| Research Article | ||
Open Vet. J.. 2024; 14(12): 3388-3396 Open Veterinary Journal, (2024), Vol. 14(12): 3388-3396 Research Article A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISAWalaa A. Gad1*, Salama A. Osman2, Khaled A. Abd El-Razik1, Ashraf H. Soror1 and Ehab A. Fouad31Department of Animal Reproduction, Veterinary Research Institute, National Research Centre, Giza, Egypt 2Department of Animal Medicine, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt 3Department of Zoonosis, Veterinary Research Institute, National Research Centre, Giza, Egypt *Corresponding Author: Walaa A. Gad. Department of Animal Reproduction, Veterinary Research Institute, National Research Centre, Egypt. Email: walaa.gad2018 [at] gmail.com Submitted: 27/09/2024 Accepted: 13/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
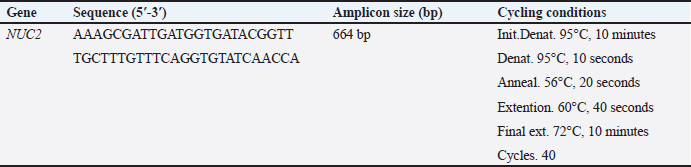
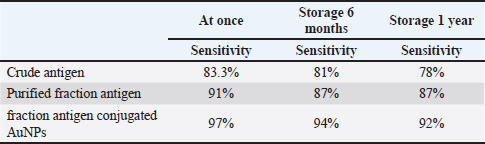
AbstractBackground: Bovine subclinical mastitis (SCM) is a significant cause of economic losses in dairy farms, primarily due to its asymptomatic nature and difficulty in early detection. The enzyme-linked immunosorbent assay (ELISA) is a widely used diagnostic tool in bioscience, facilitating antigen detection through antibody binding. Aim: The present study designed a gold nanoparticle (AuNPs)-based ELISA, to enhance the sensitivity of conventional ELISA by improving the binding efficiency of capture antibodies to purified antigens. This modified ELISA could enable more accurate detection of bovine SCM. Methods: A total of 200 milk samples from apparently healthy cows were screened for SCM using the California Mastitis Test (CMT). Positive samples were then subjected to bacteriological culture, biochemical testing, and polymerase chain reaction targeting the Nuc2 gene for confirmation of S. aureus. The purified fraction antigen of all 78 confirmed S. aureus isolates was extracted using sepharose 4B affinity column chromatography, described by SDS-polyacrylamide gel electrophoresis, and assessed for its sensitivity in S. aureus mastitis diagnosis compared with crude antigen and purified fraction antigen-conjugated gold nanoparticles within an indirect ELISA. Immunoglobulins (IGs) from positive cow serum were extracted and purified from all confirmed S. aureus. Gold nanoparticle-based indirect ELISA was used on 400 samples (200 milk and 200 serum) from the same cows. Results: Using CMT, out of 200 examined milk samples from apparently normal cows, 65% (130/200) were sub-clinically infected. Out of these 130 positive milk samples, 60% (78/130) were confirmed to be infected with S. aureus. Purified fraction antigen-conjugated gold nanoparticles achieved the highest sensitivity to ELISA at 97%, 94%, and 92% immediately before storage, 6 months at −20°C, and 1 year at −20°C, respectively. Gold nanoparticle-based indirect ELISA detected specific IgG antibodies in 97% (76/78) sera and milk samples related to confirmed S. aureus isolates. Conclusion: The utilization of purified fraction antigen with gold nanoparticles enhances the sensitivity of ELISA, increasing it from 83.3% to 97% (p < 0.01; CI: 99%). The current study establishes a valuable way for S. aureus mastitis diagnosis within the use of purified fraction antigen-conjugated gold nanoparticles instead of the classical way to improve the sensitivity and specificity of ELISA. Keywords: Indirect ELISA, Gold nanoparticle, Subclinical mastitis, S. aureus, Antigen purification. IntroductionStaphylococcus aureus is the major economically significant cause of bovine mastitis that affects dairy cattle globally (Gad et al., 2025). It poses a substantial challenge to the dairy industry, and animal welfare, and a threat to public health. A wide range of virulence genes, such as several toxins, tissue invasion, adhesions, evading the immunity defense mechanism, and evading proteins that enable bacterial colonization, are present in S. aureus, allowing it to cause severe and lasting intramammary infections (Fursova et al., 2020). The disease is characterized by an elevation in somatic cell count, inflammatory cytokines, production of antibodies, and different types of bacteria counts. When S. aureus infects the mammary glands, the immune system responds with different types of lymphocytes, as well as leucocytes and cytokines. Staphylococcus aureus virulent arsenal allows it to confuse and inhibit the immune system; enterotoxins and toxic shock syndrome toxins primarily cause the pathogenicity of S. aureus (Alluwaimi et al., 2003). The primary method for diagnosing mastitis is the quantification of milk inflammatory cells, including macrophages, lymphocytes, neutrophils, and eosinophils. The California Mastitis Test (CMT) appears to be beneficial as a screening test for the disease (Roshan et al., 2022). Mastitis has a negative economic impact since it reduces milk production, increases cow culling, raises treatment costs, and increases cow death and replacement costs (Al Emon et al., 2024). Accurate identification and determination of S. aureus virulence genes in milk samples is essential for understanding the development of bovine mastitis and designing efficient management measures. ELISA is a commonly used technique for recognizing and quantifying particular proteins or antigens in biological specimens (Sharma et al., 2023). A recent study focused on the application of nanomaterials, including nanogold particles, to ELISA-based assays in an effort to increase sensitivity, and specificity and enhance detection limits (Tabatabaei et al., 2021). Currently, nanomaterial-improved ELISA is more prevalent because the alteration has greater potential for fast on-time detection as it requires less reagent and can be kept at room temperature for a longer period of time (Juronen et al., 2018). Nanomaterials improve the detection limit of traditional ELISA assays by providing additional binding sites for detection antibodies and improving the signal intensity of this assay (Tabatabaei et al., 2021). The nano-ELISA kit achieved a sensitivity and specificity of 93.33%. This significant advancement in the sensitivity and specificity of the nano-ELISA approach is due to the increased entry of more antibodies into the antigen-antibody complex, resulting in better staining (Khodadadi et al., 2020). Gold nanoparticles are an effective biomarker for protein and deoxyribonucleic acid analysis because of their extra-ordinary absorption and optic refraction at specific wavelengths, fluorescence characteristics unique to optical detection techniques, high surface-to-volume ratio, and other particular characteristics (Gad et al., 2025). Gold nanoparticles can facilitate the rapid binding of biomolecules such as DNA, antibodies, enzymes, and others, thereby enhancing the number of biochemical detection signals (Khodadadi et al., 2020). Diagnostic systems based on gold nanoparticles (AuNPs) are gaining considerable interest due to their unique surface and extensive absorbance at 520 nanometers (nm) (Parolo et al., 2013). ELISA-based gold nanoparticles typically represent more than one enzyme for each antibody and produce a superior colorimetric signal, making it possible to detect samples with a small analytical number (Gad et al. 2025). Polymerase chain reaction (PCR) is an automated, rapid technique for detecting the most predominant mammary pathogens directly from milk (Hiitiö et al., 2015). Enhancing the diagnostics for subclinical bovine mastitis is crucial to facilitate precise and timely identification of S. aureus. In addition, this reduces financial losses and protects public health by preventing mastitis and improving dairy animal care (Mostafa Abdalhamed et al., 2022). Therefore, this study aims to evaluate a novel approach using gold nanoparticle-based ELISA for the sensitive and specific diagnosis of S. aureus in bovine subclinical mastitis (SCM), offering a promising advancement in dairy disease diagnostics. Materials and MethodsSamplesa) Milk samples: A total of 200 pooled quarter milk samples were aseptically collected from apparent healthy 200 dairy cows on different farms in Egypt between 2022 and 2023. All samples were screened for SCM using the CMT. Of these, 130 milk samples tested positive for SCM and were promptly refrigerated at 4°C for subsequent bacteriological examination. b) Serum samples: A total of 200 blood samples were collected only from the same cows that prevised the milk samples. Sera were separated and stored at −20°C until subjected to ELISA. Bacteriological examinationPositive SCM milk samples were streaked onto mannitol salt agar plates (Oxoid, UK) and then incubated at 37°C for 24–48 hours. A spherical convex golden yellow colony was sub-cultured on the same selective media plates and placed in an incubator for 48 hours, at a temperature of 37°C. Following the process of purification, the colonies were recognized morphologically and biochemically, according to Wehr and Frank (2004). Suspected positive S. aureus colonies were kept in Brain Heart Infusion (BHI) media containing 50% glycerol for further analysis (Rahim et al., 2021). The confirmation of the suspected colonies was achieved via conventional PCR for nuc2 gene recognition. DNA extractionThe purified colonies were extracted by the GF-1 Bacterial DNA Extraction Kit (Cat.no. GF-BA-100, Vivantis, Malaysia) in line with the company protocol. The nucleic acid was extracted using 50 μl of elution buffer. Extracted DNA was observed at 1.5% agarose gel electrophoresis. Molecular identification using PCR (nuc2) geneThe PCR targeting S. aureus was carried out using a GS-96 gradient thermocycler (Hercuvan, Malaysia). A total volume of 25 µl was used, containing 12.5 μl of Master Mix (Cat. W1020300X, Willofort Co., UK), 0.75 µL (10 µm) of each primer, 2 µl of DNA and 9 μl of deionized distilled water. Table 1 reports the cycling conditions and specific primer (nuc2) used to detect S. aureus according to Abd El-Razik et al. (2023). Preparation and purification of antigenPure colonies that were confirmed with PCR were cultured in BHI broth medium, incubated in a shaker incubator at 37°C for 24 hours, and centrifuged at 3,000 rpm for 20 minutes. The supernatant was removed, and the precipitate was washed three times with distilled water. The suspension of bacterial strains was diluted to a concentration of 4 × 106 CFU/ml. The crude antigen was prepared through bacterial homogenization in 0.1M pH 7.2 phosphate-buffered saline, according to Al-Mayah and Saeed (2013). Table 1. Cycling conditions and primer sequence of PCR for NUC2 gene identification.
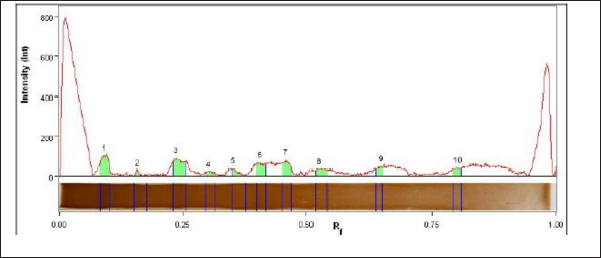
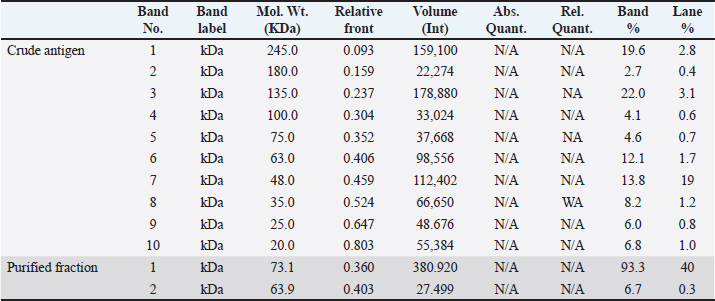
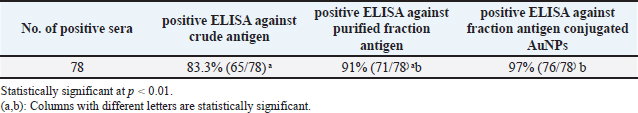
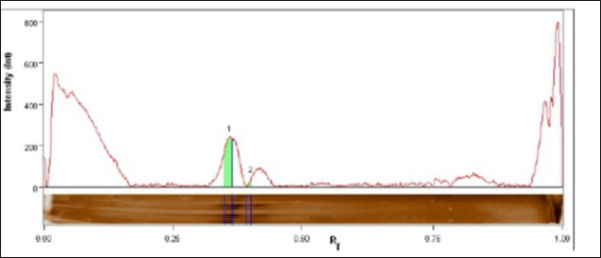
The fraction of S. aureus antigen was purified by sepharose 4B affinity column chromatography and then stored for one year at −20°C. The protein of both the crude and purified fraction was measured using a colorimetric test with folin substance following the method described by Lowry et al. (1951). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of proteinsThe crude antigen and purified fraction were separately mixed with distinct 2-mercaptoethanol sample buffers. Following the electrophoresis process using 10% reducing polyacrylamide gel, the proteins underwent staining with silver nitrate (Merck, Germany), and the molecular weights of the bands were determined using a protein marker (Sigma-Aldrich) (Laemmli, 1970). Bio Rad Gel Doc XR+ Apparatus was used to determine the molecular weights. Precipitation and affinity chromatography purification of immunoglobulins (IGs)IGs from positive cow serum were separated using 50% saturated ammonium sulphate solution, extracted by dialysis for a duration of 3 days (4 times per day at 4°C) using a NaHCO3 buffer (0.1 M, pH 8.3) supplemented with NaCl (0.5 M) and NaN3 (0.02%), and concentrated through lyophilization, following the Abdel-Rahman et al. (2017) precipitation process. IGs were purified using protein A Sepharose gel using 0.1 M glycine as an eluting buffer. The purification technique was done according to Abdel-Rahman et al. (2017). Enzyme-linked immunosorbent assaySera samples related to PCR confirmed S. aureus isolates were subjected to indirect ELISA to assess the sensitivity, and validity of the purified fraction. The concentration of antigens, dilution of antibodies, and dilution of anti-bovine horse radish peroxidase (Sigma) was assessed using checker board titration. The assay was conducted using the crude and purified fraction antigen in accordance with Nakane and Kawaoi (1974). In brief, the microtitration plate was coated with 100 μl of each antigen separately and incubated overnight at 4°C. After washing the plate was blocked with 05% Bovine Serum Albumin in phosphate-buffered saline for 1 hour at room temperature. 100 μl of each serum sample was added to each well after washing. 1.5 hours of incubation at 37°C, the plate was washed and 100 μl of diluted peroxidase conjugated anti-bovine antibodies was added to each well, and the plate was incubated for 1 hour at 37°C. Ortho-Phenlene diamine substrate buffer containing H2O2 was added and the plate was analyzed using spectrophotometry at 450 nm wavelength using the Microplate reader ELx 800 USA. Design of gold nanoparticle (AuNPs) based ELISAA total of 200 quarter milk and sera samples (negative and positive S. aureus) were subjected to indirect ELISA to assess the sensitivity, and validity of the purified fraction. Milk samples were allowed to defrost at 4°C so that fat could be separated from milk by gravity. A sample of 100 μl was taken from under the fat layer and diluted in 900 μl of sample diluent on a 96-well plate. The technique was similar to above mentioned conventional ELISA using fraction antigen conjugated gold nanoparticles in the conjugation step. In this stage, Gold (III) chloride trihydrate (HAuCl4) solutions were prepared at 1 mM concentrations and diluted in 1 mM 4-morpholinoethanesulfonic acid (MES) buffer at pH 6.0. The AuNPs were formed using H2O2− (hydrogen peroxide) as the reducing agent. The concentration of H2O2− was 100 μM and it was diluted in 1 mM MES buffer at pH 6.0. Based on the published works of Basso et al. (2024) Finally, the samples were analyzed using an ELISA reader at a wavelength of 450 nm. Statistical analysisThe obtained data was analyzed by chi-square using the SPSS for Windows (Version 15.0, USA) statistical software program, and probability (p-values) of less than 0.01 was considered significant. Also, sensitivity, specificity, positive predictive value, and negative predictive value were analyzed according to Martin et al. (1987). Ethical approvalThe Medical Research Ethics Committee approved this study (No. 13010121) at the National Research Centre, Egypt. ResultsOut of the examined 200 milk samples collected from apparently normal cow, 65% (130/200) showed SCM via the CMT. Bacteriological examinationOut of 130 positive SCM milk samples, 60% (78/130) showed spherical convex golden yellow colonies and all 60% were confirmed as S. aureus via biochemical test. Fifty-two negative S. aureus were subjected to indirect gold Nanoparticle (AuNPs) based ELISA. Molecular identification using PCRThe PCR confirmed S. aureus in 100% (78/78) of the bacteriologically positive samples as illustrated in Figure 1. SDS-PAGE profile of crude and purified fraction antigenConfirmed S. aureus crude and fraction antigen described by SDS–PAGE in Figures 2 and 3 and Table 2. The crude antigen revealed ten bands at molecular weights 245.0, 180.0, 135.0, 100.0, 75.0, 63.0, 48.0, 35.0, 25.0, and 20.0 KDa. The diagnostic fraction was characterized by two bands at 73.1 KDa and 63.9 KDa. Indirect ELISASeventy-eight sera samples related to PCR confirmed S. aureus isolates subjected to indirect ELISA to evaluate a crude, fraction, and fraction antigen conjugated gold. The OD reading at 450 nm showed that 83.3% (76/78) of the wells coated with crude antigen had S. aureus-specific IgG antibodies, while they increased to 91% (71/78) when coated with fraction antigen and to 97% (76/78) when coated with fraction antigen conjugated with gold as shown in Table 3. Validity evaluation of the stored purified fraction antigen for one year at −20°C compared with freshly purified fraction is revealed in Table 4. Gold nanoparticle-based indirect ELISA was applied on all 200 milk and serum samples to detect S. aureus specific IgG antibodies. Specific IgG antibodies were detected in 97% (76/78) sera and milk samples related to PCR and bacteriologically confirmed S. aureus isolates. IgG antibodies were found in 5.7% (3/52) positive SCM and negative S. aureus isolate bacteriologically and with PCR. IgG was not detected in any of the seventy negative SCM samples, neither in milk nor in serum. Superiority intention was applied to treat analysis, the AuNP-based ELISA was compared to standard bacteriological and PCR methods to determine its diagnostic accuracy. Gold nanoparticle-based ELISA has superiority in sensitivity and specificity over traditional methods, which revealed sensitivity, specificity, positive predictive value, and negative predictive value at rates of 97.4%, 97.5%, 96.2%, and 98.3%, respectively. DiscussionSCM is an asymptomatic form of intra-mammary inflammation that reduces milk production, quality, and overall herd health, causing severe economic losses to the dairy industry, especially that caused by S. aureus (Gad et al., 2025). Great attention has recently been given to improving the analysis of mastitis, particularly in the early subclinical stage (Gad et al., 2025). Somatic cell count estimation is one of the strategies used for the detection of SCM, either directly using automatic somatic cell counters or indirectly by gel formation because the nuclear material of somatic cells excreted in milk works in combination with a specific compound reagent (the mode of action of the CMT) (Elhaig and Selim, 2015).
Fig. 1. PCR of S. aureus nuc2 gene (664 bp). Lane 1 is a 100-bp DNA marker; Lanes 2–13 are representative positive samples. Lane 14: control positive; Lane 15: control negative.
Fig. 2. Staphylococcal aureus crude antigen SDS PAGE profile showed 10 bands (10 peaks). Table 2. SDS PAGE profile of S. aureus crude and purified fraction antigen.
Table 3. Indirect ELISA against crude, purified fraction and fraction antigen conjugated gold nanoparticles.
In the current study, the CMT revealed 65% (130/200) SCM prevalence in different farms in Egypt. This is higher than the prevalence percentage of SCM revealed in other reports conducted in Egypt (52.1%), which were informed by Algammal et al. (2020). Differences in geographic distribution, immunological station, unsanitary surroundings, and contaminated apparatuses can all afford elevated rates of SCM in dairy farms, which could be responsible for the elevated rate of S. aureus prevalence in the current report. Among 130 positive SCM milk samples, the prevalence of S. aureus in mastitis milk samples using bacteriological examination and biochemical tests was 60% (78/130), lower than that of another study accomplished in Egypt by Abd El-Razik et al. (2023) who reported a S. aureus prevalence rate of (66.7%) and higher than that of another study achieved in Egypt by Ibrahim et al. (2022) who reported a S. aureus prevalence rate of 28.92%. Table 4. Evaluation of sensitivity and validity of crude, purified fraction and fraction antigen conjugated gold nanoparticles.
Fig. 3. Staphylococcal aureus purified fraction SDS PAGE profile showed 2 bands (2 peaks). Rapid detection of S. aureus is potential throughout the use of molecular techniques like PCR and real time PCR, which are important for treatment and infection control measures to avoid the spread of diseases (Galia et al., 2019). All 78 (100%) bacteriologically positive S. aureus isolates were confirmed using nuc2 gene for S. aureus using PCR as shown in (Fig. 1). Like other bacterial species, S. aureus must adhere to host tissue for an infection to develop. Bacterial proteins are the major molecules that interact with host cells and tissues, causing this attachment (Fouad et al., 2022). The initial stage in creating vaccines and diagnostics is the isolation and identification of these proteins. The current work presents two affinity-purified protein bands that were separated from S. aureus through affinity column chromatography (CNBr-Sepharose 4B). The benefit of using CNBr-Sepharose 4B affinity chromatography is that the immunogenic fraction can be purified all at once (Aly et al., 2017). Similarly, S. aureus capsular polysaccharide (CP5) was purified using DEAE sephacel ion exchange chromatography (Sigma Chemical Co.). To demonstrate the immunogenicity of the purified element, one-dimensional SDS-PAGE and immunoblot were used after the purification process (Li et al., 2018). In the current study, the confirmed S. aureus diagnostic fraction antigen was characterized by two bands at 73.1 KDa and 63.9 KDa described by SDS–PAGE in Figure 2. The two bands of the purified antigen increase the likelihood of detecting antibodies against infection without interference from the eight nonspecific bands of the crude antigen, making the diagnosis using the purified antigen more specific and well-diagnostic than the crude one. Previously, a 210 KDa molecule was separated by affinity column chromatography and analyzed using SDSPAGE, which revealed extra smaller peptides with fibronectin-binding properties, involving 29 KDa (González-Sapienza et al., 2000). Additionally, among the isolated proteins that have been released by S. aureus proteins are 114.8 KDa in size, containing six hydrophobic segments, and 11 KD upon purification with superdex, its immunogenicity was verified through immunoblot (Aly et al., 2017). A proteomic study has identified key proteins in S. aureus extracellular vesicles, including penicillin-binding proteins (32 KDa) and bifunctional peptidoglycan hydrolase (138 KDa), and two active proteins: 62 KDa (amidase) and 51 KDa (glucosaminidase) processed from the huge one (Wang et al., 2018). Based on the result of indirect ELISA reflected by OD readings, the binding activities of fraction antigen were higher than that of crude extract and the binding activities of fraction antigen conjugated gold nanoparticles were higher than that of fraction antigen as shown in Table 3. The separated bands in this investigation demonstrated efficacy in detecting S. aureus antigen-specific antibody reactions in milk and serum samples using indirect ELISA, which indicates that these bands have been exhibited during infection and trigger humoral responses in the blood and udder. The present investigation and its predecessor verified the theory that S. aureus exhibits multiple immunogens during infection, whether in blood or milk. On the other hand, the diagnosis of mastitis might be accomplished by employing monoclonal antibodies in ELISA for antigen detection (Fouad et al., 2022). The diagnosis of mastitis involves the detection of antigens or antibodies. It is crucial to note that certain protein molecules are conserved throughout several isolates of S. aureus, as demonstrated by Misra et al. (2018). In this study, purified fraction antigen conjugated gold nanoparticles achieved the highest sensitivity to ELISA at 97%, 94%, and 92% at once before storage, 6 months at −20°C, and 1 year at −20°C, respectively. The utilization of purified fraction antigens with gold nanoparticles enhances the sensitivity of ELISA, increasing it from 83.3% to 97%. The obtained result is greater than that of Khodadadi et al. (2020), which reported a sensitivity of 93.33% for the developed Nano-ELISA kit. Specific IgG antibodies were detected in 97% (76/78) sera and milk samples related to confirmed S. aureus isolates as well as in three cows that revealed positive SCM and negative S. aureus isolates bacteriologically and with PCR using gold nanoparticle-based indirect ELISA. This is consistent with Leitner et al. (2000), who detected IgG in milk and blood and demonstrated the systemic reaction of all positive isolates following an experimental intramammary S. aureus infection. Three negative S. aureus that demonstrated little specific IgG antibodies in this study might be related to recently infected and treated cows. The two positive S. aureus isolates did not show specific IgG antibodies that might be related to recently infected or immune-suppressed cows. Researchers have investigated the possibility of utilizing the catalytic properties of AuNPs as a substitute for traditional enzymes used in experiments to improve the sensitivity of ELISA assays. Gold nanoparticles possess extreme stability and exhibit intense peroxidase-like catalytic activity, making them well-suited as nanozymes to enhance the sensitivity of ELISA assays and emerging as alternative options to horseradish peroxidase (HRP) in many biosensing applications (Zhao et al., 2019). Nanoparticles possess a major ratio of surface area to volume, enabling the binding of several enzymes with antibodies (up to 20 HRP per nanoparticle). This results in an enhanced colorimetric signal when compared to a single enzyme-conjugated antibody (Gao et al., 2019). Zhou et al. (2012) found that immobilizing capture antibodies on the AuNPs surface increased their activity by 108% compared to unmodified Elisa plates. This was due to the production of super-hydrophilic surfaces, which improved binding efficiency while preserving antibody activity. The current study establishes a valuable way for S. aureus mastitis diagnosis with the use of purified fraction antigen-conjugated gold nanoparticles instead of the traditional way. This will improve the sensitivity and the specificity of ELISA by providing additional binding sites for antibody detection and improving the signal intensity and the stability of biomolecules in complex biological milieus that decrease the reaction time of this assay. The utilization of purified fraction antigen with gold nanoparticles enhances the sensitivity of ELISA from 83.3% to 97% (p < 0.01; CI: 99%). The test may be used as a highly sensitive test for monitoring S. aureus specific IgG antibodies in milk at 37°C without the need for other laboratory testing. Increasing IgG antibodies for S. aureus can be used as a good indicator for early diagnosis of S. aureus SCM and subsequently early treatment. ConclusionThe present study validated the improvement of a novel method for quickly and cost-effectively detecting bovine mastitis using the diagnostic affinity-purified fraction, replacing traditional bacteriological culture and biochemical tests. It is also an important step for infection control. AuNPs serve a variety of purposes, including serving as three-dimensional carriers for antibodies to boost assay sensitivity. They can also replace conventional enzymes to achieve highly sensitive and stable immunological assays. Clinical applications can also utilize AuNPs to create a fast and visually detectable assay. Our study recommends using the optical properties of AuNPs to develop diagnostic systems that allow for visual detection without instruments. AcknowledgmentsSpecial thanks to Ali S. Elkelany, clinical pharmacist, department of clinical pharmacy for his great help in the statistical analysis of this study. My deepest appreciation and thanks to Prof. Ibrahim Eldaghayes, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya, and to the entire editorial board of Open Veterinary Journal for providing a waiver of the publication fees. Conflict of interestThe authors declare no conflict of interest. FundingThe study was financially supported by the National Research Centre, Egypt (Grant No. 13010121). Authors’ contributionsWalaa A. Gad—Data collection, Formal analysis, Investigation, Methodology, Visualization, Writing. Salama A. Osman, Khaled A. Abd El-Razik—supervision, reviewing, and editing, Ehab A. Fouad—Methodology, Visualization, editing. Ashraf H. Soror—Funding acquisition and reviewing. Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. ReferencesAbdel-Rahman, E.H., El-Jakee, J.K., Hatem, M.E., Ata, N.S. and Fouad, E.A. 2017. Preparation of goat and rabbit anti-camel immunoglobulin G whole molecule labeled with horseradish peroxidase. Vet. World. 10(1), 92. Abd El-Razik, K.A., Arafa, A.A., Fouad, E.A., Soror, A.H., Abdalhamed, A.M. and Elgioushy, M. 2023. Phenotypic and genotypic characterization of erythromycin-resistant Staphylococcus aureus isolated from bovine subclinical mastitis in Egypt. Vet. World. 16(7), 1562. Al Emon, A., Hossain, H., Chowdhury, M.S.R., Rahman, M.A., Tanni, F.Y., Asha, M.N., Akter, H., Hossain, M.M., Islam, M.R. and Rahman, M.M. 2024. Prevalence, antimicrobial susceptibility profiles and resistant gene identification of bovine subclinical mastitis pathogens in Bangladesh. Heliyon 10(14), e34567. Alluwaimi, A., Leutenegger, C., Farver, T., Rossitto, P., Smith, W. and Cullor, J. 2003. The cytokine markers in Staphylococcus aureus mastitis of bovine mammary gland. J. Vet. Med. Series B 50(3), 105–111. Algammal, A.M., Enany, M.E., El-Tarabili, R.M., Ghobashy, M.O. and Helmy, Y.A. 2020. Prevalence, antimicrobial resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens 9(5), 362. Al-Mayah, A.A. and Saeed, E.A. 2013. Preparation of diagnostic monovalent antisera against Staphylococcus aureus. Methods 10, 11. Aly, K.A., Anderson, M., Ohr, R.J. and Missiakas, D. 2017. Isolation of a membrane protein complex for type VII secretion in Staphylococcus aureus. J. Bacteriol. 199(23), e00482–e00417. Basso, C.R., Cruz, T.F., Vieira, L.B., Pedrosa, V.A., Possebon, F.S. and Araujo Junior, J.P. 2024. Development of a gold nanoparticle-based ELISA for detection of PCV2. Pathogens 13(2), 108. Elhaig, M.M. and Selim, A. 2015. Molecular and bacteriological investigation of subclinical mastitis caused by Staphylococcus aureus and Streptococcus agalactiae in domestic bovids from Ismailia, Egypt. Trop. Anim. Health Prod. 47, 271–276. Fouad, E., Abd El-Razik, K. and Abdel Rahman, E. 2022. Diagnosis of Staphylococcus aureus infection in bovine mastitis using its affinity purified fraction. Adv. Anim. Vet. Sci. 10(9), 1887–2089. Fursova, K., Sorokin, A., Sokolov, S., Dzhelyadin, T., Shulcheva, I., Shchannikova, M., Nikanova, D., Artem’eva, O., Zinovieva, N. and Brovko, F. 2020. Virulence factors and phylogeny of Staphylococcus aureus associated with bovine mastitis in Russia based on genome sequences. Front. Vet. Sci. 7, 135. Gad, W.A., Osman, S.A., Hegazy, Y.M., Abd El-Razik, K.A., Fouad, E.A., Soror, A.H. and Hozyen, H.F. 2025. Diagnosis of subclinical Staphylococcal aureus bovine mastitis using nanotechnology-based techniques. EJVS 56(4), 721–730. Galia, L., Ligozzi, M., Bertoncelli, A. and Mazzariol, A. 2019. Real-time PCR assay for detection of Staphylococcus aureus, panton-valentine leucocidin and methicillin resistance directly from clinical samples. AIMS Microbiol. 5(2), 138. Gao, Y., Zhou, Y. and Chandrawati, R. 2019. Metal and metal oxide nanoparticles to enhance the performance of enzyme-linked immunosorbent assay (ELISA). ACS Appl. Nano Mater. 3(1), 1–21. González-Sapienza, G., Lorenzo, C. and Nieto, A. 2000. Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein, Echinococcus granulosus antigen B. J. Clin. Microbiol. 38(11), 3979–3983. Hiitiö, H., Riva, R., Autio, T., Pohjanvirta, T., Holopainen, J., Pyörälä, S. and Pelkonen, S. 2015. Performance of a real-time PCR assay in routine bovine mastitis diagnostics compared with in-depth conventional culture. J. Dairy Res. 82(2), 200–208. Ibrahim, E.S., Arafa, A.A., Dorgam, S.M., Eid, R.H., Atta, N.S., El-Dabae, W.H. and Sadek, E.G. 2022. Molecular characterization of genes responsible for biofilm formation in Staphylococcus aureus isolated from mastitic cows. Vet. World 15(1), 205. Juronen, D., Kuusk, A., Kivirand, K., Rinken, A. and Rinken, T. 2018. Immunosensing system for rapid multiplex detection of mastitis-causing pathogens in milk. Talanta 178, 949–954. Khodadadi, A., Madani, R. and Atyabi, N. 2020. Development of Nano-ELISA method for serological diagnosis of toxoplasmosis in mice. Arch. Razi Inst. 75(4), 419. Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259), 680–685. Leitner, G., Yadlin, B., Glickman, A., Chaffer, M. and Saran, A. 2000. Systemic and local immune response of cows to intramammary infection with Staphylococcus aureus. Res. Vet. Sci. 69(2), 181–184. Li, T., Huang, M., Song, Z., Zhang, H. and Chen, C. 2018. Biological characteristics and conjugated antigens of ClfA A-FnBPA and CP5 in Staphylococcus aureus. Can. J. Vet. Res. 82(1), 48–54. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1), 265–275. Martin, S.W., Meek, A.H. and Willeberg, P. 1987. Veterinary epidemiology: principles and methods, Iowa State University Press I Amas. Full text of this book is made available by Virginia Tech Libraries at: http://hdl.handle.neU10919/72274 Misra, N., Pu, X., Holt, D., McGuire, M. and Tinker, J. 2018. Immunoproteomics to identify Staphylococcus aureus antigens expressed in bovine milk during mastitis. J. Dairy Sci. 101(7), 6296–6309. Mostafa Abdalhamed, A., Zeedan, G.S.G., Ahmed Arafa, A., Shafeek Ibrahim, E., Sedky, D. and Abdel Nabey Hafez, A. 2022. Detection of methicillin-resistant Staphylococcus aureus in clinical and subclinical mastitis in ruminants and studying the effect of novel green synthetized nanoparticles as one of the alternative treatments. Vet. Med. Int. 22(1), 6309984. Nakane, P.K. and Kawaoi, A. 1974. Peroxidase-labeled antibody a new method of conjugation. JHC 22(12), 1084–1091. Parolo, C., de la Escosura-Muñiz, A. and Merkoçi, A. 2013. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 40(1), 412–416. Rahim, A., Ahmad, G., Ijaz, M., Akhtar, H., Ahsan, K. and Rizwan, M. 2021. Isolation and identification of antibiotics susceptible Staph aureus from unprocessed milk. J. Clin. Med. Res. 3(2), 1–11. Roshan, M., Arora, D., Behera, M., Vats, A., Gautam, D., Deb, R., Parkunan, T. and De, S. 2022. Virulence and enterotoxin gene profile of methicillin-resistant Staphylococcus aureus isolates from bovine mastitis. Comp. Immunol. Microbiol. Infect. Dis. 80, 101724. Sharma, R.K., Yadav, R., Kaur, B. and Aligarh, U. 2023. Enzyme-linked immunosorbent assay (ELISA): principles, methods, and applications. J. High. Educ. Theory Pract. 23(1), 967. Tabatabaei, M.S., Islam, R. and Ahmed, M. 2021. Applications of gold nanoparticles in ELISA, PCR, and immuno-PCR assays: a review. Anal. Chim. Acta. 1143, 250–266. Wang, X., Thompson, C.D., Weidenmaier, C. and Lee, J.C. 2018. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 9(1), 1379. Wehr, H.M. and Frank, J.F. (2004). Standard methods for the examination of dairy products. Washington, DC: American Public Health Association. Zhao, C., Hong, C.Y., Lin, Z.Z, Chen, X.M. and Huang, Z.Y. 2019. Detection of malachite green using a colorimetric aptasensor based on the inhibition of the peroxidase-like activity of gold nanoparticles by cetyltrimethylammonium ions. Microchim. Acta. 186, 1–8. Zhou, F., Wang, M., Yuan, L., Cheng, Z., Wu, Z. and Chen, H. 2012. Sensitive sandwich ELISA based on a gold nanoparticle layer for cancer detection. Analyst 137(8), 1779–1784. | ||
| How to Cite this Article |
| Pubmed Style Gad WA, Osman SA, El-razik KAA, Soror AH, Fouad EA. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Vet. J.. 2024; 14(12): 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 Web Style Gad WA, Osman SA, El-razik KAA, Soror AH, Fouad EA. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. https://www.openveterinaryjournal.com/?mno=222207 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.23 AMA (American Medical Association) Style Gad WA, Osman SA, El-razik KAA, Soror AH, Fouad EA. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Vet. J.. 2024; 14(12): 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 Vancouver/ICMJE Style Gad WA, Osman SA, El-razik KAA, Soror AH, Fouad EA. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 Harvard Style Gad, W. A., Osman, . S. A., El-razik, . K. A. A., Soror, . A. H. & Fouad, . E. A. (2024) A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Vet. J., 14 (12), 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 Turabian Style Gad, Walaa A., Salama A. Osman, Khaled A. Abd El-razik, Ashraf H. Soror, and Ehab A. Fouad. 2024. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Veterinary Journal, 14 (12), 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 Chicago Style Gad, Walaa A., Salama A. Osman, Khaled A. Abd El-razik, Ashraf H. Soror, and Ehab A. Fouad. "A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA." Open Veterinary Journal 14 (2024), 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 MLA (The Modern Language Association) Style Gad, Walaa A., Salama A. Osman, Khaled A. Abd El-razik, Ashraf H. Soror, and Ehab A. Fouad. "A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA." Open Veterinary Journal 14.12 (2024), 3388-3396. Print. doi:10.5455/OVJ.2024.v14.i12.23 APA (American Psychological Association) Style Gad, W. A., Osman, . S. A., El-razik, . K. A. A., Soror, . A. H. & Fouad, . E. A. (2024) A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Veterinary Journal, 14 (12), 3388-3396. doi:10.5455/OVJ.2024.v14.i12.23 |