and antioxidant enzyme activities [SOD, CAT, POD, glutathione-S-transferases (GST) malondialdehyde (MDA)] were determined in the collected adults. Laboratory experimentsCombined effect of spinosad and high temperaturesAn investigation was conducted to examine the combined impact of spinosad and high temperature on C. capitata. A cohort of C. capitata adult insects was subjected to a temperature of 40°C for a duration of 1 hour, without being exposed to spinosad. Another group was subjected to the LC50 of spinosad, which was measured at 3.2 μg/ml, at a temperature of 25°C. The concurrent exposure to elevated temperature and spinosad was conducted as follows: adult organisms were subjected to a temperature of 40°C for a duration of 1 hour, after which they were moved to enclosures containing the LC50 of spinosad. Each group had 30 people, and each therapy had three duplicates. Antioxidant enzyme assaysThe activity levels of SOD, CAT, POD, GST, and MDA were determined using the spectrophotometric method. The processes used by the producer were applied to commercially accessible test packages from the Nanjing Jiancheng Bioengineering Institute in Jiangsu, China. The samples were mixed well in a solution of 0.9% saline at a ratio of one part sample to nine parts saline. The homogenate was centrifuged at 4°C for 15 minutes at a force 10,000 times the acceleration due to gravity. The resulting liquid located above the sediment was thereafter used for further examination. A CAT test was established using the protocols outlined by Singh et al. (2010). The measurement was conducted using a UV spectrophotometer at a wavelength of 240 nm, and the observed trend was a gradual reduction in absorbance over time, which may be attributed to the breakdown of H2O2. CAT activity was quantified as the rate at which it decomposes H2O2, measured in units per milligram of protein. Therefore, CAT activity was reported as U/mg protein. A POD test was established using the protocols outlined by Meng et al. (2009). The measurement was conducted using a UV spectrophotometer, specifically by determining the absorbance at a wavelength of 420 nm. The presence of the substrate H2O2 facilitated the oxidation process. The quantity that catalyzes the conversion of 1 μg of substrate per minute per milligram of protein was defined as a unit of POD activity. Therefore, POD activity was measured and reported as units per milligram of protein (U/mg protein). Utilizing xanthine and xanthine oxidase systems, we assessed SOD activity at 550 nm in a UV spectrophotometer in accordance with the procedures outlined by (Meng et al., 2009). The amount of enzyme that corresponds to a 50% inhibition rate in the reaction system was defined as one unit of SOD activity. Consequently, SOD activity was expressed as U/mg protein. The quantity of GST activity that facilitates the conversion of 1 μg of reduced glutathione-CDNB conjugate per minute per mg of protein was determined to be a single unit. Consequently, GST activity was quantified and reported in units per milligram of protein (U/mg protein). Quantitative real-time PCRTotal RNA was extracted using the SV Total RNA isolation method (Promega, San Luis Obispo, CA, USA), following the directions provided by the manufacturer. Using the spectrophotometry approach, the Eppendorf Bio Photometer Plus from Hamburg, Germany was used to determine the content and integrity of RNA. The CFX-96 Real-Time PCR System (Bio-Rad, Berkeley, CA, USA) was used for data analysis after the conversion of RNA to complementary DNA (cDNA) using the Prime Script RT Reagent Kit (Bio-Rad, Berkeley, CA, USA). The primers were generated using Primer 5.0 software (Table 1). Each gene-specific primer (10 μM), 2 μl of each cDNA template, 6 μl of ddH2O, and 10 μl of Bio-Rad iTaq™ Universal SYBR® Green Super mix (2 ×) made up the total reaction volume of 20 μl. The 2-ΔΔCt technique was employed to evaluate the relative mRNA levels, with actin serving as the reference gene. Each response was executed three times, and each treatment included four repetitions. The total reaction volume (20 μl) consisted of 10 μl of Bio-Rad iTaq™ Universal SYBR® Green Super mix (2 ×), 1 μl of each gene-specific primer (10 μM), 2 μl of each cDNA template, and 6 μl of ddH2O. Actin was used as a reference gene and the 2-ΔΔCt method was used to assess the relative mRNA levels. Each treatment included four replicates, and each reaction was conducted in triplicate. Table 1. List of primers used in the current study and their nucleotide sequence.
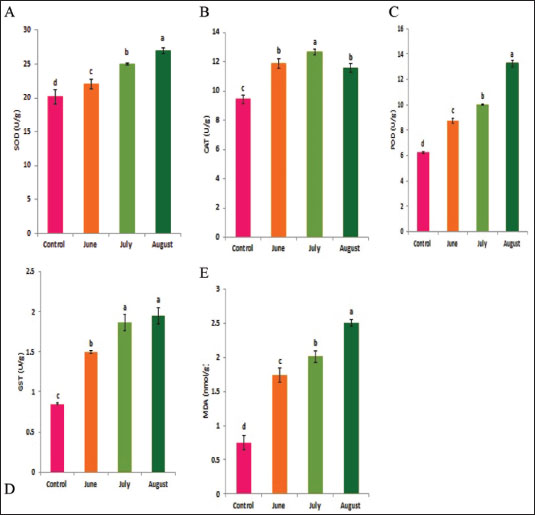
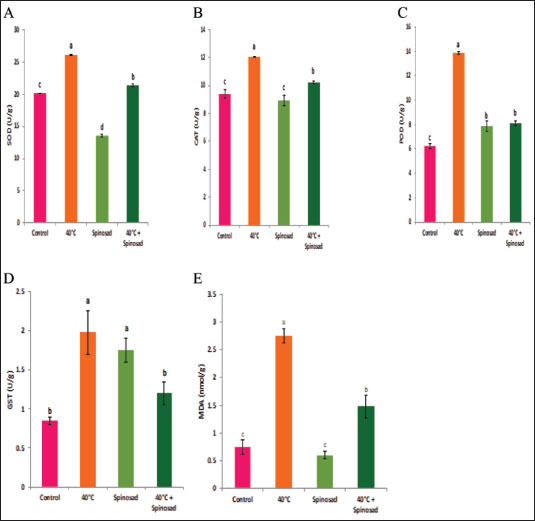
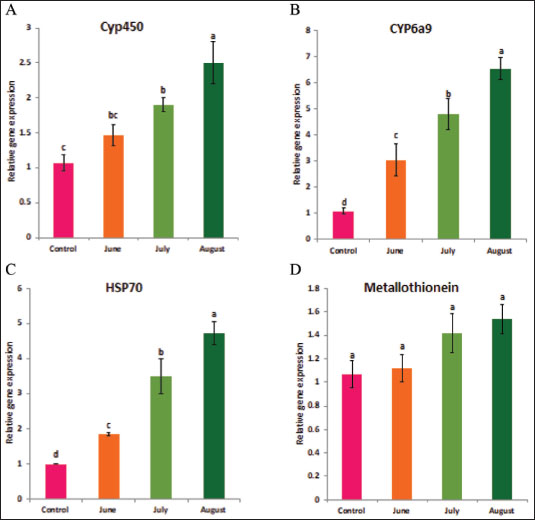
Statistical analysisA one-way ANOVA was employed to investigate statistical differences in mRNA levels among treatment groups. Tukey’s multiple comparison approach was employed to evaluate the statistical significance of gene expression levels and mortalities. The data were analyzed using SPSS v. 16.0 (SPSS, Chicago, IL, USA), and statistical significance was established at a threshold of p < 0.05. ResultsBiochemical experiments concerning antioxidant enzymatic activities were carried out for both monthly field experiments (June, July, and August 2023) and laboratory experiments (Combined effect of spinosad and high temperatures). Tolerance of the insects to high temperatures was assessed for adults by exposing the insects to 40°C for 1 hour. The temperature was chosen based on the actual temperature in fruit farms during the summer of 2022 and 2023. The antioxidant enzymes under investigation showed highly significant variation during the duration of the experiment. Similar highly significant variations in the two experiments (both monthly field experiments and laboratory experiments) were observed in SOD, CAT, POD, GST, and MDA concentration between control and treatment groups. In field experiments all enzyme activities were gradually elevated from June to August (active season), the highest activity was observed in August for SOD, POD, GST, and MDA (27.00 ± 0.23, 13.25 ± 0.14, 1.95 ± 0.05, and 2.50 ± 0.02, respectively) while the lowest activity was noticed in June (22.00 ± 0.40, 8.73 ± 0.11, 5.00 ± 0.01, and 1.74 ± 0.05) (Fig. 1) except for the CAT in which lowest activity was observed in August (11.56 ± 0. 17). In laboratory experiments also all enzyme activities showed highly significant variations between the control, group which was exposed to 40°C for 1 hour, the group was exposed to the LC50 of spinosad (LC50=3.2 μg/ml) at 25°C and the group of the combined of spinosad and high temperature. The highest activity was noticed in the group which was exposed to 40°C (high temperature only) (26.16 ± 0.57, 12.03 ± 0.01, 13.88 ± 0.05, 1.98 ± 0.16, and 2.76 ± 0.07), then gradual decrease was noticed at group which was exposed to the LC50 of spinosad (LC50=3.2μg/ml) at 25°C and the activity of all enzymes elevated again at group of the combined of spinosad and high temperature (Fig. 2) except GST activity decreased significantly (1.2 ± 0.08) comparing with both group which was exposed to 40°C (1.98 ± 0.16) and group which was exposed to the LC50 of spinosad only (1.75 ± 0.08). Analysis of mRNA expressionAs shown in Figure 3, in the field experiments, the relative gene expressions of Cyp450, HSP70, and CYP6a9 were highly significantly increased among active months (June, July, and August) as compared with control, the highest expression levels appeared in August (2.50 ± 0.17, 4.72 ± 0.19, and 6.54 ± 0.25 fold) and the lowest expression levels were observed in control. It found that metallothionein gene exhibited no significant change in expression levels among active months compared to controls. The expression levels of CYP450, HSP70, CYP6a9, and metallothionein were gradually elevated from June to August as compared with the control.
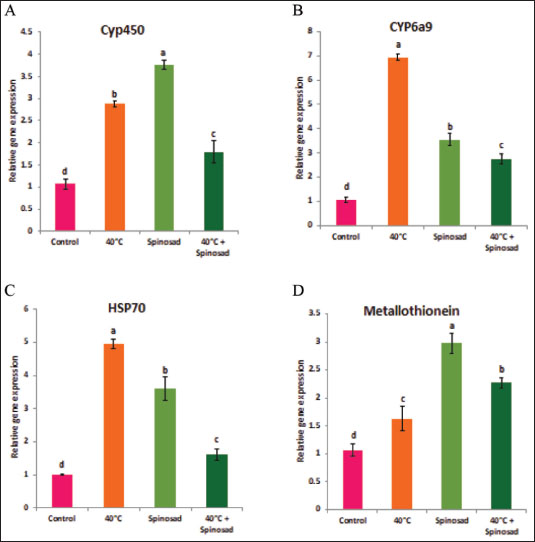
Fig. 1. Effect of temperature variation during summer seasons; June, July and August on antioxidant enzyme activities of C. capitata. A: SOD, B: CAT, C:POD, D: GST and E: MDA. In laboratory experiments, expression levels of CYP450, HSP70, CYP6a9, and metallothionein genes varied between control and different groups. Significant induction of Cyp450 mRNA was observed, increasing 2.88 ± 0.04 and 3.76 ± 0.06 fold at group that was exposed to 40°C for 1 hour and group that was exposed to the LC50 of spinosad (LC50=3.2 μg/ml) at 25°C, respectively, as compared to the control (1.07 ± 0.07), then the expression level dropped in group of the combined of spinosad and high temperature to 1.79 ± 0.14 fold. The degree of increase in the level of HSP70 and CYP6a9 mRNA was greatest at a group that was exposed to 40°C (4.96 ± 0.08 and 6.94 ± 0.07 fold, respectively) and then dropped gradually at the group that was exposed to the LC50 of spinosad (LC50 =3.2 μg/ml) at 25°C, and group of the combined of spinosad and high temperature as shown in Figure 4. The expression levels metallothionein showed the highest significant change at group which was exposed to the LC50 of spinosad (LC50=3.2 μg/ml) at 25°C (2.98 ± 0.11 fold) and then decreased again at group of the combined spinosad and high temperature (2.27 ± 0.07). DiscussionCurrent results demonstrated that heat shock induces oxidative stress in C. capitata, as evidenced by increased ROS production and elevated antioxidant enzyme activity. This aligns with findings by Heise et al. (2003), who reported that thermal stress leads to significant ROS production in insects, risking cellular damage to proteins, nucleic acids, and lipids. The protective role of antioxidant enzymes, including SOD, POD, and CAT, in counteracting oxidative stress has been widely documented in insects (Zhang et al. 2015), and our findings similarly indicate a defensive response, with increased antioxidant enzyme activities observed in C. capitata under heat stress.
Fig. 2. Combined effect of temperature and spinosad on antioxidant enzyme activities of C. capitata. A: SOD, B: CAT, C: POD, D: GST and E: MDA. Thermal stress induces an increase in oxidative stress burdens, resulting in the production of ROS compounds in greater quantities in insects (Heise et al. 2003). An excessive amount of ROS may cause the destruction of biological macromolecules, such as proteins, nucleic acids, and lipids, resulting in mutation, cell damage, and even death (Ryter et al. 2007). Antioxidant enzymes are potentially crucial for cellular adaptability. The significant rise in the activity of antioxidant enzymes indicates the presence of oxidative stress and demonstrates the capacity to effectively battle oxidative stress by removing ROS inside cells (Zhang et al. 2015). This research demonstrates that heat shock induces oxidative stress, as shown by the substantial changes in the rate of ROS production and the activity of antioxidant enzymes. Results confirmed that the antioxidant enzyme activity of SOD, POD, GST, and MDA in C. capitata was stimulated by increasing temperature in all experimental groups. This suggests that these enzymes have a defensive role in reducing the harmful impact of ROS produced by thermal stress. This aligns with several studies indicating that elevated temperatures activate the immune responses of insects, including antioxidants and HSPs (Yang et al. 2010; Kang et al. 2017). The rise in total peroxide concentrations during the active season may be attributed to the heightened activity of C. capitata and their flight to gather food (Detzel and Wink 1993). Yan and Sohal (2000) also agreed with these results and concluded that when flight activity was experimentally prevented, there was a reduction in the rate of H2O2 formation and a decrease in oxidative damage to certain mitochondrial proteins. Thus, antioxidants play a crucial role in protecting insects from both external and internal oxidative radicals (Krishnan and Kodrίk 2006). Superoxide dismutases serve as the primary defense against oxygen-free radicals, namely O2•−. They possess potent antioxidant characteristics and have shown the ability to safeguard both normal cells and various pathogens against ROS (Oliveira et al. 2011). Insects have been shown to possess the enzymatic activity of superoxide dismutase, which helps them counteract the harmful effects of ROS (Suh et al. 2010). Furthermore, it is worth noting that the SOD activity was influenced by both temperature and relative humidity. The elevation was in the active season, which was marked by elevated temperatures and relative humidity. In contrast, the moderately active season exhibited lower levels of relative humidity and temperature.
Fig. 3. Effect of temperature variation during summer seasons; June, July and August on gene expression of C. capitata. A: Cyp450, B: Cyp6a9, C: HSP70and D: metallothionein. In August, during the active season, the amount of CAT activity in C. capitata decreased, indicating a reduction in total peroxide concentrations. This might be attributed to the extraordinary ability of CAT to efficiently remove H2O2, particularly when it is created at low levels. Clavaron-Mathews et al. (1997) suggested that CAT is not effective in reducing H2O2 to low levels. This inefficiency may be attributed to increased oxidative stress caused by flight activity, as well as the metabolic activity that generates H2O2, which is counteracted by the production of more antioxidants. Consistent with this finding, Corona et al. (2005) documented that some insects had elevated levels of antioxidant gene expression, which is likely associated with increased flying activity. This refers to the coordination and control of enzymes, which work together to maintain the redox state of the cell and prevent free radicals from exceeding typical activity levels. The heightened level of activity led to an elevation in the metabolic pathways responsible for detoxifying peroxides and allelochemicals via cytochrome (P-450, b, c) (Claudianos et al. 2006). This is in agreement with the results of this study, which showed that heat shock treatments (40°C) combined with spinosad treatment increased the expression of HSPs, CYP450, HSP70, CYP6a9, and metallothionein genes as compared to control, eventually leading to a reduction in spinosad activity. There is a strong correlation between how insects react to heat and the presence of HSPs. The HSPs function as chaperones to assist in the creation and restructuring of proteins after experiencing stress, and they may also improve immunological responses (Wojda, 2017). The heightened expression of HSPs, particularly HSP70, in response to elevated temperatures, is consistent with studies showing HSPs’ essential role in insect thermal tolerance (King and MacRae 2015; Kang et al. 2017). In fruit flies, for instance, HSP expression assists in protein stabilization and cellular repair following stress, as noted by Jones et al. (2018), underscoring their crucial function as molecular chaperones during thermal stress (Wojda 2017). Additionally, HSP23 and HSP27, associated with heat tolerance, have shown increased expression in response to heat stress in related insect species (Martínez-Paz et al. 2014; Khurshid et al. 2021), supporting the idea that various HSPs contribute to the survival of C. capitata under adverse conditions.
Fig. 4. Combined effect of temperature and spinosad on gene expression of C. capitata. A: Cyp450, B: Cyp6a9, C: HSP70and D: metallothionein. To safeguard themselves from elevated temperatures, insects have developed intricate protective proteins, as evidenced by a multitude of studies on the relationship between thermal stress and insects. This process has been primarily influenced by HSPs and antioxidant enzymes (Yang et al. 2010; King and MacRae, 2015). The abridged versions of HSPs, specifically Hsp22, Hsp23, and Hsp27, hormone-triggered shortened versions in Drosophila cells are about 100 nucleotides shorter, and the same is true for their associated messenger RNAs. Khurshid et al (2021) revealed that the expression of HSP genes in cells of the green peach aphid Myzus persicae may be stimulated via exposure to high-temperature shock (Jones et al. 2018). The peptide substrate is released from Hsp70 in an ATP-dependent manner by the Hsp110 genes, which operate as a nucleotide exchange factor. (Jones et al. 2018) conducted a recent review that found that species within the same genus exhibit varying responses to heat stress. This phenomenon may be attributed to the influence of heat stress on animal expression (Jones et al. 2018). Alterations in the activity of enzymatic indicators in insect tissues are indicative of the enzymatic defense response (Dampc et al. 2020). HSPs are accountable for safeguarding proteins from oxidative and hypertonic stress, which can be caused by exposure to foreign substances, severe temperatures, UV radiation, and parasitic infestations (Kang et al. 2017). As a result, it is advantageous for future agricultural practices to comprehend the transcriptional level of HSPs in a variety of heat stress scenarios. The gene expression of Hsp27 in Chironomus riparius was substantially elevated when subjected to heat stress at a temperature of 35°C for a duration of 2 hours, as per Martínez-Paz et al. (2014). The Hsp23 gene is essential for heat tolerance, as evidenced by numerous experiments. Insects that exhibited elevated Hsp gene expression exhibited a higher survival rate when exposed to thermal stress (Zhao and Jones, 2012). ConclusionThe current study demonstrates that elevated temperatures significantly influenced antioxidant enzyme activities and gene expression of key heat stress-related genes in Ceratitis capitata. Up-regulation of studied genes under various heat stress conditions suggests their critical role in the insect’s heat tolerance and cellular stress response. Additionally, the efficacy of spinosad was influenced when combined with temperature stress, highlighting the complexity of pest management under changing environmental conditions. These findings underscore the importance of further research for more effective management strategies in response to environmental changes. AcknowledgmentThe authors extend their appreciation to the Deanship of Scientific Research at Jouf University, Saudi Arabia, for funding this work through research grant number (DSR2021-03-03151). Conflict of interestThe authors declare no conflict of interest. FundingThis study was funded by the Deanship of Scientific Research at Jouf University, Saudi Arabia, grant number (DSR2021-03-03151). Consent to participateAll authors agree to participate in this paper. Consent to publishThe final version of the manuscript was reviewed and approved by all authors. Ethical approvalNot applicable. Author contributionsMaged Fouda: Resources, Data curation, Funding acquisition, Project administration, writing-review & editing. Amira Negm: Data curation, Formal analysis, Writing-review & editing. M. Germoush: Data curation, Formal analysis. Shaymaa Mahmoud Conceptualization, Data curation, Formal analysis, Supervision, Writing-review & editing. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data generated during this study are included in this article. ReferencesAli, A., Rashid, M.A., Huang, Q.Y. and Lei, C.L. 2017. Influence of UV-A radiation on oxidative stress and antioxidant enzymes in Mythimnaseparata (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. Int. 24(9), 8392–8398. https://doi.org/10.1007/s11356-017-8514-7 Bale, J., Masters, G., Hodkinson, I., Awmack, C., Bezemer, T.m., Brown, V., Butterfield, J., Buse, A., Coulson, J., Farrar, J., Good, J., Harrington, R., Hartley, S., Jones, T., Lindroth, R., Press, M., Symrnioudis, I., Watt, A. and Whittaker, J. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16. https://doi.org/10.1046/j.1365-2486.2002.00451.x Chen, B. and Kang, L. 2005. Implication of pupal cold tolerance for the northern over-wintering range limit of the leafminerLiriomyzasativae (Diptera: Agromyzidae) in China. Appl. Entomol. Zool. 40, 437–446. https://doi.org/10.1303/aez.2005.437 Chen, H., Solangi, G.S., Guo, J., Wan, F. and Zhou, Z. 2018. Antioxidant responses of ragweed leaf beetle Ophraellacommuna (Coleoptera: Chrysomelidae) exposed to thermal stress. Front. Physiol. 9, 808. https://doi.org/10.3389/fphys.2018.00808 Claudianos, C., Ranson, H., Johnson, R.M., Biswas, S., Schuler, M.A., Berenbaum, M.R., Feyereisen, R. and Oakeshott, J. G.2006. Adeficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect. Mol. Biol. 15(5), 615–636; doi: 10.1111/j.1365-2583.2006.00672.x Clavaron-Mathews, M., Summers, C.B. and Felton, G.F.1997. Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch. Insect. Biochem. Physiol. 34, 57–68. https://doi.org/10.1002/(SICI)1520-6327(1997)34:1<57::AID-ARCH5>3.0.CO;2-T Dampc, J., Kula-Maximenko, M., Molon, M. and Durak, R. 2020. Enzymatic defense response of apple Aphid Aphis pomi to increased temperature. Insects 11(7), 436. https://doi.org/10.3390/insects11070436 Detzel, A. and Wink, M.1993. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4, 8–18. https://doi.org/10.1007/BF01245891 Deutsch, C.A., Tewksbury, J.J., Huey, R.B., Sheldon, K.S., Ghalambor, C.K., Haak, D.C. and Martin, P.R. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. U.S.A. 105(18), 6668–6672. https://doi.org/10.1073/pnas.0709472105 Dixon, A., Honek, A., Keil, P., Kotela, M., Sizling, A. and Jarošík, V. 2008. Relationship between the minimum and maximum temperature thresholds for development in insects. Funct. Ecol. 23, 257–264. https://doi.org/10.1111/j.1365-2435.2008.01489.x Dubrez, L., Causse, S., Borges Bonan, N., Dumétier, B. and Garrido, C. 2020. Heat-shock proteins: chaperoning DNA repair. Oncogene 39(3), 516–529. https://doi.org/10.1038/s41388-019-1016-y Heise, K., Puntarulo, S., Pörtner, H.O. and Abele, D. 2003. Production of reactive oxygenspecies by isolated mitochondria of the Antarctic bivalve Laternula elliptica(King and Broderip) under heat stress. Comp. Biochem. Physiol. C 134, 79–90; doi: 10.1016/s1532-0456(02)00212-0 Hu, C., Yang, J., Qi, Z., Wu, H., Wang, B., Zou, F., Mei, H., Liu, J., Wang, W. and Liu, Q. 2022. Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm 3(3), e161. https://doi.org/10.1002/mco2.161 Jia, F.X., Dou, W., Hu, F. and Wang, J.J. 2011. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactroceradorsalis (Diptera: Tephritidae). Florida Entomol. 94(4), 956–963. https://doi.org/10.1653/024.094.0432 Jones, L.M., Eves-van den Akker, S., van-OostenHawle, P., Atkinson, H.J. and Urwin, P.E. 2018. Duplication of hsp-110 is implicated in differential success of globodera species under climate change. Mol. Biol. Evol. 35(10), 2401–2413. https://doi.org/10.1093/molbev/msy132 Käfer, H., Kovac, H., Simov, N., Battisti, A., Erregger, B., Schmidt, A.K.D. and Stabentheiner, A. 2020. Temperature tolerance and thermal environment of European seed bugs. Insects 11(3), Article 3. https://doi.org/10.3390/insects11030197 Kang, Z.W., Liu, F.H., Liu, X., Yu, W.B., Tan, X.L., Zhang, S.Z., Tian, H.G. and Liu, T.X. 2017. The potential coordination of the heat- shock proteins and antioxidant enzyme genes of Aphidius gifuensis in response to thermal stress. Front. Physiol. 8, 976. https://doi.org/10.3389/fphys.2017.00976 Khurshid, A., Inayat, R., Tamkeen, A., Ul Haq, I., Li, C., Boamah, S. and Liu, C. 2021. Antioxidant enzymes and heat-shock protein genes of green peach aphid (Myzus persicae) under short-time heat stress. Front. Physiol. 12, 805509; doi: 10.3389/fphys.2021.805509 King, A.M. and MacRae, T.H. 2015. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 60, 59–75. https://doi.org/10.1146/annurev-ento-011613-162107 Kramer BH, Nehring V, Buttstedt A, Heinze J, Korb J, Libbrecht R, Meusemann K, Paxton RJ, Séguret A, Schaub F, Bernadou A. Oxidative stress and senescence in social insects: a significant but inconsistent link? Philos Trans R Soc Lond B Biol Sci. 2021 Apr 26;376(1823):20190732. doi: 10.1098/rstb.2019.0732. Krishnan, N. and Kodrίk, D. 2006. Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protectgut tissues during oxidative stress? J. Insect. Physiol. 52(1), 11–20; doi: 10.1016/j.jinsphys.2005.08.009 Lalouette, L., Williams, C.M., Hervant, F., Sinclair, B.J. and Renault, D. 2011. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 158(2), 229–234. https://doi.org/10.1016/j.cbpa.2010.11.007 Lanneau, D., Brunet, M., Frisan, E., Solary, E., Fontenay, M. and Garrido, C. 2008. Heat shock proteins: essential proteins for apoptosis regulation. J. Cell. Mol. Med. 12(3), 743–761. https://doi.org/10.1111/j.1582-4934.2008.00273.x Liu, T., Han, Y., Liu, Y. and Zhao, H. 2019. Genomewide identification and analysis of heat-shock proteins 70/110 to reveal their potential functions in Chinese soft-shelled turtle Pelodiscussinensis. Ecol. Evol. 9(12), 6968–6985. https://doi.org/10.1002/ece3.5264 Lopez-Martinez, G., Elnitsky, M.A., Benoit, J.B., Lee, R.E. and Denlinger, D.L. 2008. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect. Biochem. Mol. Biol. 38(8), 796–804. https://doi.org/10.1016/j.ibmb.2008.05.006 Martínez-Paz, P., Morales, M., Martín, R., Martínez-Guitarte, J.L. and Morcillo, G. 2014. Characterization of the small heat shock protein Hsp27 gene in Chironomus riparius (Diptera) and its expression profile in response to temperature changes and xenobiotic exposures. Cell Stress Chaperones 19(4), 529–540; doi: 10.1007/s12192-013-0479-y Meng, J.Y., Zhang, C.Y., Zhu, F., Wang, X.P. and Lei, C.L. 2009. Ultraviolet light-induced oxidative stress: effects on antioxidant response of Helicoverpa armigera adults. J. Insect. Physiol. 55(6), 588–592. https://doi.org/10.1016/j.jinsphys.2009.03.003 Oliveira, J.H., Goncalves, R.L., Lara, F.A., Dias, F.A.,Gandara, A.C., Menna-Barreto, R.F., Edwards, M.C., Laurindo, F.R., Silva-Neto, M.A., Sorgine, M.H. and Oliveira, P.L. 2011. Blood meal -derived heme decreases ROS levels in the midgut of Aedes aegyptian dallows proliferation of intestinal microbiota. PLoS Pathog. 7, e1001320. Rao, J., Zhang, Y., Zhao, H., Guo, J., Wan, F., Xian, X., Yang, N. and Liu, W. 2024. Projecting the global potential geographical distribution of Ceratitis capitata (Diptera: Tephritidae) under current and future climates. Biology 13(3), 177. https://doi.org/10.3390/biology13030177 Ryter, S.W., Kim, H.P., Hoetzel, A., Park, J.W., Nakahira, K. and Choi, A.M. 2007. Mechanism of cell death in oxidative stress. Antioxid. Redox Signal. 9(1), 49–89; doi: 10.1089/ars.2007.9.49 Schieber, M. and Chandel, N.S. 2014. ROS function in redox signaling and oxidative stress. Curr. Biol. 24(10), R453–462. https://doi.org/10.1016/j.cub.2014.03.034 Singh, B., Sharma, S. and Singh, B. 2010. Antioxidant enzymes in cabbage: variability and inheritance of superoxide dismutase, peroxidase and catalase. Sci. Hortic. 124, 9–13. https://doi.org/10.1016/j.scienta.2009.12.011 Suh, H.J., Kim, S.R., Lee, K.S., Park, S. and Kang, S.C. 2010. Antioxidan t activity of various solven t extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. J. Photochem. Photobiol. B. 99(2), 67–73; doi: 10.1016/j.jphotobiol.2010.02.005 Wang, Y., Oberley, L.W. and Murhammer, D.W. 2001. Antioxidant defense systems of two lipidopteran insect cell lines.Free Radic. Biol. Med. 30(11), 1254–1262. https://doi.org/10.1016/s0891-5849(01)00520-2 Wojda, I. 2017. Temperature stress and insect immunity. J. Therm. Biol. 68(Pt A), 96–103. https://doi.org/10.1016/j.jtherbio.2016.12.002 Yan, L.J. and Sohal, R.S. 2000. Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associ ated oxidative damage to specific mitochondrial proteins. Free Radic. Biol. Med. 29(11), 1143–1150; doi: 10.1016/s0891-5849(00)00423-8 Yang, L.H., Huang, H. and Wang, J.J. 2010. Antioxidant responses of citrus red mite, Panonychuscitri (McGregor) (Acari: Tetranychidae), exposed to thermal stress. J. Insect. Physiol. 56(12), 1871–1876. https://doi.org/10.1016/j.jinsphys.2010.08.006 Zhang, S., Fu, W., Li, N., Zhang, F. and Liu, T.X. 2015. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect. Physiol. 73, 47–52. https://doi.org/10.1016/j.jinsphys.2015.01.004 Zhao, L. and Jones, W. 2012. Expression of heat shock protein genes in insect stress response. Invertebr. Surviv. J. 9, 93–101. | ||
| How to Cite this Article |
| Pubmed Style Fouda M, Negm A, Germoush M, Mahmoud S. Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. Open Vet. J.. 2025; 15(1): 108-117. doi:10.5455/OVJ.2025.v15.i1.10 Web Style Fouda M, Negm A, Germoush M, Mahmoud S. Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. https://www.openveterinaryjournal.com/?mno=221355 [Access: January 09, 2026]. doi:10.5455/OVJ.2025.v15.i1.10 AMA (American Medical Association) Style Fouda M, Negm A, Germoush M, Mahmoud S. Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. Open Vet. J.. 2025; 15(1): 108-117. doi:10.5455/OVJ.2025.v15.i1.10 Vancouver/ICMJE Style Fouda M, Negm A, Germoush M, Mahmoud S. Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. Open Vet. J.. (2025), [cited January 09, 2026]; 15(1): 108-117. doi:10.5455/OVJ.2025.v15.i1.10 Harvard Style Fouda, M., Negm, . A., Germoush, . M. & Mahmoud, . S. (2025) Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. Open Vet. J., 15 (1), 108-117. doi:10.5455/OVJ.2025.v15.i1.10 Turabian Style Fouda, Maged, Amira Negm, Mousa Germoush, and Shaymaa Mahmoud. 2025. Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. Open Veterinary Journal, 15 (1), 108-117. doi:10.5455/OVJ.2025.v15.i1.10 Chicago Style Fouda, Maged, Amira Negm, Mousa Germoush, and Shaymaa Mahmoud. "Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata." Open Veterinary Journal 15 (2025), 108-117. doi:10.5455/OVJ.2025.v15.i1.10 MLA (The Modern Language Association) Style Fouda, Maged, Amira Negm, Mousa Germoush, and Shaymaa Mahmoud. "Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata." Open Veterinary Journal 15.1 (2025), 108-117. Print. doi:10.5455/OVJ.2025.v15.i1.10 APA (American Psychological Association) Style Fouda, M., Negm, . A., Germoush, . M. & Mahmoud, . S. (2025) Temperature and spinosad-induced modulation of antioxidant enzyme activity and gene expression of adaptive stress-related genes in Ceratitis capitata. Open Veterinary Journal, 15 (1), 108-117. doi:10.5455/OVJ.2025.v15.i1.10 |