| Research Article | ||
Open Vet. J.. 2025; 15(1): 126-138 Open Veterinary Journal (2025), Vol. 15(1): 126-138 Research Article Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolisMarwa B. M. Gomaa1†, Khaled M. A. Abdelhameed2, Sherien E. Sobhy3, Hanan M. A. Konper1, Zienab A. E. Hassanein1, Ahmed A. Saleh4,5†*, Mamdoh T. Jamal6 and Elsayed E. Hafez31Plant Protection Research Institute, Agricultural Research Center, Dokki, Giza, Egypt 2Apicalture Department, Plant Protection Research Institute, Agricultural Research Center, Giza, Egypt 3Plant Protection and Bimolecular Diagnosis Department, Arid Lands Cultivation Research Institute, City of Scientific Research and Technological Applications, New Borg El.Arab, 21934, Egypt 4College of Animal Science and Technology, Yangzhou University, Yangzhou, 225009, Jiangsu, China 5Animal and Fish Production Department, Faculty of Agriculture (Al-Shatby), Alexandria University, Alexandria City, 11865, Egypt 6Department of Marine Biology, Faculty of Marine Sciences, King Abdul-Aziz University, Jeddah, 21589, Saudi Arabia †These authors contributed equally to the current work. *Corresponding Author: Ahmed A. Saleh. Animal and Fish Production Department, Faculty of Agriculture (Al-Shatby), Alexandria University, Alexandria City, 11865, Egypt. Email: Elemlak1339 [at] Gmail.Com Submitted: 27/09/2024 Accepted: 03/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractBackground: Propolis, a resinous substance produced by bees, exhibits significant phytochemical and biological properties, which have been explored for various therapeutic applications. Aim: This study investigates the phytochemical composition, antioxidant activity, antibacterial efficacy, and anticancer potential of ethanolic extracts from three propolis samples (P1, P2, and P3). Methods: Phytochemical screening was conducted to determine the presence of bioactive compounds, such as ascorbic acid, saponins, and tannins. Antioxidant activity was evaluated using the phosphomolybdate (PMA) and ferric reducing power (FRP) assays. The antibacterial efficacy against Salmonella Typhimurium and Staphylococcus aureus was assessed using the well diffusion method. Cytotoxicity and anticancer effects were investigated using the MTT assay on red blood cells (RBCs) and various carcinoma cell lines (HepG2, MDA, and A549). Gene expression analysis was performed using RT-qPCR to assess the upregulation of immune response genes (P53, Bcl2, Bax, Ca125, and C3). Results: Phytochemical screening revealed considerable quantities of ascorbic acid, saponins, and tannins in the propolis samples. The P1 sample exhibited the most substantial antioxidant activity, with FRP values at 62.9 mg/g DM and PMA content at 20.7 mg/g DM. In antibacterial assays, P1 demonstrated the highest inhibitory zones at the maximum concentration (400 mg/ml), outperforming standard antibiotic treatments. In cytotoxicity and anticancer assays, P1 preserved the highest percentage of RBCs from hemolysis and showed marked anticancer activity, with the lowest cell viability observed at 3.9 µg/ml. Gene expression analysis revealed significant upregulation of immune response genes, particularly in MDA and HepG2 cell lines upon P1 treatment. Conclusion: This study underscores the potent antioxidant, antibacterial, and anticancer properties of propolis, highlighting its potential as a natural therapeutic agent. The observed activities suggest promising applications for propolis in combating bacterial infections and various cancer types, warranting further exploration into its molecular mechanisms and potential clinical uses. Keywords: Propolis, Anti-cancer, Lung cancer, Liver cancer, Immune response. IntroductionThe escalation in antibiotic resistance among microbes, particularly pathogenic bacteria, poses a significant current public health challenge with the potential to trigger a global pandemic (Chen et al., 2022). Consequently, there is an urgent need to explore innovative approaches for combating drug-resistant bacteria. Common bacterial pathogens, such as Klebsiella pneumoniae, Salmonella typhimurium, Acinetobacter baumannii, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Clostridium sp., have developed resistance to a broad spectrum of antibiotics (Docquier and Mangani, 2018). Globally, E. coli, in conjunction with Salmonella species, stands out as one of the most significant human pathogens, causing severe infections (Ramos et al., 2020). Furthermore, S. aureus, which has long been recognized for causing a wide array of infectious diseases, ranging from mild to fatal, is notorious for its ability to resist numerous antibiotics (Chen et al., 2022). In addition to the challenge of multidrug-resistant bacteria, the incidence of cancer continues to escalate. Cancer, a multifaceted disease, presents numerous complexities in modern medicine. Annually, an estimated 10 million individuals globally lose their lives to cancer, with breast, lung, colon, and prostate cancers showing the highest prevalence (Lin et al., 2021; El Fawal et al., 2024). The pursuit of effective cancer therapies is ongoing, with chemotherapy and radiation therapy standing out as the predominantly utilized treatment modalities. Despite their common usage, these therapeutic approaches are frequently plagued by significant drawbacks, including toxicity, resistance, poor selectivity, and severe systemic side effects, underscoring the urgent need for alternative treatment avenues (Cheville et al., 2021). Leveraging natural extracts as anticancer agents offers several advantages when compared to conventional chemotherapy and radiation therapy (Elnasr et al., 2024). A promising area of research involves the exploration of natural products as potential sources of antibacterial and anticancer agents (Naeem et al., 2022). Recently, the use of natural extracts as antimicrobials has garnered significant attention due to their safety, eco-friendliness, and cost-effectiveness (Rabiee et al., 2023; Gebreslassie and Gebremeskel, 2024). Propolis, a natural bee product composed of a resinous mixture with a diverse chemical composition, has been used in folk medicine since ancient times (Barsola and Kumari, 2022). The properties of propolis are influenced by various factors such as climate zones, seasons, vegetation, and environmental conditions (Silva-Carvalho et al., 2015). Propolis exhibits antibacterial, antifungal, hepatoprotective, anti-proliferative, antimicrobial, antioxidative, anti-inflammatory, antiviral, immunomodulatory, and regenerative activities (Tatli Seven et al., 2018). The active components of propolis have been identified as polyphenols, flavonoids, and numerous other ingredients (Daleprane and Abdalla, 2013). Understanding these processes involves the application of various molecular techniques. Quantitative real-time polymerase chain reaction (qRT-PCR) stands out as a reliable, sophisticated, and reproducible technology that offers distinct advantages for gene expression analysis over traditional methods (Singh and Ali, 2016). Furthermore, the simplicity and widespread applicability of this technique have revolutionized the study by elucidating gene induction/suppression under both controlled and stress conditions (Chaudhary et al., 2024; Ijaz et al., 2024). Additionally, qRT-PCR is recognized as the most precise and commonly used method for gene quantification due to its broad dynamic range, allowing the detection of rare transcripts and subtle changes in gene expression (Nolan et al., 2006). Moreover, assessing gene expression in response to stress and various environmental stressors enables a more accurate evaluation of antioxidant/immune-responsive gene activation compared to enzyme activity and physiological parameters (Liu et al., 2012; Sobhy et al., 2019). The aim of the present study was to evaluate the effectiveness of propolis as an antibacterial agent against multidrug-resistant bacteria and assess its inhibitory impact on their growth. Additionally, the research aims to investigate the mode of action of propolis on various cell lines to support its potential as an anticancer agent as an alternative to chemotherapy, along with examining molecular-level changes. Furthermore, the study seeks to quantify the antioxidant compounds present in propolis extract and assess their antioxidant activity. Materials and MethodsPropolis sampling and extract preparationThree propolis samples labelled as P1, P2, and P3 were collected for the study. The phytochemical screening of active compounds in these samples followed the methodology outlined in the study by Sobhy et al. (2023). To prepare the propolis extracts, a 10-g aliquot of propolis dry matter (DM) from each sample was dissolved in 100 ml of 80% ethanol as the solvent. The mixture was vigorously mixed and then placed in a dark location for 3 days to allow for extraction. Subsequently, the samples were centrifuged to separate the solid components, and the resulting ethanolic extract was used for further analysis. Estimation of antioxidant compoundsAscorbic acid estimationThe quantification of ascorbic acid was conducted as per Damon (1966) with minor adjustments. For the determination of ascorbate levels, a reaction mixture comprising 2% Na-molybdate, 0.15 N H2SO4, 1.5 mM Na2HPO4, and the plant extract in the presence of sulfosalicylic acid was prepared. The mixture was then incubated at 60°C in a water bath for 40 minutes followed by cooling and centrifugation at 4000 rpm for 10 minutes. The absorbance was measured at 660 nm, and the content of ascorbic acid was calculated as mg per gram of DM using a calibration curve prepared with ascorbic acid. Saponin estimationSaponin quantification was carried out using the method outlined by Hiai et al. (1975) with modifications. A volume of 100 µl of the extract was combined with 100 µl of vanillin reagent (8% w/v in 99.9% ethanol). This mixture was placed in an ice-cold water bath, and 2.5 ml of 72% (v/v) sulphuric acid was slowly added along the inner wall of the test tube. After thorough mixing, the contents were heated at 60°C for 10 minutes using a water bath and subsequently cooled in an ice-cold water bath. The absorbance was measured at 544 nm using a spectrophotometer against the reagent blank, and the total saponin content was expressed as mg per gram of DM. Estimation of tanninsThe tannin levels were assessed following the procedure outlined by Sereme et al. (1994) with slight modifications. In a clean test tube, the extract was combined with a freshly prepared solution of ammonium ferric citrate and 20% ammonia solution. The mixture was left at room temperature for 10 minutes and then measured spectro-photometrically at 525 nm. The tannin content was expressed as mg per gram of DM. Estimation of antioxidant activityThe antioxidant scavenging activity of the propolis samples was evaluated using two distinct methods: PMA and FRP. Assessment of phosphomolybdate reagent (PMA)The total antioxidant capacity of the extracts was evaluated using the PMA method with ascorbic acid as the reference standard (Jayaprakasha et al., 2006) with slight modifications. In this procedure, 0.1 ml of the extract solution was mixed with 3 mL of PMA reagent containing 0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate. Subsequently, the tubes were subjected to incubation in a boiling water bath for 30 minutes. After cooling to room temperature, the absorbance was measured at 695 nm against the blank. Assay of ferric reducing power (FRP)The FRP was assessed following the method proposed by Oyaizu, (1986) with certain modifications. The reaction was conducted in a mixture consisting of 1 ml of the sample extract, 1 ml of 0.2 M sodium phosphate buffer (pH 6.6), and 1 ml of potassium ferricyanide K3Fe(CN)6 (1% w/v), with incubation at 50°C for 20 minutes. Subsequent to the addition of 1 ml of trichloroacetic acid (20% w/v), the mixture was centrifuged at 5000 rpm for 15 minutes. The upper layer (1 ml) was then combined with 0.2 ml of freshly prepared FeCl3 (0.1% w/v), and the absorbance was measured at 700 nm against a blank without Pot. Ferricyanide. A higher absorbance value indicates a greater reducing power. Propolis antibacterial activityTo assess the antibacterial efficacy of propolis, tests were conducted against two human pathogens: S. typhimurium and S. aureus using the well diffusion method on solid LB media (Luria-Bertani agar) plates. Wells of 6 mm diameter were carefully positioned on the agar plates containing the suitable media, with the bacterial density adjusted to approximately 107 CFU/ml. Subsequently, 50 μl of the propolis extract was inoculated into the wells on the plates, and the agar plates were then incubated for 24 hours at 37 °C. The size of the inhibition zone was determined by measuring the diameter around the well (mm), incorporating the diameter of the well itself. Measurements were conducted in three fixed directions for all triplicates, and the average values were recorded. Gentamicin was employed as the positive control in this study. Cytotoxicity and anticancer effectIn this study, all cell lines used were GPEx cell lines obtained from Catalent Biologics in Anagni, Italy. The cytotoxic and anticancer effects of the extracts were assessed on red blood cells (RBCs), liver (HepG2), breast (MDA), and lung (A549) carcinoma cells sourced from the American Type Culture Collection in Manassas, VA. Cells (100 μl) were seeded at a density of 1 × 105 cells/mL into a 96-well tissue culture plate (cell passage number 103) and incubated at 37°C for 24 hours to form a complete monolayer (Mani et al., 2021). Cytotoxicity was evaluated using the MTT protocol (Alley et al., 1988), which is a widely accepted method for assessing cell viability and cytotoxic effects of substances at varying concentrations. Both normal and carcinomatous cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) under a humidified atmosphere of 5% CO2 and 95% air at 37°C. For cytotoxicity assessment, carcinomatous cells were cultured in 96-well plates with 100 μl of DMEM at a concentration of 5 × 108 cells/well for 24 hours at 37°C. Each sample at concentrations of 3.9, 7.8, 15.6, 31.2, 62.5, 125, 250, and 500 μg/ml was added to the respective wells, followed by incubation for 24 hours at 37°C. After centrifugation and washing with phosphate-buffered saline, 15 μl of MTT reagent (0.5 mg/ml) was added, and the plate was incubated for 4 hours at 37°C. To solubilize the formazan crystals, 150 μl of DMSO was added to each well, followed by stirring for 10 minutes on a shaker. The optical density (OD) of formazan products was measured at 570 nm using a spectrophotometer. The percentage of cell viability was calculated using the formula as follows. % Cell viability=(OD Sample/OD Control) * 100 where OD Control is the absorbance of the negative control and OD Sample is the absorbance of the test sample. Molecular evaluationsIn order to assess the effect of the P1 sample on three cancer cell lines (HepG2, MDA, and A549) by examining a panel of immune genes (P53, Bcl2, Bax, Ca125, and C3), primers for the five target genes are detailed in Table 1. Both treated and untreated cell lines were harvested, followed by RNA extraction and RT-qPCR analysis to quantify the expression levels of these genes. RNA extraction and cDNA synthesisTotal RNA from both control and treated cell lines was extracted using the RNeasy Mini Kit (Qiagen, Germany) following the manufacturer’s instructions. The cDNA synthesis was carried out in a total volume of 20 μl using a SureCycler 8800 thermocycler (Agilent Technologies, USA), with the oligo (dT) primer present at a concentration of 10 pmol/μl. The reaction components included 3 μl of RNA (500 ng), 2.0 μl of dNTPs (10 mM), 2.0 μl of Buffer, 5 μl of primer (10 pmol/μl), and 0.3 μl of reverse transcriptase enzyme (Biolabs, New England, Ipswich, MA) (5U/µl), and the remainder filled with ddH2O. The reaction conditions involved an initial cycle of enzyme activation at 42°C for 1 hour, followed by a final cycle of enzyme inactivation at 95°C for 5 minutes. Real-time RT-qPCRReal-time reverse transcription quantitative polymerase chain reaction (RT-qPCR) was conducted using the SYBR Green PCR Master Mix (Fermentas, USA). Each reaction was composed of a 25 μl mixture, comprising 1.5 μl of 10 pmol/l forward primer, 1.5 μl of 10 pmol/l reverse primer, 1 μl of template cDNA (50 ng), 12.5 μl of 2× SYBR Green, and 3.5 μl of nuclease-free water. All samples were run in triplicate. The reactions were carried out using a Rotor-Gene 6000 system (QIAGEN, ABI System, USA), with an amplification program consisting of an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30 sec, and final extension at 72°C for 30 sec. Subsequent to the 40 cycles, melting curves were generated to verify the absence of non-specific products. The data were normalized to the expression of GAPDH mRNA, a housekeeping gene, using the 2(−∆∆CT) method (Santangelo et al., 2018). Statistical analysisAll experiments, including those for antioxidant evaluation, antibacterial activity, cytotoxicity, and anticancer effects, were performed in triplicate, and the results are reported as mean ± standard deviation (SD). A one-way analysis of variance was carried out using the CoSTAT software on a Windows platform at a significance level of 0.05. Additionally, the LSD test was used to assess the significance of the results. Ethical approvalNot needed for this study. ResultsAntioxidant of propolis samplesThe evaluation of potential antimicrobial and anticancer activities of various propolis ethanolic extracts involved comprehensive screening of their antioxidant activities. These were assessed using methods such as the FRP and PMA assay , alongside the measurement of specific compounds such as saponins, tannins, and ascorbic acid, which are known indicators of antioxidant activity. As demonstrated in Table 2, the P1 propolis ethanolic extract emerged as the most effective, with an FRP value of 62.9 mg/g DM and the highest PMA content at 20.7 mg/g DM, in comparison to the P2 and P3 samples. Table 1. Phytochemical screening of three different propolis active compounds.
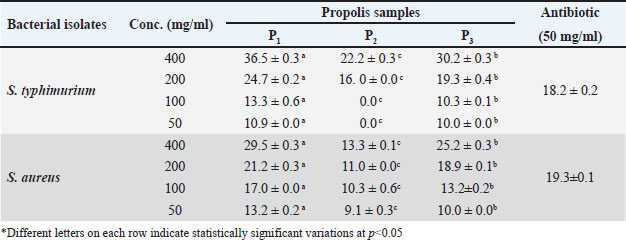
Table 2. The effect of different concentrations (400, 200, 100, and 50 mg/ml) of three propolis samples (P1, P2, and P3) on inhibition growth (mm) of different human bacterial isolates (S. typhimurium, and S. aureus) compared to antibiotic effect.
The superior antioxidant performance of the P1 sample may be attributed to its significantly high concentrations of bioactive compounds. The presence of saponins and tannins at 115.5 and 252 mg/g DM, respectively, in the northern sample, suggests a robust profile for combating oxidative stress. Ascorbic acid content, noted at 65.8 mg/g DM in the P1 extract, further enhances its antioxidant potential by providing additional mechanisms for neutralizing free radicals, which could play a role in inhibiting microbial growth and reducing cancer cell proliferation. These findings highlight the synergistic interactions between these compounds, indicating that P1’s effectiveness likely stems from a combination of these bioactives rather than a single constituent. Such a profile suggests that P1 propolis has profound potential in the realm of both antimicrobial and anticancer applications, offering a natural resource with substantial therapeutic promise. By better understanding these intricate interactions, the benefits of the P1 extract can be more effectively harnessed in relevant medical and pharmaceutical contexts. Antibacterial activityThe data presented in Table 3 evaluated the antibacterial effects of three propolis samples against S. typhimurium and S. aureus, offering insights into their potential as antibacterial agents. The analysis focused on varying concentrations (400, 200, 100, and 50 mg/ml) of the propolis samples (P1, P2, and P3) compared to a standard antibiotic concentration (50 mg/ml). At the highest concentration of 400 mg/ml, the P1 propolis sample demonstrated the strongest antibacterial activity across both bacterial strains. It produced significant inhibition zones of 36.5 ± 0.3 mm for S. typhimurium and 29.5 ± 0.3 mm for S. aureus, surpassing the inhibitory effects of the antibiotic treatment. This suggests that the bioactive components in P1 are highly effective at these concentrations, potentially due to synergistic interactions that enhance bacterial growth inhibition. Sample P3 also exhibited commendable antibacterial properties, creating inhibition zones of 30.2 ± 0.3 mm and 25.2 ± 0.3 mm for S. typhimurium and S. aureus, respectively, though it was slightly less effective than P1. The performance differences between P1 and P3 may hint at variations in the composition and concentration of active compounds within the extracts, influencing their antimicrobial potency. Conversely, P2 exhibited the least antibacterial efficacy among the three propolis samples, with notably smaller inhibition zones and being ineffective at lower concentrations for S. typhimurium (e.g., 50 mg/ml). This variability could be attributed to different geographical or botanical origins of the propolis, affecting the concentration and effectiveness of its antibacterial constituents. The remarkable antibacterial effectiveness of propolis, especially P1, highlights its potential to surpass conventional antibiotics in certain scenarios, marking it as a promising natural alternative for managing bacterial infections. The concentration-dependent increase in inhibition zones for P1, particularly the pronounced impact on S. typhimurium with a 36.5 mm zone at 400 mg/ml, underscores the importance of optimizing extract concentration to maximize antibacterial efficacy. In contrast, the minimal inhibition exhibited by P2 against S. aureus at the same concentration (13.3 mm) emphasizes the significant variability in propolis efficacy, which should be carefully considered in application contexts. Cytotoxic activityFigure 1 illustrates the concentration-dependent effects of three propolis extracts on the viability of RBCs, focusing on their potential cytoprotective properties. Notably, the extract from the northern sample (P1) exhibited the strongest protective effect against hemolysis, showcasing its ability to preserve cell integrity more effectively than the other samples. At a concentration of 80 µg/ml, the percentage of hemolytic cells with P1 was approximately 38%, which is significantly lower compared to 69% for the middle sample (P2) and 64% for the southern sample (P3). Table 3. The oligonucleotide sequences of the primers used in RT-PCR study.
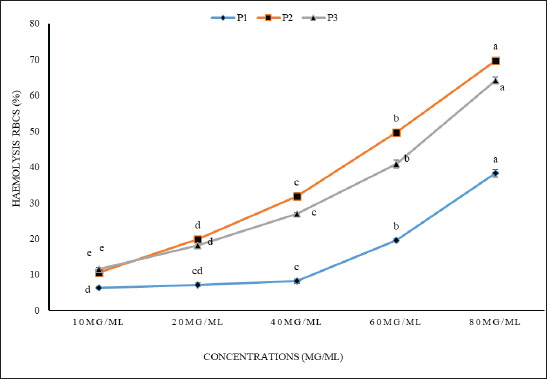
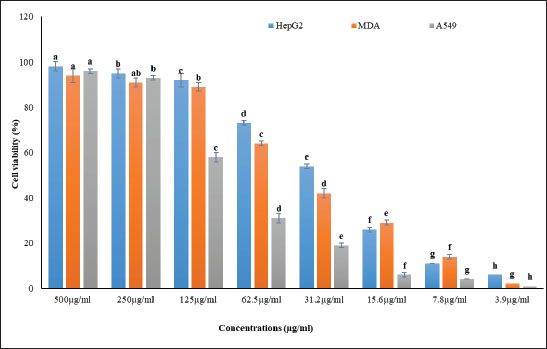
Fig. 1. The hemolytic effect of various concentrations (10, 20, 40, 60, and 80 μg/ml) of P1, P2, and P3 propolis samples on human RBC cell activity is investigated. Different letters in each row indicate statistically significant differences at p < 0.05. The substantially lower hemolysis observed with P1 suggests its potential superiority in mitigating oxidative stress or membrane destabilization, which are common causes of hemolysis in RBCs. This effect might stem from a higher concentration or more potent activity of bioactive compounds present in P1, which enhance erythrocyte membrane stability or act as free radical scavengers. The extracts’ inhibitory concentrations (IC50), the point at which 50% hemolysis occurred, were also notably different among the samples, with values at 104.6 µg/ml for P1, 60.5 µg/ml for P2, and 73.5 µg/ml for P3. The higher IC50 value for P1 corroborates its cytoprotective potential, implying that it requires a greater concentration to induce hemolysis compared to P2 and P3. The differences in IC50 values among the samples reflect the varied composition of propolis from different geographical or botanical origins, affecting their cytotoxic profiles. This variation in bioactivity is a critical consideration in evaluating the therapeutic applications of propolis extracts, particularly in formulations aimed at minimizing cytotoxic effects while maintaining efficacy. Overall, the northern propolis extract demonstrates a promising profile for applications necessitating reduced cytotoxicity, reinforcing its potential benefits, particularly in therapeutic environments where cell viability is paramount. Anticancer activityThe anticancer efficacy of the P1 propolis extract was evaluated based on the antioxidant and cytotoxicity data presented in Table 3 and Figure 2. This analysis focused on its impact on three carcinoma cell lines: HepG2 (liver carcinoma), MDA (breast carcinoma), and A549 (lung carcinoma). The findings, illustrated in Figure 2, highlighted the efficacy of the P1 sample, with distinct concentration-dependent effects on cell viability observed across the cell lines. At a concentration of 500 µg/ml, the P1 extract achieved the highest cell viability among the HepG2, MDA, and A549 cell lines, while the lowest cell viability was recorded at 3.9 µg/ml. Notably, the A549 cell line consistently exhibited the lowest cell viability across most treatments, with the exception being the higher concentrations of 500 and 250 µg/ml, where it showed comparatively better resilience than the MDA line. Conversely, the HepG2 cell line demonstrated the highest viability across all concentration treatments, except at 15.6 and 7.8 µg/ml, where the response was less favorable than MDA. These results underline the importance of concentration in determining the cytotoxic potential of P1, emphasizing the variability in response among different carcinoma types.
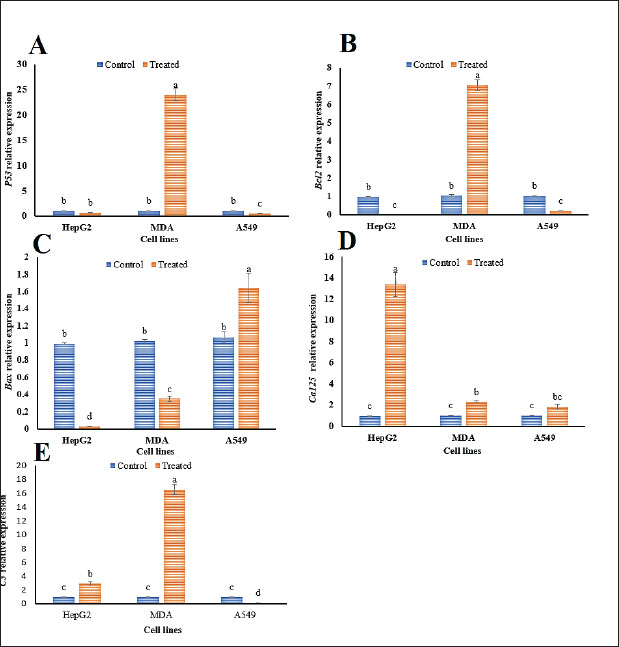
Fig. 2. The anticancer effect of various concentrations (500, 250, 125, 62.5, 31.2, 15.6, 7.8, and 3.9 μg/ml) of Propolis 1 (P1) extract on HepG2, MDA, and A549 carcinoma cell lines. Different letters in each row signify statistically significant differences at p < 0.05. The pronounced concentration-dependent responses observed, particularly in the A549 and MDA cell lines at higher concentrations, suggest a therapeutic window where anticancer efficacy could be maximized while mitigating adverse impacts on non-cancerous cells. This potential for targeted therapy is further underscored by the HepG2 cell line’s unique viability patterns at specific concentrations, indicating that individual carcinoma types may require tailored treatment regimens to optimize therapeutic outcomes. These findings highlight the importance of understanding the nuanced interactions between propolis extract concentrations and cancer cell viability. The distinct behavior seen in each cell line stresses the need for personalized approaches in anticancer treatments, considering the specific vulnerabilities and resistance profiles of different carcinoma cells. This comprehensive assessment of P1’s anticancer activity offers valuable insights into its potential as a versatile and effective therapeutic agent. qRT-PCRqRT-PCR was used to investigate the transcriptional responses of five critical immune-related genes (P53, Bcl2, Bax, Ca125, and C3) in three carcinoma cell lines: HepG2, MDA, and A549 following treatment with the P1 propolis extract compared to untreated controls. As demonstrated in Figure 3, P53 gene exhibited a substantial increase in expression in MDA cells, showing a 23.29-fold elevation under the influence of P1 treatment. This upregulation indicates a potential mechanism by which P1 induces apoptosis or cell cycle arrest, reflecting its role in tumor suppression. In contrast, P53 expression was significantly reduced in A549 cells, suggesting a differential response that could be influenced by the unique genetic makeup or stress-resilience pathways intrinsic to these cells (Fig. 3A). The Bcl2 gene, known for its role in inhibiting apoptosis, exhibited a similar trend to P53, with a 6.73-fold increase in MDA cells, potentially contributing to cell survival and proliferation in these cells under P1 treatment. Meanwhile, Bcl2 expression was suppressed in both HepG2 and A549 cells, aligning with an enhanced apoptotic response in these lines (Fig. 3B). The Bax gene, a pro-apoptotic marker, was notably upregulated in A549 cells with a 1.53-fold change, suggesting a boosted apoptotic pathway activation, which might counteract the reduced P53 expression (Fig. 3C). This implies a complex interaction where Bax-mediated apoptosis might still proceed effectively despite modifications in P53 regulation. Ca125 expression increased significantly across all cell lines, with the HepG2 line experiencing a remarkable 13.53-fold change. This upregulation might be linked to the cellular stress response or altered cell adhesion and migration properties associated with cancer progression (Fig. 3D). For the C3 gene, an essential component of the complement system, expression was prominently induced in HepG2 (2.84-fold change) and MDA (16.38-fold change) cells, highlighting an enhanced immune response or a heightened state of inflammation under P1 treatment. A549 cells, however, showed decreased C3 expression, which may reflect an evasion of immune-mediated cell death or a modified inflammatory environment (Fig. 3E). These differential gene expression patterns underscore the complex, cell-specific responses to P1 treatment, illustrating the potential for tailored therapeutic strategies that harness gene-specific modulation. The distinct behaviors of each gene across the cell lines provide valuable insights into the molecular mechanisms governing P1’s anticancer effects, which could inform the development of targeted cancer therapies. DiscussionAs chronic diseases continue to rise and global health faces threats from increasing rates of antibiotic resistance among microbes, particularly infectious bacteria, extensive research has been undertaken to explore natural products and their constituents as alternative therapies to conventional chemical approaches for chronic diseases (Docquier and Mangani, 2018). Propolis, a complex mixture of phytoconstituents with a resinous texture produced by honeybees from plant resins, has been used since ancient times for various purposes and has gained recognition in pharmaceutical applications in recent years (Chavda et al., 2023). The chemical composition of propolis varies based on factors, such as honeybee species, plant sources used, climate conditions, and harvesting seasons (Barsola and Kumari, 2022). Our results revealed a rich content of antioxidants and secondary metabolites in propolis, aligning with previous findings (Chavda et al., 2023). Given the growing interest in antioxidants among health and medical researchers, the abundance of antioxidants in propolis is noteworthy. Additionally, the presence of saponins and tannins in propolis, known antioxidant compounds, suggests its potential as an effective antibacterial agent. Saponins are recognized for their antimicrobial properties and role in protective mediation against potential illnesses (Ghafar et al., 2010). Tannins, as secondary metabolites, function as powerful biological antioxidants, playing a crucial role in defending against oxidative damage (Kehrer, 1993).
Fig. 3. The alteration in the relative expression of genes (P53, Bcl2, Bax, Ca, and C3) in control and P1-treated cancer cell lines (HepG2, MDA, and A549) is presented. The data represent the means of three replicates ± SD, with different letters in each column indicating statistically significant variances at p < 0.05. The current study elucidated that the antioxidant capacity of propolis is influenced by its reducing capability, serving as a crucial indicator of its potential antioxidant efficacy. This property plays a key role in converting free radicals into more stable products, thereby interrupting free radical-induced chain reactions (Ardestani and Yazdanparast, 2007). The FRP method is renowned for offering reliable insights into the antioxidant capacity of distinct extracts, products, or compounds, with all substances analyzed in our study demonstrating activity. The antioxidant prowess of propolis, as evidenced by its FRP and PMA values, corresponds with the outcomes described by Mahmoudi et al., (2016), underscoring propolis’ effectiveness as an antimicrobial agent. Propolis extracts exhibited antimicrobial activity against a broad spectrum of bacteria, including antibiotic-resistant species, corroborating the findings of previous studies ( Lee and Cha, 2010; Aref et al., 2011;). In the present study, the efficacy of different propolis samples as antibacterial agents revealed a noteworthy concentration-dependent inhibitory effect, particularly pronounced in sample P1. At the highest concentration (400 mg/ml), propolis sample P1 demonstrated superior antibacterial activity against both S. typhimurium and S. aureus, with inhibition zones of 36.5 and 29.5 mm, respectively, significantly exceeding the effectiveness of the standard antibiotic treatment. Additionally, the broad-spectrum antibacterial effect of propolis sample P1 underscores its potential as a powerful alternative to conventional antibiotics, particularly in cases of antibiotic resistance. Conversely, the minimal inhibitory effect observed with propolis sample P2, especially against S. aureus with an inhibition zone of only 13.3 mm at the same concentration, highlights the variability in antibacterial potency among different propolis samples. This variability emphasizes the need for further research to standardize and optimize propolis formulations for therapeutic applications. In addition to shielding cells from free radical damage, natural compounds also contribute to combating various diseases, including cancer (El Fawal et al., 2024). Another significant role of propolis is its containment of phytocompounds such as phenolics, crucial for inhibiting tumor growth. The P53 gene acts as a crucial tumor suppressor gene, playing a vital role in preventing cancer development by regulating cell division and inducing apoptosis in damaged cells (Kung and Weber, 2022). Suppressing P53 expression could potentially impair tumor suppressor effectiveness, leading to uncontrolled cell proliferation and potentially facilitating cancer growth or advancement (Hassin and Oren, 2023). Interestingly, the observed similarity in P53 inhibition by both extracts suggests that these treatments may impact P53-regulated pathways in a similar way (Sp et al., 2021). On the other hand, the impact of P1 treatment on gene expression varied markedly across different cell lines, reflecting the heterogeneity in cellular responses. In terms of Bcl2, these findings indicate that the treatments might influence apoptosis regulation differently and potentially affect the survival of cancer cells (Hafezi and Rahmani, 2021; Yao et al., 2022). Bcl2 is well-known for its anti-apoptotic role. Thus, inhibiting its expression could induce programmed cell death in cancer cells, leading to the elimination of abnormal or impaired cells. This could potentially help hinder the growth and survival of cancer cells (Ploumaki et al., 2023). For instance, Bax gene upregulation was most pronounced in the A549 cell line, suggesting enhanced apoptosis in this lung cancer model under P1 influence. This differential expression highlights the potential specificity of P1 in inducing apoptotic pathways primarily in A549 cells. In contrast, the Ca125 gene exhibited a consistent upregulation across all tested cell lines, with HepG2 cells showing a significant increase. This uniform response across cell lines positions Ca125 as a potentially universal marker of P1 treatment efficacy, especially noteworthy in HepG2 cells, implicating a broader regulatory mechanism at play. Moreover, C3 gene expression shows a dichotomous response as significantly upregulated in HepG2 and MDA cells but reduced in A549 cells. This suggests that while P1 may stimulate complement or inflammatory pathways in HepG2 and MDA cells, A549 cells react by downregulating these pathways, indicating a potentially unique adaptive or stress response mechanism in lung cancer cells. The results confirmed that genes influenced by propolis treatment significantly contribute to immune surveillance pathways and have the potential to regulate key signaling pathways, as previously reported by Cakir et al. (2023). Furthermore, the utilization of natural extracts led to changes in the fold change of apoptosis-associated genes, indicating that sustained release of propolis active components from the extract enhanced its anti-cancer efficacy by reinforcing cellular apoptosis. These findings align with the study by Mohebian et al. (2021) which also used natural extracts for breast cancer treatment. Briefly, propolis has the ability to influence cancer cells through three distinct pathways. First, it induces apoptosis by potentially triggering this process through different pathways, including the intrinsic mitochondrial or extrinsic death receptor pathways (Jeivad et al., 2020). This action may involve the activation of pro-apoptotic proteins such as Bax and the inhibition of anti-apoptotic proteins such as Bcl-2 (Cakir et al., 2023). Second, propolis is implicated in cell cycle arrest by potentially halting cell cycle progression at specific checkpoints, thus impeding the uncontrolled proliferation of cancer cells (Oršolić and Jazvinšćak Jembrek, 2022). This effect could be achieved by modulating cyclins, cyclin-dependent kinases, and checkpoint proteins such as p53 (Chung and Bunz, 2010). Finally, propolis components may also inhibit angiogenesis by impeding the formation of new blood vessels that nourish tumors, depriving them of vital resources for growth (Mostafaei, 2011). Concerning the controversies in the field, the therapeutic use of propolis is not without its controversies, primarily stemming from the variability in its composition and the resulting inconsistencies in research findings. Critics argue that differences in geographic and botanical origins lead to variability in the bioactive compounds present in propolis, causing significant challenges in standardization and comparability across studies (Przybyłek and Karpiński, 2019; Forma and Bryś, 2021; Elumalai et al., 2022). This inconsistency is exacerbated by the lack of standardized extraction methods and dosing, which complicates the assessment of propolis’s true therapeutic potential. Additionally, some skeptics question the reproducibility of propolis’s effects in clinical settings, highlighting the urgent need for rigorous and uniform methodologies in future research. Regarding recent and important achievements in the field, notwithstanding these controversies, there have been significant advancements in our understanding of propolis’s pharmaceutical potential. Recent studies have revealed its multifaceted biological activities, such as its ability to induce apoptosis, cause cell cycle arrest, and inhibit angiogenesis in cancer cells (Surek et al., 2021; Zulhendri et al., 2021; Bouchelaghem, 2022). Our investigation into Egyptian propolis further underscores these findings, demonstrating superior antioxidant capabilities and pronounced cytotoxic effects on specific cancer cell lines. These advancements reflect a deeper understanding of propolis’s mechanisms, paving the way for its inclusion in novel therapeutic strategies aimed at treating a range of diseases. The novelty of our study lies in its comprehensive evaluation of the bioactivity of distinct Egyptian propolis samples, employing techniques such as RT-qPCR to explore its impact on immune response pathways. Therefore, this research is pioneering in integrating advanced gene expression analyses to demonstrate how propolis modulates immune response genes, offering deeper insights into its anticancer mechanisms. The potent bioactivities observed, particularly in the P1 sample, highlight the potential of regional propolis varieties to outperform traditional treatments, marking a significant leap forward in natural therapeutics research. Regarding the practical considerations and future directions, while propolis’s potential is evident, practical considerations for its application in clinical settings remain a topic of discussion. Key questions about optimal dosing, delivery methods, and interactions with other treatments must be addressed to ensure its safe and effective use. Standardizing propolis formulations to overcome variability due to environmental factors is crucial for its widespread adoption in oncology and other medical fields. Continued research and collaboration in these areas will be essential to fully realize the therapeutic potential of propolis and incorporate it into modern healthcare practices. ConclusionThis study highlights the multifaceted therapeutic potential of propolis, specifically its potent antioxidant, antibacterial, and anticancer properties. The comprehensive analysis of ethanolic extracts from the three propolis samples (P1, P2, and P3) revealed significant quantities of bioactive compounds, including ascorbic acid, saponins, and tannins, with P1 standing out as the most effective sample across various assays. The P1 extract demonstrated exceptional antioxidant activity, indicated by its high FRP value of 62.9 mg/g DM and PMA content of 20.7 mg/g DM. Such strong antioxidant properties are likely crucial in neutralizing oxidative stress, which plays a pivotal role in both microbial inhibition and cancer suppression. In antibacterial evaluations, P1 exhibited superior efficacy, generating the largest inhibitory zones against S. typhimurium and S. aureus at 400 mg/mL, outperforming even standard antibiotic treatments. This suggests that P1 propolis could serve as a potent natural antibacterial agent, potentially broadening the arsenal against resistant bacterial strains. Regarding anticancer activity, P1 demonstrated robust effects, significantly inhibiting cell viability in carcinoma cell lines (HepG2, MDA, and A549). Notably, the lowest cell viability was achieved at a concentration of 3.9 µg/ml, pointing to its cytotoxic potential against cancer cells. The upregulation of immune response genes, such as P53, Bcl2, Bax, Ca125, and C3, particularly in MDA and HepG2 cell lines, indicates a pathway through which propolis may augment immune-mediated cancer cell destruction. Overall, these findings underscore the promise of P1 propolis as a versatile natural therapeutic agent. However, to fully leverage its potential, further detailed investigations are required to elucidate the precise molecular mechanisms at play. Additionally, studies testing the clinical applicability and safety of propolis in human subjects are essential to transition from in vitro analyses to real-world medical applications. Future research should also consider the variability in propolis composition due to geographical and botanical differences, which could impact its efficacy and consistency as a therapeutic product. AcknowledgmentsThe authors gratefully thank the working team of the Plant Protection and Bio-Molecular Diagnosis Department, Arid Lands Cultivation Research Institute (ALCRI), The City of Scientific Research and Technology Applications. Conflict of interestThe authors declare that there is no conflict of interest. FundingNot applicable. Authors’ contributionsThe work presented here was carried out in collaboration between all authors. M.G., Kh.A., Z.H., A.A.S., and E.E.H. defined the research theme. M.G., H.K., A.A.S., S.E.S., M.T.J., and E.E.H. designed methods and experiments. M.G., Kh.A., S.E.S., A.A.S., and Z.H. carried out the field experiments. M.G. and A.A.S. carried out the genetic investigation. M.G., A.A.S., M.T.J., and E.E.H. organized the data. A.A.S. and E.E.H. interpreted the results and wrote the manuscript. All authors read, reviewed, and approved the final manuscript. Data availabilityAll data generated or analyzed during this study are included in this manuscript and its information files. ReferencesAlley, M.C., Scudiero, D.A., Monks, A., Hursey, M.L., Czerwinski, M.J., Fine, D.L., Abbott, B.J., Mayo, J.G., Shoemaker, R.H. and Boyd, M.R. 1988. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 48(3), 589–601. Ardestani, A. and Yazdanparast, R. 2007. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 104(1), 21–29. Aref, H.L., Bahri-Sahloul, K.B., Fekih, A., Chemli, R., Mars, M., Aouni, M., Chaumon, J.P. and Said, K. 2011. Variability in antimicrobial activity of latex from two varieties of Ficus carica. Afr. J. Microbiol. Res. 5, 1361–1367. Barsola, B. and Kumari, P. 2022. Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: a review. Green Process Synth. 11(1), 659–673. Bouchelaghem, S. 2022. Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: a review. Saudi J. Biol. Sci. 29(4), 1936–1946. https://doi.org/10.1016/j.sjbs.2021.11.063 Cakir, M.O., Bilge, U., Naughton, D. and Ashrafi, G.H. 2023. Ficus carica latex modulates immunity-linked gene expression in human papillomavirus positive cervical cancer cell lines: evidence from RNA Seq transcriptome analysis. Int. J. Mol. Sci. 24(17):13646. https://doi.org/10.3390/ijms241713646 Chaudhary, D., Jeena, A.S., Rohit, Gaur, S., Raj, R., Mishra, S., Kajal, Gupta, O.P. and Meena, M.R. 2024. Advances in RNA interference for plant functional genomics: unveiling traits, mechanisms, and future directions. Appl. Biochem. Biotechnol. 1–30. Chavda, V.P., Chaudhari, A.Z., Teli, D., Balar, P. and Vora, L. 2023. Propolis and thier active constituents for chronic diseases. Biomedicines 11(2), 259. Chen, H., Zhang, J., He, Y., Lv, Z., Liang, Z., Chen, J., Li, P., Liu, J., Yang, H. and Tao, A. 2022. Exploring the role of Staphylococcus aureus in inflammatory diseases. Toxins 14(7), 464. Cheville, A., Smith, S., Barksdale, T. and Asher, A. 2021. Cancer rehabilitation. Braddom’s Phys. Med. Rehabil. 568–593.e567. Chung, J.H. and Bunz, F. 2010. Cdk2 is required for p53-independent G2/M checkpoint control. PLoS Genet. 6(2), e1000863. Daleprane, J.B. and Abdalla, D.S. 2013. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid. Based Complement. Alternat. Med. 2013(1), 175135. Damon, C. 1966. Hawk’s physiological chemistry. 14th Ed. Edited by Bernard L. Oser. McGraw-Hill, New York, xv + 1472 pp. J. Pharm. Sci. 55, 455. https://api.semanticscholar.org/CorpusID:97131779. Docquier, J.D. and Mangani, S. 2018. An update on β-lactamase inhibitor discovery and development. Drug Resist. Updat. 36, 13–29. El Fawal, G., Sobhy, S.E. and Hafez, E.E. 2024. Biological activities of fig latex-loaded cellulose acetate/poly (ethylene oxide) nanofiber for potential therapeutics: anticancer and antioxidant material. Int. J. Biol. Macromol. 270, 132176. Elnasr, T.A.S., Ibrahim, O.M., Alhumaimess, M.S., Alsohaimi, I.H., El-Ossaily, Y.A., Hussein, M.F., Rafea, M.A., Hassan, H.M., Sobhy, S.E. and Hafez, E.E. 2024. Olive leaf extract-derived chitosan-metal nanocomposite: green synthesis and dual antimicrobial-anticancer action. Int. J. Biol. Macromol. 270, 132252. Elumalai, P., Muninathan, N., Megalatha, S.T., Suresh, A., Kumar, K.S., Jhansi, N., Kalaivani, K. and Krishnamoorthy, G. 2022. An insight into anticancer effect of propolis and its constituents: a review of molecular mechanisms. Evid. Based Complement. Alternat. Med. 2022, 5901191. https://doi.org/10.1155/2022/5901191 Forma, E. and Bryś, M. 2021. Anticancer activity of propolis and its compounds. Nutrients 13(8). https://doi.org/10.3390/nu13082594 Gebreslassie, Y.T. and Gebremeskel, F.G. 2024. Green and cost-effective biofabrication of copper oxide nanoparticles: exploring antimicrobial and anticancer applications. Biotechnol. Rep. 12;41:e00828. doi: 10.1016/j.btre.2024.e00828. PMID: 38312482; PMCID: PMC10835232. Ghafar, M., Prasad, K.N., Weng, K.K. and Ismail, A. 2010. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr. J. Biotechnol. 9(3). Hafezi, S. and Rahmani, M. 2021. Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers 13(6), 1292. Hassin, O. and Oren, M. 2023. Drugging p53 in cancer: one protein, many targets. Nat. Rev. Drug Discov. 22(2), 127–144. Hiai, S., Oura, H., Hamanaka, H. and Odaka, Y. 1975. A color reaction of panaxadiol with vanillin and sulfuric acid. Planta Med. 28(06), 131–138. Ijaz, S., Khan, V.S., Ghazanfar, A. and Khan, Z. 2024. Regulatory Roles of Plant MicroRNAs, in: plant MicroRNAs and stress response. Taylor & Francis. CRC Press, pp. 50–64. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003322214-3 Jayaprakasha, G.K., Rao, L.J. and Sakariah, K.K. 2006. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 98(4), 720–724. Jeivad, F., Yassa, N., Ostad, S.N., Hassannejad, Z., Gheshlaghi, G.H. and Sabzevari, O. 2020. Ficus carica L. Latex: possible chemo-preventive, apoptotic activity and safety assessment. Iran. J. Pharm. Res. 19(3), 231. Kehrer, J.P. 1993. Free radicals as mediators of tissue injury and disease. Crit. Rev. Toxicol. 23(1), 21–48. Kung, C.P. and Weber, J.D. 2022. It’s getting complicated—a fresh look at p53-MDM2-ARF triangle in tumorigenesis and cancer therapy. Front. Cell Dev. Biol. 10, 818744. Lee YS and Cha J.D. 2010. Synergistic antibacterial activity of fig (Ficus carica) leaves extract against clinical isolates of methicillin-resistant Staphylococcus aureus. Korean J. Microbiol. Biotechnol. 38(4), 405–413. Lin, L., Li, Z., Yan, L., Liu, Y., Yang, H. and Li, H. 2021. Global, regional, and national cancer incidence and death for 29 cancer groups in 2019 and trends analysis of the global cancer burden, 1990–2019. J. Hematol. Oncol. 14, 1–24. Liu, Z.W., Li, H.P., Cheng, W., Yang, P., Zhang, J.B., Gong, A.D., Feng, Y.N., Fernando, W.D. and Liao, Y.-C. 2012. Enhanced overall resistance to Fusarium seedling blight and Fusarium head blight in transgenic wheat by co-expression of anti-fungal peptides. Eur. J. Plant Pathol. 134, 721–732. Mahmoudi, S., Khali, M., Benkhaled, A., Benamirouche, K. and Baiti, I. 2016. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac. J. Trop. Biomed. 6(3), 239–245. Mani, V.M., Kalaivani, S., Sabarathinam, S., Vasuki, M., Soundari, A.J.P.G., Das, M.A., Elfasakhany, A. and Pugazhendhi, A. 2021. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ. Res. 201, 111502. Mohebian, Z., Babazadeh, M., Zarghami, N. and Mousazadeh, H. 2021. Anticancer efficiency of curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for potential postsurgical breast cancer treatment. J. Drug Deliv. Sci. Technol. 61, 102170. Mostafaei, A. 2011. Augmenting trabeculectomy in glaucoma with subconjunctival mitomycin C versus subconjunctival 5-fluorouracil: a randomized clinical trial. Clin. Ophthalmol. 5:491–494. doi: 10.2147/OPTH.S17328. Epub 2011 Apr 18. PMID: 21573097; PMCID: PMC3090304. Naeem, A., Hu, P., Yang, M., Zhang, J., Liu, Y., Zhu, W. and Zheng, Q. 2022. Natural products as anticancer agents: current status and future perspectives. Molecules 27(23), 8367. Nolan, T., Hands, R.E. and Bustin, S.A. 2006. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1(3), 1559–1582. Oršolić, N. and Jazvinšćak Jembrek, M. 2022. Molecular and cellular mechanisms of propolis and its polyphenolic compounds against cancer. Int. J. Mol. Sci. 23(18), 10479. Oyaizu, M. 1986. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 44(6), 307–315. Ploumaki, I., Triantafyllou, E., Koumprentziotis, I.A., Karampinos, K., Drougkas, K., Karavolias, I., Trontzas, I. and Kotteas, E.A. 2023. Bcl-2 pathway inhibition in solid tumors: a review of clinical trials. Clin. Transl. Oncol. 25(6), 1554–1578. https://doi.org/10.1007/s12094-022-03070-9 Przybyłek, I. and Karpiński, T.M. 2019. Antibacterial properties of propolis. Molecules 24(11), 2047. https://doi.org/10.3390/molecules24112047 Rabiee, N., Ahmadi, S., Iravani, S. and Varma, R.S. 2023. Natural resources for sustainable synthesis of nanomaterials with anticancer applications: a move toward green nanomedicine. Environ. Res. 216, 114803. Ramos, S., Silva, V., Dapkevicius, M.d.L.E., Caniça, M., Tejedor-Junco, M.T., Igrejas, G. and Poeta, P. 2020. Escherichia coli as commensal and pathogenic bacteria among food-producing animals: Health implications of extended spectrum β-lactamase (ESBL) production. Animals 10(12), 2239. Santangelo, C., Vari, R., Scazzocchio, B., De Sanctis, P., Giovannini, C., D’Archivio, M. and Masella, R. 2018. Anti-inflammatory activity of extra virgin olive oil polyphenols: which role in the prevention and treatment of immune-mediated inflammatory diseases? Endocr. Metab. Immune Disord.-Drug Targets. 18(1), 36–50. Sereme, A., Kouda-Bonafos, M. and Nacro, M. 1994. Tannins in utilization of sorghum grains in Burkina Faso. Plant Foods Hum. Nutr. 46, 331–334. Silva-Carvalho, R., Baltazar, F. and Almeida-Aguiar, C. 2015. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid. Based Complement. Alternat. Med. 2015(1), 206439. Singh, V. and Ali, M. 2016. Analysis of differential gene expression under salinity through differential display reverse transcription polymerase chain reaction (DDRT-PCR) technique: a review. Electron. J. Biol. 12, 394–401. Sobhy, S., Al-Askar, A.A., Bakhiet, E.K., Elsharkawy, M.M., Arishi, A.A., Behiry, S.I. and Abdelkhalek, A. 2023. Phytochemical characterization and antifungal efficacy of camphor (Cinnamomum camphora L.) extract against phytopathogenic fungi. Separations 10(3), 189. Sobhy, S., Allah, K., Kassem, E., Hafez, E. and Sewelam, N. 2019. Seed priming in natural weed extracts represents a promising practice for alleviating lead stress toxicity. Egypt. J. Exp. Biol. 15, 453. Sp, N., Kang, D.Y., Lee, J.M., Bae, S.W. and Jang, K.J. 2021. Potential antitumor effects of 6-gingerol in p53-dependent mitochondrial apoptosis and inhibition of tumor sphere formation in breast cancer cells. Int. J. Mol. Sci. 22(9), 4660. Surek, M., Fachi, M.M., de Fátima Cobre, A., de Oliveira, F.F., Pontarolo, R., Crisma, A.R., de Souza, W.M. and Felipe, K.B. 2021. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 269, 113662. https://doi.org/10.1016/j.jep.2020.113662 Tatli Seven, P., Seven, I., Gul Baykalir, B., Iflazoglu Mutlu, S. and Salem, A.Z. 2018. Nanotechnology and nano-propolis in animal production and health: an overview. Ital. J. Anim. Sci. 17(4), 921–930. Yao, W., Bai, L., Wang, S., Zhai, Y. and Sun, S.Y. 2022. Mcl-1 levels critically impact the sensitivities of human colorectal cancer cells to APG-1252-M1, a novel Bcl-2/Bcl-XL dual inhibitor that induces Bax-dependent apoptosis. Neoplasia 29, 100798. Zulhendri, F., Chandrasekaran, K., Kowacz, M., Ravalia, M., Kripal, K., Fearnley, J. and Perera, C.O. 2021. Antiviral, antibacterial, antifungal, and antiparasitic properties of propolis: a review. Foods 10(6), 1360. https://doi.org/10.3390/foods10061360 | ||
| How to Cite this Article |
| Pubmed Style Gomaa MBM, Abdelhameed KMA, Sobhy SE, Konper HMA, Hassanein ZAE, Saleh AA, Jamal MT, Hafez EE. Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Vet. J.. 2025; 15(1): 126-138. doi:10.5455/OVJ.2025.v15.i1.12 Web Style Gomaa MBM, Abdelhameed KMA, Sobhy SE, Konper HMA, Hassanein ZAE, Saleh AA, Jamal MT, Hafez EE. Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. https://www.openveterinaryjournal.com/?mno=222035 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.12 AMA (American Medical Association) Style Gomaa MBM, Abdelhameed KMA, Sobhy SE, Konper HMA, Hassanein ZAE, Saleh AA, Jamal MT, Hafez EE. Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Vet. J.. 2025; 15(1): 126-138. doi:10.5455/OVJ.2025.v15.i1.12 Vancouver/ICMJE Style Gomaa MBM, Abdelhameed KMA, Sobhy SE, Konper HMA, Hassanein ZAE, Saleh AA, Jamal MT, Hafez EE. Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 126-138. doi:10.5455/OVJ.2025.v15.i1.12 Harvard Style Gomaa, M. B. M., Abdelhameed, . K. M. A., Sobhy, . S. E., Konper, . H. M. A., Hassanein, . Z. A. E., Saleh, . A. A., Jamal, . M. T. & Hafez, . E. E. (2025) Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Vet. J., 15 (1), 126-138. doi:10.5455/OVJ.2025.v15.i1.12 Turabian Style Gomaa, Marwa B. M., Khaled M. A. Abdelhameed, Sherien E. Sobhy, Hanan M. A. Konper, Zienab A. E. Hassanein, Ahmed A. Saleh, Mamdoh T. Jamal, and Elsayed E. Hafez. 2025. Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Veterinary Journal, 15 (1), 126-138. doi:10.5455/OVJ.2025.v15.i1.12 Chicago Style Gomaa, Marwa B. M., Khaled M. A. Abdelhameed, Sherien E. Sobhy, Hanan M. A. Konper, Zienab A. E. Hassanein, Ahmed A. Saleh, Mamdoh T. Jamal, and Elsayed E. Hafez. "Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis." Open Veterinary Journal 15 (2025), 126-138. doi:10.5455/OVJ.2025.v15.i1.12 MLA (The Modern Language Association) Style Gomaa, Marwa B. M., Khaled M. A. Abdelhameed, Sherien E. Sobhy, Hanan M. A. Konper, Zienab A. E. Hassanein, Ahmed A. Saleh, Mamdoh T. Jamal, and Elsayed E. Hafez. "Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis." Open Veterinary Journal 15.1 (2025), 126-138. Print. doi:10.5455/OVJ.2025.v15.i1.12 APA (American Psychological Association) Style Gomaa, M. B. M., Abdelhameed, . K. M. A., Sobhy, . S. E., Konper, . H. M. A., Hassanein, . Z. A. E., Saleh, . A. A., Jamal, . M. T. & Hafez, . E. E. (2025) Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Veterinary Journal, 15 (1), 126-138. doi:10.5455/OVJ.2025.v15.i1.12 |