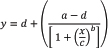| Research Article | ||
Open Vet. J.. 2024; 14(12): 3397-3403 Open Veterinary Journal, (2024), Vol. 14(12): 3397-3403 Research Article Study of the effect of different diluents for syringe immersion test on Rhipicephalus microplus larvae against macrocyclic lactonesDiego Robaina*, Jessica Caballero and Gonzalo SuárezUnidad de Farmacología y Terapéutica, Departamento Hospital y Clínicas Veterinarias, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay *Corresponding Author: Diego Robaina. Unidad de Farmacología y Terapéutica, Departamento Hospital y Clínicas Veterinarias, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay. Email: diego.robaina [at] fvet.edu.uy Submitted: 01/10/2024 Accepted: 18/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
AbstractBackground: Macrocyclic lactones (MLs) are pharmaceutical compounds extensively utilized in the management of Rhipicephalus microplus tick infestations in bovine populations. It is of paramount importance to prevent or delay the development of drug resistance to ML. In vitro techniques are validated by FAO and can serve as an orientative diagnosis of the resistance developed in field conditions. Diluent selection must be considered when sensitivity on field strains is being studied. The syringe immersion test (SIT) is a modification of the larval immersion test where syringes are used seeking to reduce the workload. Aim: Study the interchangeability of two diluents in the diagnosis of sensitivity to MLs on R. microplus larvae using the SIT technique. Methods: Dose-response curves were adjusted using SIT with MLs, on different diluents [acetone (ACT) and dimethyl sulfoxide (DMSO)] on Mozo strain (standard susceptible strain). Slope, potency, and discriminating concentration were estimated for each drug on both diluents. A four-parameter log-logistic model was applied for model fitting. The ratio between estimated parameters was used to compare results. Field strains were tested on both diluents for each drug, using the discriminating concentration estimated for Mozo strain. Results: For the Mozo strain, dose-response models were adjusted for each drug on both diluents using SIT. Ivermectin (IVM) and doramectin (DRM) showed no significant difference in slope when comparing diluents (p > 0.05); moxidectin (MOX) presents a higher sensitivity for DMSO versus IVM (p < 0.05). Significant differences occur when comparing DRM with MOX in both diluents. Potency does not differ for avermectins using ACT 1%, and MOX has a higher potency than avermectins (p < 0.05). On field populations, we found an increase in larval mortality when using DMSO as opposed to ACT (p < 0.05) for IVM, DRM, and MOX, a differential sensitivity to detect larvae with survival capacity at equal levels of lethal concentration in both diluents for the same drug on Mozo strain. Conclusion: We conclude that the SIT technique is a tool capable of detecting susceptibility/resistance in R. microplus populations regardless of the diluent used. Keywords: Diagnostic, Ectoparasiticides, Pharmacodynamics, Resistance, Ticks. IntroductionRhipicephalus microplus ticks are an obligate and temporary external parasite of vertebrate animals. They are responsible for serious direct economic losses due to their hematophagous feeding, as well as indirect losses due to being vectors of pathogens (Eckstein et al., 2015; Molento, 2020), in addition to the costs involved in ectoparasite control strategies (labor, purchase of veterinary products, and appropriate instruments). Given that chemical treatments are currently almost the only resource available to producers for the control of this parasite (Fiel and Nari, 2013), it is imperative to carry out studies to ensure the efficacy of the tool for as long as possible, avoiding or delaying the generation of resistance, which is becoming a global problem (Cuore et al., 2012; Reck et al., 2014; Vilela et al., 2020; Villar et al., 2020). Major contributing factors to the development of resistance may be the misuse of drugs (Bianchi et al., 2003) and the use of the wrong concentration of acaricide, leading to the failure of the tick control programs (Pegram et al., 2000). The macrocyclic lactones (MLs) family consists of avermectins [ivermectin (IVM) and doramectin (DRM)] and milbemycins [moxidectin (MOX)]. There is concern about the possibility of cross-resistance between MLs, given their similar molecular structures and mechanisms of action (Prichard et al., 2012), with an increasing need to maximize the use of the pharmacological tool. Aspects of potency and peak efficacy, the main parameters of pharmacodynamics, must be taken into account when performing sensitivity diagnostics in field populations. Early characterization of the ML sensitivity profile is a starting point for the strategic use of the available tools. Robaina et al. (2023) performed in vitro dose-response assays with R. microplus larvae using a modification of the immersion techniques called syringe immersion test (SIT), working with a diluent based on distilled water and 1% acetone (ACT), for IVM, DRM, and MOX. The adjusted models showed that MOX was the most potent ML (lowest effective dose 50, ED50), while DRM had the lowest slope, indicating a lower degree of sensitivity to variations in concentrations. Diluent selection and preparation is one of the variables to consider when aiming to standardize an in vitro study. The most commonly used solvent vehicles in both in vivo and in vitro models include dimethyl sulfoxide (DMSO), ACT, methanol, ethanol, and the detergents Tween-20 and Tritox X-100 (Castro et al., 1995; Gonçalves et al., 2007; Ravindran et al., 2011). The solvent used to dissolve the active ingredient must have little or no acaricidal effect. The effect of ethanol on membranes and the displacement of cell-bound water has been documented (Jones, 1989). Conversely, DMSO readily crosses most animal membranes and is easily absorbed through the skin (Jacob and Herschler, 1986). These differences may be of interest when considering the excipients in the formulations and how the active ingredients are dissolved. The absorption properties at the tick level could represent a barrier that modifies the concentrations at the parasite biophase. This may ultimately result in a determinant variable for establishing efficacy parameters in dose-response curves for the active ingredients in a formulation, as well as for diagnosing the sensitivity of field strains. The present study aims to study the interchangeability of two diluents (ACT 1% vs. DMSO 1%) in the diagnosis of sensitivity to IVM, DRM, and MOX on R. microplus larvae using the SIT technique. Materials and MethodsChemicalsIVM (Lot 49450511) and DRM (Lot 07492109) were donated by Compañia Cibeles S.A. (Uruguay). MOX (Lot MX-A2007025) was obtained from Laboratorio Pasteur S.A. (Uruguay). All other reagents used in this work were from SIGMA Chemical Company. Stock solutions were prepared in ACT or DMSO [IVM (2,000 ppm), DRM (2,000 ppm), and MOX (100 ppm)]. Rhipicephalus microplus larvaeMozo strain In Uruguay, the standard susceptible strain Mozo is used by the regulatory authorities. Briefly, adult female ticks are conditioned in Petri dishes and incubated with controlled temperature and humidity (27°C and 90%). After 14 days of incubation, the xenogyns are removed and new 25-day incubation period is continued for hatching of viable larvae. Larvae with 14–16 days old are used on the tests, allowing the comparison of different populations of larvae synchronized at the same time of development in terms of vitality and survival time. Field strain Larvae were obtained from engorged ticks from each of five different animals, from nine different farms, and stored individually to assess for drug sensitivity variance among the field strain. Adult ticks, eggs, and larvae were managed by applying the same protocols as Mozo strain. The farm manager reported reduced efficacy when using IVM for R. microplus control. Syringe immersion testSyringe assembly and immersion time was performed as described by Robaina et al. (2023). Corresponding drug dilutions were prepared daily according to the methodology published by FAO (2004). Briefly, the diluent was used as a control solution, formulated from 1% ACT or DMSO in distilled water. After 5 minutes of immersion, the syringes are removed, dried on drying paper, and placed in a flow hood for 1 hour prior to incubation for 24 hours. Incubation conditions are the same as those established for adult ticks. After 24 hours, larval mortality was determined by counting both live and dead larvae. Larvae that were paralyzed or that moved only their appendages, but were unable to walk, were considered dead. Mozo strainThree dose-response curves were performed by triplicate on different days. When using ACT 1%, IVM and DRM concentrations ranged from 20 to 0.155 ppm, and in the case of MOX, the final concentrations were between 1 and 0.01 ppm (data report according to Robaina et al., 2023). For DMSO 1%, the dose-response model for IVM was in the range from 1 to 0.03 ppm; DRM ranged from 2 to 0.06 ppm and from 0.4 to 0.012 ppm for MOX. Pharmacodynamic function for SITAccording to Ritz et al. (2015), the pharmacodynamic profile for increasing acaricide concentrations (four-parameter log-logistic model, equation 1) was adjusted using larvae mortality and log-transformed concentration data. For both ACT and DMSO, the effect (Emax) for each curve was set to 1 (100% mortality), being that the maximum possible mortality cannot exceed 100%. The estimated values for the lower limit of efficacy, slope (indicating the sensitivity of the technique), ED50 (as an estimation of the potency), and ED95 (as a discriminating dose). Equation 1. Four-parameter log-logistic equation
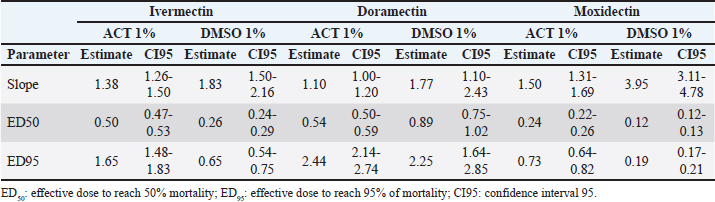
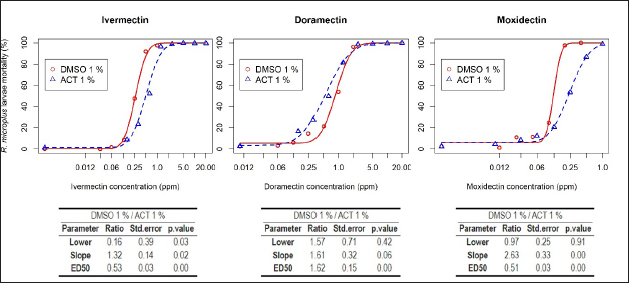
where “y” is the response variable (alive/dead), “a” is the theoretical response at infinite concentration, “b” is the slope factor, “c” is the mid-range concentration (inflection point), and “d” is the theoretical response at zero concentration. Field strainField strains (alphabetical order A–I, n=9) were tested as paired samples at the discriminating dose (ED95) estimated for IVM, DRM, and MOX for each diluent (ACT 1% and DMSO 1%) (Section “Pharmacodynamic function for SIT”). Statistical analysisThe pharmacodynamic parameters were shown in mean (estimate), standard error (std. error), and the 95% confidence interval using the dose-response models fitted for the Mozo strain using R software (R Core Team, 2024) and the drc package (Ritz et al., 2015). The adjusted model for each diluent was performed following the analysis published by Robaina et al. (2023). The ratio for the pharmacodynamic parameters (slope, ED50, and ED95) was calculated for each drug between both diluents (ACT 1% vs. DMSO 1%). All tests were performed with a statistical significance set to 95%. Ethical approvalNot needed for this study. ResultsDose-response fittingTable 1 summarizes the dose-response model for R. microplus Mozo larvae on each drug (IVM, DRM, and MOX) for the diluents used in this study (ACT 1% and DMSO 1%). CI95 is shown next to each pharmacodynamic parameter estimate. Diluent comparison for model adjustmentFigure 1 and Table 2 show the model adjusted for each drug included in the study as well as the ratio between the main pharmacodynamic parameters (slope and ED50) when using both DMSO 1% and ACT 1% as diluents for the SIT technique, on R. microplus Mozo strain larvae. For each diluent on each drug, dose-response models are summarized, considering the three adjusted curves as one. Figure 1 and Table 2 indicate the differences between the pharmacodynamic parameters when using both diluents. When we focus on the slope of the curve, the avermectins (IVM and DRM) no longer show a significant difference between them (p > 0.05) when changing the diluent from ACT 1% to DMSO 1%; these differences are reversed in the slope ratio for MOX versus IVM, with a higher sensitivity for MOX in DMSO (1.5 in ACT to 3.95 in DMSO, p < 0.05). Significant differences occur when comparing DRM with MOX in both diluents. Table 1. Dose-response model for R. microplus larvae (Mozo) by IVM, DRM, and MOX for ACT 1% and DMSO 1% for SIT.
Fig. 1. Comparison of parameter log-logistic model for R. microplus larvae (Mozo) between ACT 1% and DMSO 1% by IVM, DRM, and MOX for SIT. Green line=95 mortality. Vertical red line=ED95 for DMSO1%. Vertical blue line=ED95 for ACT1%. Table 2. Ratio between the main pharmacodynamic parameters when using IVM, DRM, or MOX for the SIT technique in ACT 1% and DMSO 1% on R. microplus Mozo strain larvae.
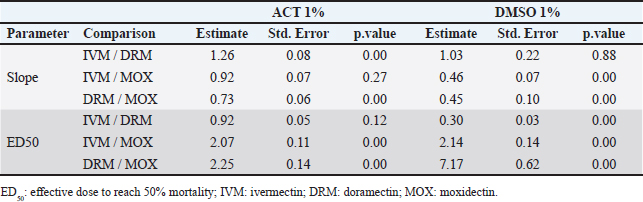
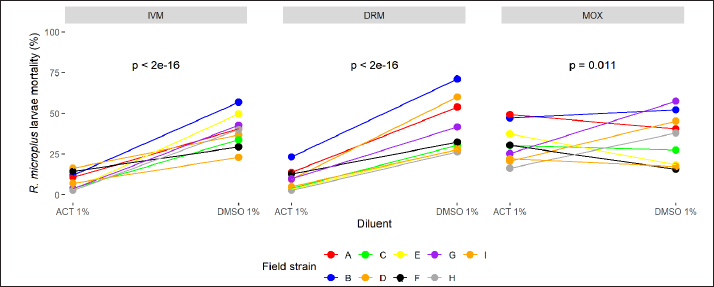
While the pharmacological potency (ED50) does not differ for avermectins using ACT 1%, MOX has a higher potency than avermectins (ratio >1 and p < 0.05). However, differences in the pharmacological potency between avermectins are identified, where the DRM curve shifts to the right ( Figure 2 shows the mortality variation for R. microplus field strain larvae when tested by SIT using IVM, DRM, and MOX ED95 concentrations, for both ACT1% and DMSO 1% adjusted models. ML concentrations used were estimated by the adjusted model for each diluent on R. microplus Mozo strain larvae. For the three MLs, we found an increase in larval mortality when using DMSO as opposed to ACT (p < 0.05), which we interpret as a differential sensitivity to detect larvae with survival capacity at equal levels of lethal concentration in both diluents (ED95) for the same ML. Visually, the situation is more relevant in the case of avermectins. The use of SIT as a technique to construct dose-response curves for R. microplus larvae using MLs offers an alternative for the detection of sensitivity loss in cattle tick field strains. Robaina et al. (2023) fitted in vitro pharmacodynamic models for IVM, DRM, and MOX using ACT 1% as diluent, managing to estimate the main pharmacodynamic parameters [slope (sensitivity) and ED50 (potency)]. The selection of a suitable solvent for bioassays is governed by the solubility of the samples (extracts, fractions, or isolated compounds) and by the stress imposed on the test organisms by organic solvents (Gonçalves et al., 2007). DMSO is a polar organic molecule widely used as a solvent for small molecule hydrophobic drugs (Galvao et al., 2013), with low toxicity and a tendency to conduct compounds across biological barriers (White et al., 2004). Our results indicate potential changes and variability according to the choice of diluent used but show that the MLs used in the study behaved differentially in terms of pharmacological potency with DMSO. A comparison of parameters (Fig. 1) between diluents for each drug was performed by calculating pharmacodynamic parameters and DMSO 1%/ACT 1% ratios between MLs and between diluents for each drug. The slopes showed significant differences, except for DRM. This indicates that the sensitivity for IVM and MOX in SIT is modified when using 1% DMSO as diluent, where increases in drug concentration lead to a rapid increase in mortality of R. microplus larvae compared to what is observed for ACT 1%. The estimated potency (ED50) in all MLs by the adjusted models in both diluents presented statistically different behaviors. Gonçalves et al. (2007) carried out comparisons of different solvents and surfactants on R. microplus adult females and larvae. In their trials, they did not test for active ingredients, the object of the study being the effect of the solvents on different stages of R. microplus. The tests carried out with ACT on adult females resulted in 100% mortality, except that they used 100% ACT and did not carry out work with lower concentrations of the solvent. The aforementioned authors used DMSO 1% for immersion of adult females of R. microplus, detecting 0% mortality, with oviposition similar to the control group (water). The work with larvae was done applying the larval immersion test (LIT) technique using DMSO 1% and ACT (without indication of concentration), finding larval mortalities of 5% and 1%, respectively, concluding that ACT should not be used in tests such as LIT. Our work detected basal mortality ranging from 0% to 3% for DMSO 1% and from 0% to 11% for ACT 1%. Among the techniques for determining the sensitivity of R. microplus to ectoparasiticides, we have in vivo tests such as the stable test (Wharton et al., 1970), which is considered a defining methodology. As alternatives, there are in vitro techniques, bioassays that are performed on both adult parasites and larvae; both techniques are validated by FAO (2004) and are capable of achieving an indicative diagnosis of the resistance profile developed in the field (Cuore, 2013). Concern about the generation of resistance by R. microplus requires us to have tools that are capable of early detection of the loss of sensitivity in field populations, to facilitate decision-making for each situation. The differences that may arise from the use of different organic solvents for in vitro assays should be evaluated for each acaricide drug, as well as for each parasitic species. Fig. 2. Comparison of R. microplus larvae mortality (%) based on two different diluents (ACT 1% and DMSO 1%) using IVM, DRM, and MOX by SIT. The use of DMSO as a solvent for in vitro tests was also studied on cestodes by Ahmad and Nizami (1983), who published the effects of DMSO on the metabolism of the cestode A. lahorea. DMSO has no metabolic effect on the cestode A. lahorea and is considered a suitable solvent for in vitro tests on this parasite. Resende et al. (2012) tested Amblyomma cajennense and Dermacentor nitens larvae treated with 1% DMSO. The mortality obtained was less than 4%, revealing its low toxicity to the larvae of both parasite species. Similar results were reported by White et al. (2004) working with different solvents, including DMSO (1%, 3%, and 5%) when using acaricides such as amitraz, propoxur, and IVM on Amblyomma americanum larvae. Although the in vitro test performed by White et al. (2004) differs from the one applied in our trial, the mortality results for DMSO at the different concentrations evaluated did not exceed 0%. The dose-response model fits for MLs in each diluent (ACT 1% and DMSO 1%) reveal that MOX is the drug with the highest potency (ED50MOX < ED50IVM=ED50DRM and ED50MOX < ED50IVM < ED50DRM < ED50DRM, for ACT 1% and DMSO 1%, respectively). A possible explanation could be that MOX has a higher lipophilicity than IVM (logPMOX=6 and logPIVM=4.8) (Prichard et al., 2012), which would favor penetration of the acaricide upon contact with the larvae during immersion, resulting in higher mortality at lower concentrations. There are no reports on the solubility of MLs in ACT, so we cannot make clear comparisons on possible alterations in drug entry into larvae. The discriminant dose (ED95) then selected to test the efficacy of the drugs on field strains behaved the same for both diluents regardless of solvent, where ED95MOX < ED95IVM < ED95DRM for both ACT 1% and DMSO 1%. For these parasite populations where resistance is not diagnosed, but there are reports of low therapeutic efficacy (<95% efficacy), there may be a zone of uncertainty around sensitivity for avermectins according to the solvent used in SIT. When ACT 1% is used as a solvent in the diagnostic test in populations where mortality is slightly lower than expected for the reference strain (ranging from 80% to 95% field strain vs. 95% reference strain), the field strain would be detected as “resistant.” A different situation would arise when using the diluent based on DMSO 1%, where the same field population could be diagnosed as “sensitive.” Finally, when dealing with resistant populations, the mortality obtained was always lower than expected with the sensitive strain (ED95). We conclude that the SIT technique is a tool capable of detecting susceptibility/resistance to ML in R. microplus populations regardless of the diluent used. Nevertheless. ACT 1% should be considered as the recommended diluent when carrying out resistance development studies for the sustainable use of MLs on R. microplus. Laboratorios Compañia Cibeles S.A. and Pasteur S.A. (Uruguay) for the donation of the active principles used in the studies. Departamento de Parasitologia, DILAVE (Ministerio de Ganadería Agricultura y Pesca, Uruguay), for providing the Mozo strain. To the owners of the production establishments that participated in the trial by submitting tick samples. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. This work was funded by the Universidad de la República, Uruguay. GS and DR designed this study and performed the data analysis. JC performed the laboratory tests. GS and DR wrote and edited the manuscript. JC edited the manuscript. GS and DR finalized the manuscript and all the authors approved the final version. The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Ahmad, M. and Nizami, W.A. 1983. Dimethyl sulfoxide? A safe drug solvent for in vitro screening against cestode parasite. Ann. N. Y. Acad. Sci. 411, 347–351. Bianchi, M.W., Barre, N. and Messad, S. 2003. Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Vet. Parasitol. 112, 75–89. Castro, C.A., Hogan, J.B., Benson, K.A., Shehata, C.W. and Landauer, M.R. 1995. Behavioral effects of vehicles: DMSO, ethanol, tween-20, tween-80, and emulphor-620. Pharmacol. Biochem. Behav. 50(4), 521–526. Cuore, U. 2013. Determinación de eficacia de productos garrapaticidas. Pruebade Establo. Available via https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/sites/ministerio-ganaderia-agricultura-pesca/files/2020-10/DGSG_N%C2%BA_153A_24_03_2017_Procedimiento_ver2_res.pdf Data acces: 06/09/2023. Cuore, U., Altuna, M., Cicero, L., Fernández, F., Luengo, L., Mendoza, R., Nari, A., Pérez Rama, R., Solar, M. and Trelles, A. 2012. Aplicación del tratamiento generacional de la garrapata en la erradicación de una población multiresistente de Rhipicephalus (Boophilus) microplus en Uruguay. Veterinaria (Montevideo) 48, 5–13. Eckstein, C., Lopes, L., Romero Nicolino, R., Oliveira, C.S and Haddad, J. 2015. Economic impacts of parasitic diseases in cattle. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 10(51), 1. FAO. 2004. Module 1. Ticks: acaricide resistance: diagnosis, management and prevention. Rome, Italy: FAO. Fiel, C. and Nari, A. 2013. Enfermedades Parasitarias de Importancia Clínica y Productiva en Rumiantes. Buenos Aires, Argentina: Hemisferio Sur, pp: 752. Galvao, J., Davis, B., Tilley, M., Normando, E., Duchen, M.R. and Cordeiro, M.F. 2013. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 28(3), 1317–1330. Gonçalves, K., Toigo, E., Ascoli, B.M., Von Poser, G. and Ribeiro, V.L.S. 2007. Effects of solvents and surfactant agents on the female and larvae of cattle tick Boophilus microplus. Parasitol. Res. 100(6), 1267–1270. Jacob, S.W. and Herschler, R. 1986. Pharmacology of DMSO. Cryobiology 23, 14–27. Jones, R.P. 1989. Biological principles for the effects of ethanol. Enzyme Microb. Technol. 11, 130–153. Molento, M. 2020. Avaliação seletiva de bovinos para o controle do carrapato. Brasilia, Brasil: Ministério da Agricultura, Pecuária e Abastecimento. Available via https://www.gov.br/agricultura/pt-br/assuntos/producao-animal/arquivos-publicacoes-bem-estar-animal/CARRAPATOS2.pdf (Accessed 21 July 2023) Pegram, R.G., Wilson, D.D. and Hansen, J.W. 2000 Past and present national tick control programs. Why they succeed or fail? Ann. N.Y. Acad. Sci. 916, 546–554. Prichard, R., Ménez, C. and Lespine, A. 2012. Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2, 134–153. R Core Team. 2024. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available via https://www.R-project.org/ Ravindran, R., Juliet, S., Gopalan, A.K.K., Kavalimakkil, A.K., Ramankutty, S.A., Nair, S., Narayanan, P.M. and Ghosh, S. 2011. Toxicity of DMSO, Triton X 100 and Tween 20 against Rhipicephalus (Boophilus) annulatus. J. Parasit. Dis. 35(2), 237–239. Reck, J., Klafke, G.M., Webster, A., Dall’Agnol, B., Scheffer, R., Souza, U.A., Corassini, V.B., Vargas, R. and Santos, J.S. 2014. First report of fluazuron resistance in Rhipicephalus microplus: a eld tick populations resistant to six classes of acaricides. Vet. Parasitol. 201, 128–136. Resende, J.D., Daemon, E., Monteiro, C.M., Maturano, R., Azevedo Prata, M.C. and Rodrigues, A.F.S.F. 2012. Toxicity of solvents and surfactants to Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) and Dermacentor nitens (Neumann, 1897) (Acari: Ixodidae) larvae. Exp. Parasitol. 131(2), 139–142. Ritz, C., Baty, F., Streibig, J.C. and Gerhard, D. 2015. Dose-response analysis using R. PLoS One 10(12), e0146021. Robaina, D., Caballero, J. and Suarez, G. 2023. Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: macrocyclic lactones dose-response relationship. Open Vet. J. 13(10), 1259–1267. Vilela, V.L.R., Feitosa, T.F., Bezerra, R.A., Klafke, G.M. and Riet-Correa, F. 2020. Multiple acaricide-resistant Rhipicephalus microplus in the semi-arid region of Paraíba State, Brazil. Ticks Tick-borne Dis. 11(4), 101413. Villar, D., Klafke, G.M., Rodríguez-Durán, A., Bossio, F., Miller, R., Perez de Leon, A.A. and Chaparro-Gutiérrez, J.J. 2020 Resistance prole and molecular characterization of pyrethroid resistance in a Rhipicephalus Microplus strain from Colombia. Med. Vet. Entomol. 34, 105–115. Wharton, R.H., Roulston, W.J., Utech, K.B.W. and Kerr, J.D. 1970. Assessment of the efficiency of acaricides and their mode of application against the cattle tick (Boophilus microplus). Aust. J. Agric. Res. 21(6), 985–1006. White, W.H., Plummer, P.R., Kemper, C.J., Miller, R.J., Davey, R.B., Kemp, D.H., Hughes, S., Smith, C.K. and Gutierrez, J.A. 2004. An in vitro larval immersion microassay for identifying and characterizing candidate acaricides. J. Med. Entomol. 41(6), 1034–1042. | ||
| How to Cite this Article |
| Pubmed Style Robaina D, Caballero J, Suárez G. Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. Open Vet. J.. 2024; 14(12): 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 Web Style Robaina D, Caballero J, Suárez G. Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. https://www.openveterinaryjournal.com/?mno=222265 [Access: January 11, 2026]. doi:10.5455/OVJ.2024.v14.i12.24 AMA (American Medical Association) Style Robaina D, Caballero J, Suárez G. Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. Open Vet. J.. 2024; 14(12): 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 Vancouver/ICMJE Style Robaina D, Caballero J, Suárez G. Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. Open Vet. J.. (2024), [cited January 11, 2026]; 14(12): 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 Harvard Style Robaina, D., Caballero, . J. & Suárez, . G. (2024) Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. Open Vet. J., 14 (12), 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 Turabian Style Robaina, Diego, Jessica Caballero, and Gonzalo Suárez. 2024. Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. Open Veterinary Journal, 14 (12), 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 Chicago Style Robaina, Diego, Jessica Caballero, and Gonzalo Suárez. "Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones." Open Veterinary Journal 14 (2024), 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 MLA (The Modern Language Association) Style Robaina, Diego, Jessica Caballero, and Gonzalo Suárez. "Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones." Open Veterinary Journal 14.12 (2024), 3397-3403. Print. doi:10.5455/OVJ.2024.v14.i12.24 APA (American Psychological Association) Style Robaina, D., Caballero, . J. & Suárez, . G. (2024) Study of the effect of different diluent for Syringe Immersion Test on Rhipicephalus microplus larvae against macrocyclic lactones. Open Veterinary Journal, 14 (12), 3397-3403. doi:10.5455/OVJ.2024.v14.i12.24 |