| Research Article | ||
Open Vet. J.. 2024; 14(12): 3404-3416 Open Veterinary Journal, (2024), Vol. 14(12): 3404-3416 Research Article The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat modelsYos Adi Prakoso1, Achmadi Susilo2 and Sitarina Widyarini3*1Department of Pharmacology, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, Surabaya, Indonesia 2Department of Agrotechnology, Faculty of Agriculture, University of Wijaya Kusuma Surabaya, Surabaya, Indonesia 3Department of Pathology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Sitarina Widyarini. Department of Pathology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: w.sitarina [at] gmail.com Submitted: 30/09/2024 Accepted: 26/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
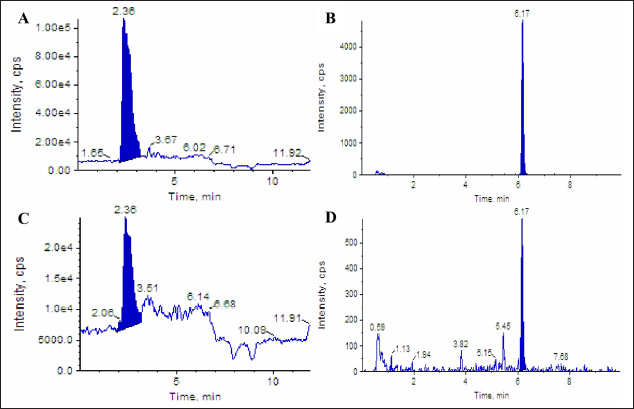
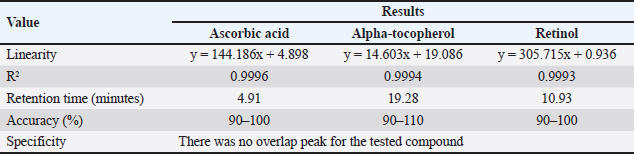
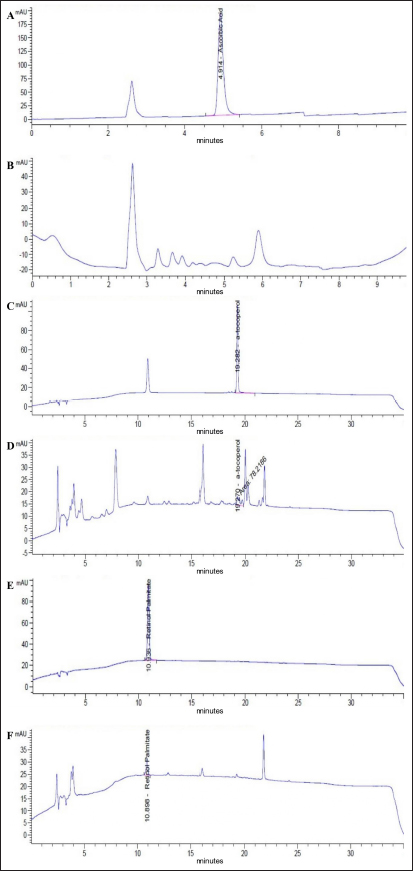
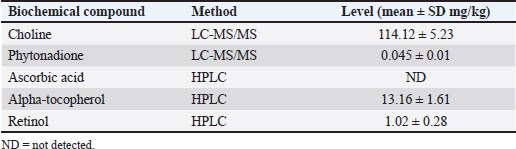
AbstractBackground: Pasteurella multocida is an opportunistic bacterium that causes pneumonic pasteurellosis (PP). The common treatment against PP is using antibiotics in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs). This combination presents various complications, i.e., immune-depression. Hence, the alternative therapy to replace the effects of NSAIDs needs to be clarified. One of them is using fermented calabash [Crescentia cujete (L.)] (FCC). Aim: This study aimed to elucidate the efficacy of FCC in combination with enrofloxacin against artificially induced PP in rat models. Methods: The calabash was collected and fermented. Moreover, the product of FCC was standardized regarding its biochemical compounds using Liquid chromatography-tandem mass spectrometry and high-performance liquid chromatography. This study used 30 male Sprague Dawley rats, weighing 251.52 ± 2.65 grams, 6 months old. The rats were divided into six groups as follows: G1 (control); G2 (infected with Pasteurella multocida + untreated); G3 (infected + 20 mg/kg enrofloxacin); G4 (infected + 20 mg/kg enrofloxacin + 30 mg/kg ibuprofen); G5 (infected + 20 mg/kg enrofloxacin + 2.96 mg/kg FCC); and G6 (infected + 20 mg/kg enrofloxacin + 5.92 mg/kg FCC). The treatment was given once daily for 7 days. On day eight, the rats were radiographed. The serum was collected and tested against C-reactive protein (CRP) and procalcitonin. The rats were euthanized and lung tissue was collected for histopathology and immunohistochemistry against CD4+, CD8+, and COX-2. The data were analyzed using SPSS. Results: This study indicated that FCC contains choline, phytonadione, alpha-tocopherol, and retinol. Moreover, using FCC as a combination therapy with enrofloxacin against PP in group G6 promotes a repair of radiology image compared to other treatments (p < 0.05). Group G5 and G6 showed increased activity of bronchial-associated lymphoid tissue, immune expression of CD4+ and COX-2, and the level of CRP and procalcitonin within the lung tissue (p < 0.05). Group G6 indicated better effects in various parameters in this study. However, the FCC has not influenced the immune expression of CD8+ during PP (p > 0.05). Conclusion: This study proved that FCC could be used in rat models as an alternative anti-inflammatory treatment in combination with enrofloxacin against PP. Further research is needed to explore other effects of FCC to support the current findings. Keywords: Anti-inflammatory, Fermented Crescentia cujete (L.), Lung, Pasteurella multocida, Pneumonia. IntroductionPasteurella multocida is a Gram-negative bacterium commonly found in animals’ upper respiratory systems and can potentially cause opportunistic infections in humans (Piorunek et al., 2023). Pasteurella multocida is most frequently associated with infections resulting from bites inflicted by pet animals. A bite wound creates an environment conducive to P. multocida colonization, biofilm formation (Patel et al., 2021), and dissemination to various organs, including the lungs (Yang et al., 2022). This bacterium can lead to severe infections in the pulmonary system, culminating in acute and chronic pneumonia (Martin et al., 2018). Notably, prevalent research indicates that P. multocida primarily incites pneumonia in immunocompromised individuals rather than those with an immunocompetent status (Schlichthaar et al., 1995; Abreu et al., 2018; Boadu et al., 2021; Mahony et al., 2023). This realization has disconcerted the medical community, leading to pneumonic pasteurellosis (PP) emerging as a critically neglected malady, as evidenced by escalated mortality rates due to pasteurellosis in the United States in 2005 (Kofteridis et al., 2009). One commonly utilized treatment for PP conditions involves the administration of antibiotics such as ampicillin-sulbactam, carbapenem, ceftriaxone, and enrofloxacin in conjunction with non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and piroxicam. The rationale behind using NSAIDs in treating PP lies in their ability to alleviate pain and reduce fever by inhibiting the enzymatic activity of cyclooxygenase (COX), particularly COX-2 (Ahmadi et al., 2022). However, the combined use of antibiotics and NSAIDs presents various complications, including compromised bacterial clearance (Voiriot et al., 2019), prolonged risk of hospitalization and antibiotic dependency (Öztürk et al., 2021), exacerbation of pulmonary tissue damage (Lands and Stanojevic, 2019), diminished immune response in patients (Kuchar et al., 2022), and heightened mortality rates (Micallef et al., 2020). The increase of complications due to the inhibition of CD4+ (Odeny et al., 2022) and the increase of CD8+ immune expression (Sengupta et al., 2023), which is crucial in maintaining lung immunity (Laidlaw et al., 2016). Hence, the alternative herbal therapy with anti-inflammatory potential to replace the impacts of NSAIDs needs to be clarified. The calabash [Crescentia cujete (L.)] is one of alternative herbal therapy with anti-inflammatory properties. This fruit contains several biochemical components, such as tannin, saponin, alpha-tocopherol, glycoside, ascorbic acid, and apigenin (Das et al., 2014). According to Buthelezi et al. (2024), ascorbic acid is crucial in reducing inflammation. Additionally, alpha-tocopherol, categorized as an antioxidant component, aids in reducing inflammation (Saeed et al., 2022). Wilujeng et al. (2023) have indicated that fermented calabash (FCC) contains choline. Moreover, the choline from FCC has been proven to decrease microgliosis associated with neuroinflammation (Hidayah et al., 2023). Nevertheless, the standardization of the biochemical compound of FCC and its potential benefit against PP has not been explored. Hence, this study aimed to clarify the efficacy of FCC in combination with enrofloxacin against artificially induced PP in rat models. Materials and MethodsHerbal preparationThe calabash was collected from the local garden at the University of Wijaya Kusuma Surabaya. The fruit was fermented using a previously demonstrated procedure by Wilujeng et al. (2023). The fermentation was conducted at 25°C for 30 days. The cocktail was stirred once daily without opening the lid. After 30 days, the suspension was filtered, and the FCC was collected. The product was then stored in the fridge at 4°C. Liquid chromatography tandem mass spectrometry (LC-MS/MS)In this study, the analyzed biochemical compounds were choline, phytonadione, alpha-tocopherol, ascorbic acid, and retinol. The choline and phytonadione were comprehensively analyzed using LC-MS/MS (SCIEX Triple Quad 5500+, SCIEX, Framingham, MA, USA). The procedure meticulously followed the previous study by Wilujeng et al. (2023). The choline was detected on the retention time at 2.36 minutes, and the calibration curve was y=2.73e + 5x + 2.8e + 4 with r2=0.998, providing a thorough understanding of the compound’s behavior. The concentration ranged between 0 and 24 ng/ml, and the analyte peak area for choline from FCC was 3.13e + 005, with the analyte peak height at 1.04e + 004. Moreover, phytonadione was detected at a retention time of 6.17 minutes, with the calibration curve y=2.99e + 3x + 5.8e + 2, r2=0.999. The concentration range was between 0 and 10 ng/ml. The analyte peak area and height were 2.18e + 004 and 6.68e + 003, respectively. The standard chromatogram of choline and phytonadione was embedded in Figure 1A and 1B. In addition, Figure 1C and 1D show the chromatograms of choline and phytonadione from FCC, respectively. High-performance liquid chromatography (HPLC)Ascorbic acid, alpha-tocopherol, and retinol were tested using HPLC (Shimadzu version 6.1, Japan). The HPLC was performed following the manufacturer’s standard procedure. The validation method for ascorbic acid, alpha-tocopherol, and retinol is embedded in Table 1. Moreover, the peak area for ascorbic acid, alpha-tocopherol, and retinol is shown in Figure 2. Artificially induced PP in rat modelsThe clinical isolate of P. multocida was gathered from the Laboratory of Bacteriology FIKES UMSIDA. The isolate was made into suspension in 108 CFU/ml. Furthermore, the suspension was transformed into an agar-based inoculum using nutrient agar by adding 1 ml bacterial suspension to the 9 ml nutrient agar. This procedure was conducted using a water bath at 42°C. Moreover, the rats were anesthetized using ketamine (50 mg/kg BW) and xylazine (4 mg/kg BW). The rats were then inserted into a cannula with polyethylene tube into the trachea. The tube was pushed until the bifurcation and the 100 µl agar-based inoculum was inserted (Hoover et al., 2017). The rats were then maintained for 48 hours until they were treated. Research design and treatmentThis study used 30 male Sprague Dawley rats weighing 251.52 ± 2.65 g, 6 months old. The rats were divided into six groups, as shown in Table 2. The treatment was given at 48 hours PI. The treatment was performed using a gastric probe, repeated once daily for 7 days.
Fig. 1. Chromatogram of choline and phytonadione from FCC using LC-MS/MS. Standard chromatogram of choline (A); and phytonadione (B); the chromatogram of choline (C) and phytonadione from FCC (D). Table 1. Validation methods in determining ascorbic acid, phytonadione, alpha-tocopherol, and retinol using HPLC.
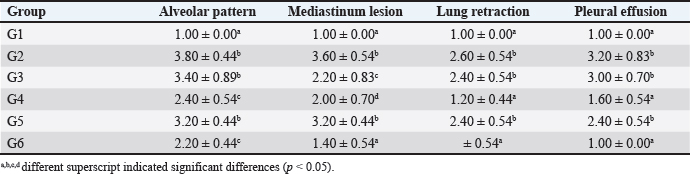
Radiology testOn day eight post-treatment, the rats were then anesthetized using the combination of ketamine and xylazine with a similar dose to the artificially induced PP. The rats’ lung imaging was performed using a radiography system (Dong-mun, DM Series, Korea). The lung image was then analyzed and scored by a veterinary radiologist. The score was determined using several parameters, including alveolar pattern, mediastinum lesion, lung retraction, and pleural effusion (Fouriez-Lablée et al., 2017). The lesions’ score was given as follows: 1=the lesion was not observed, 2=focal, 3=lobular, and 4=diffuse. Sample collectionAfter the thoracic imaging, the rats’ blood was collected using microhematocrit through the ophthalmic vein. The blood was then inserted into the plain tube, and the serum was collected. Furthermore, the rats were euthanized by cervical dislocation. The rats were necropsied, and the lung tissue was collected and stored at 10% neutral buffer formalin. Histopathology and immunohistochemistryThe lung tissue was processed for routine histopathology using H&E staining and immunohistochemistry (IHC) against CD4+, CD8+, and COX-2. The IHC procedure was performed following the demonstrated procedure by Prakoso et al. (2020). Histopathology was reported as semi-quantitative and quantitative data. The semi-quantitative data were collected from the scoring of histopathological changes. The histopathology score was reported as follows: 1=no histopathological changes; 2=mild; 3=moderate; and 4=severe. Moreover, the quantitative data were collected from the measurement of the bronchial-associated lymphoid tissue (BALT). However, the IHC was reported as the score as follows: 1=no immune expression; 2=minimal; 3=mild, 4=moderate, and 5=severe/extensive.
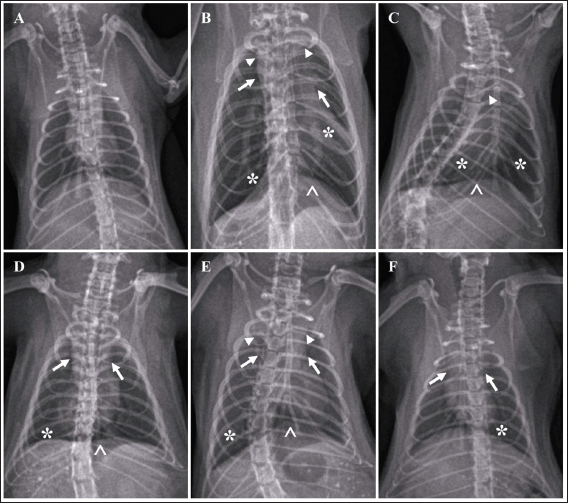
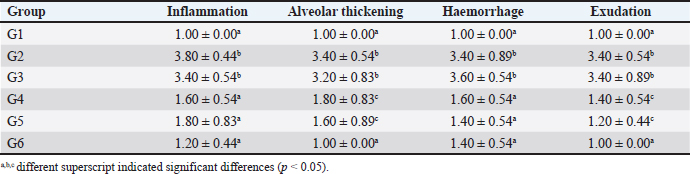
Fig. 2. Chromatogram of ascorbic acid, alpha-tocopherol, and retinol from FCC using LC-MS/MS. Standard chromatogram of ascorbic acid (A); and the peak of ascorbic acid from FCC was not detected (B); the chromatogram of alpha-tocopherol (C); and the peak of alpha-tocopherol from FCC was observed in the retention time at 19.27 minutes (D); the chromatogram of retinol (E); and the peak of retinol from FCC was observed in the retention time at 10.89 minutes (F). Enzyme-linked immunosorbent assays (ELISA)Using ELISA, the serum was tested against C-reactive protein (CRP) and procalcitonin. The chemical used for ELISA was purchased from Elabscience, USA. The product’s catalogue number was E-MSEL-M0059 for CRP and E-EL-R2400 for procalcitonin. The ELISA was performed following the manufacturer’s standard procedure. Analysis dataThe data were analyzed using SPSS version 26. The thoracic imaging, histopathology, and IHC data were tested using Kruskal Wallis and Man Whitney-U tests. However, the BALT area and ELISA data were tested using ANOVA and Duncan tests. This study used a p-value of 0.05. Ethics approvalThis study was approved by the ethical clearance committee for the animal model from the Faculty of Veterinary Medicine Universitas Wijaya Kusuma Surabaya. The registration number was 181-KKE/2024. ResultsPhytochemical compounds of FCCAnalyzing biochemical compounds from FCC using LC-MS/MS and HPLC has yielded significant results. The fermentation product contains important compounds such as choline, phytonadione, alpha-tocopherol, and retinol, essential for health. Notably, ascorbic acid is absent in FCC. Detailed levels of these biochemical compounds are provided in Table 3. Score of lung imagingA significant difference was found between group G2 (untreated) and group G1 in terms of lung radiograph scores (p < 0.05). Groups G3 and G5 exhibited lung radiographs similar to those of group G2 (p > 0.05). Moreover, groups G4 and G6 showed lung repair after treatment compared to group G2 (p < 0.05). In several parameters, groups G4 and G6 did not exhibit differences compared to G1, especially in lung retraction scores and pleural effusion (p > 0.05). Group G6 also displayed better outcomes in lung radiographs than G4, particularly regarding the alveolar pattern and widened mediastinum (p < 0.05). Furthermore, group G6 did not differ from G1 in terms of mediastinal lesions (p > 0.05) (Table 4). A qualitative assessment of the lung radiograph is presented in Figure 3. Histopathology and BALT areaThe semi-quantitative analysis of histopathology revealed that group G1 has a similar histopathological score in all parameters to the G6 (p > 0.05). However, groups G2 and G3 have the most severe histopathological scores compared to the other groups (p < 0.05). Group G4 and G5 were not different ones to each other in all histopathology parameters (p > 0.05) (Table 5). There was no difference in the BALT area between groups G1 and G2 (p > 0.05). Notably, treatment with enrofloxacin alone in group G3 significantly promoted the hyperplasia of BALT and bronchus and bronchioles epithelial within the lung tissue compared to the other groups (p < 0.05). Conversely, the combination treatment of enrofloxacin and ibuprofen in group G4 induced significant BALT atrophy compared to the other groups (p < 0.05). Furthermore, the BALT area increased in groups G5 and G6 compared to G1 and G2 (p < 0.05) (Table 6). The histopathology and BALT area are qualitatively embedded in Figure 4A–H. Table 2. Grouping of the animal models.
Table 3. Level of phytochemical compounds from FCC.
Table 4. Comparison of lung imaging using radiography.
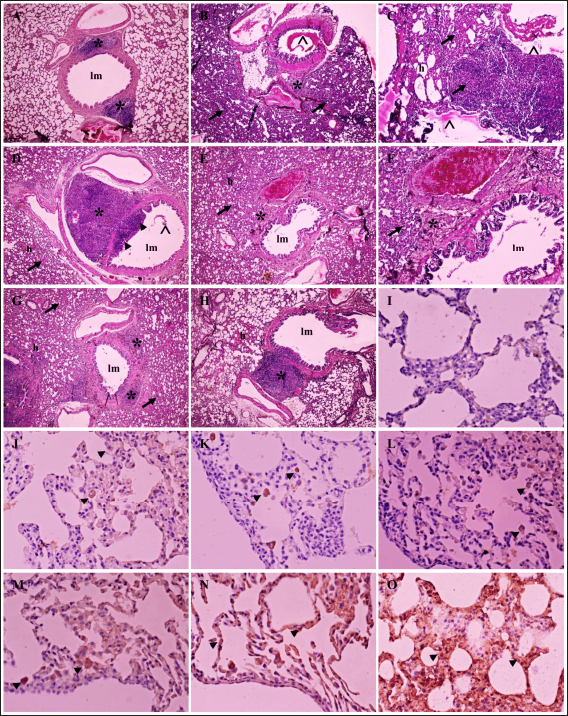
Fig. 3. Radiograph of rats’ lung after treatment. Typical lung image of the healthy rats from group G1 (A); the infected rat without treatment from group G2 showed a diffuse lesion on the lung marked by the widened of the mediastinum (arrow) with the retraction of the caudal portion of the lung (arrowhead), alveolar pattern (*) with the pleural effusion (^) (B); the group G3 showed the similar radiographic pattern with group G2 (C); group G4 indicated the focal alveolar pattern (*) with minimal pleural effusion (^) and mediastinum is widened (arrow) (D); moderate lesion of the lung from group G5 marked by widened of mediastinum (arrow), lung retraction (arrowhead), lobular alveolar lesion (*) with pleural effusion (^) (E); and minimal alveolar lesion (*) and the retraction of lung and widened of mediastinum still observed (arrow) from group G6 (F). Table 5. Histopathology of lung tissue.
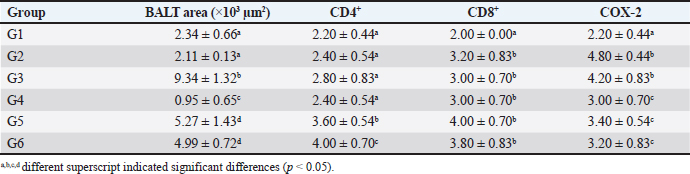
Table 6. BALT area and immune expression of CD4+, CD8+ and COX-2 of lung tissue.
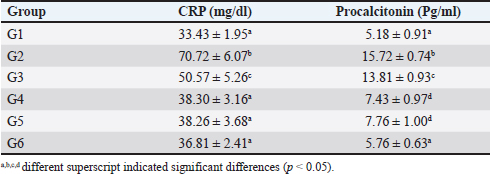
Immune expression of CD4+, CD8+ and COX-2The immune expression of CD4+ in groups G1, G2, G3, and G4 did not differ significantly from one another (p < 0.05). However, groups G5 and G6 displayed higher immune expression of CD4+ compared to the other groups (p < 0.05). The immune expression of CD8+ and COX-2 significantly increased in groups G2, G3, G4, G5, and G6 compared to G1 after treatment (p < 0.05). However, the increase in CD8+ levels in these groups did not differ significantly from one another (p > 0.05). In terms of immune expression of COX-2, group G2 (untreated) and G3 (treated with single enrofloxacin) exhibited higher COX-2 levels compared to the other groups (p < 0.05). However, groups G4, G5, and G6 displayed similar immune expression of COX-2 (p > 0.05) (Table 6). The immunohistochemistry of CD4+, CD8+, and COX-2 can be found in Figure 4I–O. Level of CRP and procalcitoninIn comparison to group G1, the levels of CRP and procalcitonin significantly increased in rats in group G2 following the induction of PP (p < 0.05). The CRP and procalcitonin levels in group G3 significantly decreased (p < 0.05), albeit not as significantly as in group G1. Groups G4, G5, and G6 displayed CRP levels similar to group G1 (p > 0.05). Additionally, the levels of procalcitonin in groups G4 and G5 were similar to each other (p < 0.05), while the level of procalcitonin in group G6 was similar to that of G1 (p > 0.05) (Table 7). DiscussionThe FCC, a herbal remedy, has been previously utilized for the management of ischemic stroke in rat models. A study by Wilujeng et al. (2023) highlighted that FCC contains choline, an essential compound for neurons. Additionally, this study found that FCC comprises choline and several other compounds, such as phytonadione, alpha-tocopherol, and retinol. These compounds serve as antioxidants (Lewis et al., 2019), support the immune system, regulate proliferation, cell growth, and cell differentiation (O’Byrne and Blaner, 2013), address coagulation disorders (Popa et al., 2021), and enhance neuronal function (Derbyshire and Obeid, 2020). Furthermore, our study has provided evidence of FCC’s efficacy in managing PP when used in conjunction with enrofloxacin, a standard therapy for PP. FCC has shown promise in alleviating the cellular mechanisms of inflammation and the clinical presentation of PP. These benefits and the phytochemical compounds present in FCC establish a robust foundation for its usage in PP treatment. Choline, a primary compound found in FCC, is significant in promoting cell integrity and preserving membrane function. Choline-rich FCC has been shown to enhance immunity by influencing BALT, the local immune system in lung tissue (Baumgarth et al., 2024). A previous study by Liu et al. (2017) indicates that choline can improve cardiovascular health by downregulating the expression of TNF-α and IL-6 in rat models. Additionally, choline can be metabolized into acetylcholine, which has the capacity to reduce the activity of COX-2, a crucial enzyme implicated in inflammation associated with various chronic diseases (Moussa and Dayoub, 2023).
Fig. 4. Histopathology and immune expression of CD4+, CD8+ and COX-2 of rats’ lungs after treatment. A typical morphology of lung tissue from rat with clear bronchioles lumen (lm), interalveolar septum and BALT (*) in group G1 (A); severe haemorrhage (h) and exudation within the bronchioles’ lumen (^), thickened interalveolar lumen septum with severe inflammatory cells infiltration (arrow), and BALT (*) in group G2 (B,C); proliferation of BALT (*) and bronchioles epithelium (arrowhead), thickened interalveolar septum (arrow) in group G3 (D); group G4 indicated atrophy of BALT (*), haemorrhage (h) and the alveolar septum still thickening (arrow) (E,F); increased area of BALT (*) with mild haemorrhage (h) within the interalveolar septum (arrow) in group G5 (G); a mild haemorrhage (h), increased BALT area (*) and typical interalveolar septum in group G6 (H); no immune expression in lung tissue which is stained without antibody (I); the immune expression of CD4+ (arrowhead) from group G1 (J); and G6 (K); immune expression of CD8+ (arrowhead) from group G1 (L); and G6 (M); and COX-2 (arrowhead) in group G1 (N); and G6 (O). H&E, 40× (A,B,D,E,G,H), 100× (C,F); IHC without antibody, 400× (I); IHC antibody anti-CD4+, 400× (J,K); IHC antibody anti-CD8+, 400× (L,M); IHC antibody anti-COX-2, 400× (N,O). Table 7. Level of CRP and procalcitonin in serum from.
Similarly, alpha-tocopherol, another compound present in FCC, is essential for activating BALT and regulating the repair of bronchopulmonary dysplasia (Stone et al., 2018). Alpha-tocopherol exhibits free radical scavenging activity, effectively suppressing reactive oxygen species (ROS) (Kopańska et al., 2022). Furthermore, the suppression of ROS is believed to inhibit inflammation. In the pathogenesis of PP, lung airway inflammation is primarily infiltrated by neutrophils due to Pasteurella infection. Using FCC-containing alpha-tocopherol has been associated with a reduction in neutrophilic inflammation, correlated with decreased COX-2 activity (Burbank et al., 2017). Phytonadione, an essential phytochemical compound, is crucial in repairing coagulation disorders and is commonly used as an anti-hemorrhage agent. It is integral to the posttranslational modification of seven proteins within cells, activities that are vital for blood coagulation and mitigating disease-related aging (Mladěnka et al., 2022). Notably, a study by Simes et al. (2020) established that phytonadione can potentially prevent aging and osteoarthritis. Simes et al. (2019) further emphasized the significance of vitamin K-dependent proteins in suppressing NF-kB transduction during inflammation. Additionally, Xie et al. (2024) highlighted the role of phytonadione in maintaining a low grade of chronic inflammation in arthritis. Moreover, phytonadione contributes to the increased matrix gamma-carboxyglutamic acid protein within lung epithelium and concurrently promotes the reduction of immune expression of IL-6 (Janssen and Vermeer, 2017). The decreased expression of IL-6 correlates with improved prognosis of lung infection and lung tissue repair, ultimately leading to enhanced ventilatory capacity (Jespersen et al., 2023). Another compound of interest, retinol, a transport form that can be activated into retinoic acid through oxidation, is essential for the growth and differentiation of cells across various tissues (Colt et al., 2021). Retinoic acid binds to nuclear receptors and triggers cellular differentiation (Chen and Ross, 2004). Retinol exhibits antioxidant and anti-inflammatory properties (Suri et al., 2021), and influences the reduction of COX-2 within lung tissue, promoting healing and recovery (Oliveira et al., 2018). The study demonstrates the benefits of choline, alpha-tocopherol, phytonadione, and retinol derived from FCC as anti-inflammatory agents. Their effectiveness is shown by their ability to increase the BALT area within lung tissue and reduce the immune expression of COX-2 during the development of PP in a rat model. The decrease of COX-2 influences the proliferation of healing factors like CD4+. CD4+ is a lymphocyte involved in healing, attracting various cytokines to penetrate the infected and inflamed area (Kolls, 2013). CD4+ then regulates B cell and antibody synthesis. The increase of CD4+ within lung tissue is crucial in regulating lung immunity and protecting against bacterial infections (Qin et al., 2022). Another study by Shenoy et al. (2020) found that the increase of CD4+ within the lung epithelium accelerates the lung immune system’s response to the initial stage of pneumonia. This aligns with the study’s results, which show an increase in CD4+ immune expression following COX-2 depletion after treatment using FCC compared to the other groups. Enrofloxacin combined with ibuprofen was found to cause the atrophy of BALT and did not affect the immune expression of CD4+ compared to other treatments. These findings suggest that combining enrofloxacin and ibuprofen can deplete the local immune system due to enrofloxacin’s non-selective inhibition of COX during the progression of a disease. This result is consistent with a previous study by Chen et al. (2023) that found ibuprofen use in pups led to the disruption of blood vessel density in lung tissue, particularly affecting CD31, Willebrand factor, and reducing alveolar macrophages. Alveolar macrophages are crucial for maintaining lung tissue repair and ventilation. These mechanisms can lead to endothelial cell apoptosis by arresting the cell cycle in the S-phase (Leksomboon et al., 2022) and disrupting endothelial DNA through oxidative stress (Sohail et al., 2023). Prolonged atrophy of BALT and CD4+ may also lead to severe immunodeficiency in patients. The analysis revealed that there is no significant difference in the immune expression of CD8+ between the treated and untreated groups. This suggests that artificial infection of PP leads to increased CD8+ levels. The heightened immune expression of CD8+ suggests that the immune system is working to defend the lungs against infection (Chen and Kolls, 2013). However, an excessive increase in CD8+ levels can also contribute to tissue damage (Peiris et al., 2010). Connors et al. (2016) have reported that an elevated level of CD8+ exacerbates the severity of lung disease during viral infections. Furthermore, Williams et al. (2021) have explained that a significant increase in CD8+ during the acute phase leads to alveolar destruction. Therefore, the increase in immune expression of CD8+ needs to be balanced by CD4+, which promotes healing and acts as a T helper in the lungs (Yuan et al., 2021). These findings correlate with the results of this study, which found a significant increase in CD8+ in the untreated group. In contrast, the untreated group exhibited mild immune expression of CD4+ and higher levels of COX-2, indicating greater disease severity. Ultimately, the cellular mechanism during the pathogenesis of PP in this study can be observed clinically through lung imaging using radiology. Improvement in lung imaging was observed in the groups treated with a combination of enrofloxacin with ibuprofen and enrofloxacin with FCC. However, the group treated with a combination of enrofloxacin and FCC exhibited better improvement in lung imaging, aligning with the results of histopathology and immunohistochemistry. The repair of lung tissue following an increase in the BALT area led to decreased immune expression of COX-2 and increased immune expression of CD4+, which had a systemic impact on serum CRP and procalcitonin levels. The CRP is an acute-phase protein synthesized in the liver as an inflammation biomarker. CRP is primarily induced by the over-expression of IL-6 (Ngwa et al., 2022), TNF-α (Ng et al., 2018), and COX-2 (Elisia et al., 2022). A decrease in CRP after treatment indicates a good prognosis (Stanimirovic et al., 2022). Additionally, FCC systemically acts as an anti-inflammatory agent via its phytonadione compound to decrease the level of CRP. This is supported by Shioi et al. (2020), who explained that phytonadione blocks the NF-kB transduction level, directly affecting the CRP level. Furthermore, procalcitonin is a protein used as a biomarker of systemic bacterial infection as well as bacterial pneumonia (Kamat et al., 2020). A previous study by Samsudin and Vasikaran (2017) described that the success of therapy using antibiotics against bacterial pneumonia could be marked by decreased procalcitonin levels. Referring to the result of this study, a decreased level of procalcitonin after therapy using a combination of enrofloxacin and FCC indicates that the therapy is efficient. In this study, we also demonstrated that the use of enrofloxacin as a monotherapy without combination using analgesia did not improve the prognosis of bacterial pneumonia, marked by the stagnation of immune expression of COX-2, CD4+, CRP, and procalcitonin. Our finding is similar to a previous report by Nayar et al. (2019), which demonstrated that the morbidity and mortality of evidence of bacterial pneumonia is still high after treatment using antibiotics as a single therapy. It can be concluded that FCC contains essential compounds, including choline, phytonadione, alpha-tocopherol, and retinol. This study suggests that using FCC in combination with enrofloxacin could beneficially improve the prognosis of bacterial pneumonia, specifically for PP at a 5.92 mg/kg BW dose. FCC in combination with enrofloxacin was found to enhance lung radiology’s clinical presentation, BALT area, immune expression of CD4+, and decreasing COX-2, CRP, and procalcitonin. However, further research is needed to explore other mechanisms in this study to support the current findings fully. AcknowledgmentThe authors acknowledged Kemdikbudristek, Indonesia Republic, for funding this study. All the laboratory technicians from the Integrated Laboratory of FIKES UMSIDA were acknowledged for their assistance. FundingThis study was supported and funded by Kemdikbudristek, Indonesia Republic with Decree number: 0459/E5/PG.02.00/2024, National grand number: 109/E5/PG.02.00.PL/2024, derivative grand number: 007/SP2H/PT/LL7/2024, 07/LPPM/UWKS/VI/2024. Authors’ contributionYAP supervised the study, performed animal experimentation, collected the specimen, and interpretated the analysis data. AS performed the fermentation and analysis data. SW conducted histopathology and immunohistochemistry. All authors wrote the draft of the publication, and revised and agreed to the submitted paper. Conflict of interestThe authors declared that they have no conflict of interest. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbreu, F., Rodríguez-Lucas, C., Rodicio, M.R., Vela, A.I., Fernández-Garayzábal, J.F., Leiva, P.S., Cuesta, F., Cid, D. and Fernández, J. 2018. Human Pasteurella multocida infection with likely zoonotic transmission from a pet dog, Spain. Emerg. Infect. Dis. 24(6), 1145–1146. Ahmadi, M., Bekeschus, S., Weltmann, K.D., von Woedtke, T. and Wende, K. 2022. Non-steroidal anti-inflammatory drugs: recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem. 13(5), 471–496. Baumgarth, N., Prieto, A.C., Luo, Z. and Kulaga, H. 2024. B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine. Res. Sq. rs.3, rs-4421566. Boadu, C., Hernandez, A., Zeidan, B., Jr, Young, J.T. and Frunzi, J. 2021. Pasteurella multocida bacteremia in an immunocompromised patient after multiple cat scratches. Cureus 13(1), e12938. Burbank, A.J., Duran, C.G., Almond, M., Wells, H., Jenkins, S., Jiang, Q., Yang, C., Wang, T., Zhou, H., Hernandez, M.L. and Peden, D.B. 2017. A short course of gamma-tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo. Inhal. Toxicol. 140(4), 1179–1181. Buthelezi, L.G., Mavengahama, S., Sibiya, J., Mchunu, C.N. and Ntuli, N.R. 2024. Phytochemical composition of Lagenaria siceraria fruits from KwaZulu-Natal and Limpopo, South Africa. Food Chem. X. 22, 101338. Chen, K. and Kolls, J.K. 2013. T cell-mediated host immune defenses in the lung. Annu. Rev. Immunol. 31, 605–633. Chen, Q. and Ross, A.C. 2004. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp. Cell Res. 297(1), 68–81. Chen, X., Han, D., Wang, X., Huang, X., Huang, Z., Liu, Y., Zhong, J., Walther, F.J., Yang, C. and Wagenaar, G.T.M. 2023. Vascular and pulmonary effects of ibuprofen on neonatal lung development. Respir. Res. 24(1), 39. Colt, S., Gannon, B.M., Finkelstein, J.L., Zambrano, M.P., Andrade, J.K., Centeno-Tablante, E., August, A., Erickson, D., Cárdenas, W.B. and Mehta, S. 2021. Vitamin A status, inflammation adjustment, and immunologic response in the context of acute febrile illness: a pilot cohort study among pediatric patients. Clin. Nutr. 40(5), 2837–2844. Connors, T.J., Ravindranath, T.M., Bickham, K.L., Gordon, C.L., Zhang, F., Levin, B., Baird, J.S. and Farber, D.L. 2016. Airway CD8(+) T cells are associated with lung injury during infant viral respiratory tract infection. Am. J. Respir. Cell. Mol. Biol. 54(6), 822–830. Das, N., Islam, M.E., Jahan, N., Islam, M.S., Khan, A., Islam, M.R. and Parvin, M.S. 2014. Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds. BMC Complement. Altern. Med. 14, 45. Derbyshire, E. and Obeid, R. 2020. Choline, neurological development and brain function: a systematic review focusing on the first 1000 days. Nutrients 12(6), 1731. Elisia, I., Yeung, M., Kowalski, S., Wong, J., Rafiei, H., Dyer, R.A., Atkar-Khattra, S., Lam, S. and Krystal, G. 2022. Omega 3 supplementation reduces C-reactive protein, prostaglandin E2 and the granulocyte/lymphocyte ratio in heavy smokers: an open-label randomized crossover trial. Front. Nutr. 9, 1051418. Fouriez-Lablée, V., Vergneau-Grosset, C., Kass, P.H. and Zwingenberger, A.L. 2017. Comparison between thoracic radiographic findings and postmortem diagnosis of thoracic diseases in dyspneic companion rats (Rattus norvegicus). Vet. Radiol. Ultrasound 58(2), 133–143. Hidayah, J.H., Prakoso, Y.A. and Widyarini, S. 2023. Histopathological changes after treatment using calabash fruit (Crescentia cujete L.) in rat model with artificially induced ischemic stroke. Adv. Anim. Vet. Sci. 11(12), 2003–2009. Hoover, J.L., Lewandowski, T.F., Mininger, C.L., Singley, C.M., Sucoloski, S. and Rittenhouse, S. 2017. A robust pneumonia model in immunocompetent rodents to evaluate antibacterial efficacy against S. pneumoniae, H. influenzae, K. pneumoniae, P. aeruginosa or A. baumannii. J. Vis. Exp. 119, 55068. Janssen, R. and Vermeer, C. 2017. Vitamin K deficit and elastolysis theory in pulmonary elasto-degenerative diseases. ERJ Open Res. 108, 38–41. Jespersen, T., Kampmann, F.B., Dantoft, T.M., Jørgensen, N.R., Kårhus, L.L., Madsen, F., Linneberg, A. and Thysen, S.M. 2023. The association of vitamin K status with lung function and disease in a general population. ERJ Open Res. 9(5), 00208–2023. Kamat, I.S., Ramachandran, V., Eswaran, H., Guffey, D. and Musher, D.M. 2020. Procalcitonin to distinguish viral from bacterial pneumonia: a systematic review and meta-analysis. Clin. Infect. Dis. 70(3), 538–542. Kofteridis, D.P., Christofaki, M., Mantadakis, E., Maraki, S., Drygiannakis, I., Papadakis, J.A. and Samonis, G. 2009. Bacteremic community-acquired pneumonia due to Pasteurella multocida. Int. J. Infect. Dis. 13(3), e81–e83. Kolls, J.K. 2013. CD4(+) T-cell subsets and host defense in the lung. Immunol. Rev. 252(1), 156–163. Kopańska, M., Batoryna, M., Banaś-Ząbczyk, A., Błajda, J. and Lis, M.W. 2022. The effect of α-tocopherol on the reduction of inflammatory processes and the negative effect of acrylamide. Molecules 27(3), 965. Kuchar, E., Karlikowska-Skwarnik, M. and Wawrzuta, D. 2022. Anti-inflammatory therapy of infections. Encyclop. Infect. Immun. 4, 791–797. Laidlaw, B.J., Craft, J.E. and Kaech, S.M. 2016. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 16(2), 102–111. Lands, L.C. and Stanojevic, S. 2019. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst. Rev. 9(9), CD001505. Leksomboon, R., Kumpangnil, K., Pangjit, K. and Udomsuk, L. 2022. The effects of ibuprofen, naproxen and diclofenac on cell apoptosis, cell proliferation and histology changes in human cholangiocarcinoma cell lines. Asian Pac. J. Cancer Prev. 23(4), 1351–1358. Lewis, E.D., Meydani, S.N. and Wu, D. 2019. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 71(4), 487–494. Liu, L., Lu, Y., Bi, X., Xu, M., Yu, X., Xue, R., He, X. and Zang, W. 2017. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 7, 42553. Mahony, M., Menouhos, D., Hennessy, J. and Baird, R.W. 2023. Spectrum of human Pasteurella species infections in tropical Australia. PLOS One 18(1), e0281164. Martin, T.C.S., Abdelmalek, J., Yee, B., Lavergne, S. and Ritter, M. 2018. Pasteurella multocida line infection: a case report and review of literature. BMC Infect. Dis. 18(1), 420. Micallef, J., Soeiro, T., Jonville-Béra, A.P. and French Society of Pharmacology, Therapeutics (SFPT). 2020. Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie 75(4), 355–362. Mladěnka, P., Macáková, K., Kujovská Krčmová, L., Javorská, L., Mrštná, K., Carazo, A., Protti, M., Remião, F., Nováková, L. and OEMONOM Researchers and Collaborators. 2022. Vitamin K—sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 80(4), 677–698. Moussa, N. and Dayoub, N. 2023. Exploring the role of COX-2 in Alzheimer’s disease: potential therapeutic implications of COX-2 inhibitors. Saudi Pharm. J. 31(9), 101729. Nayar, S., Hasan, A., Waghray, P., Ramananthan, S., Ahdal, J. and Jain, R. 2019. Management of community-acquired bacterial pneumonia in adults: limitations of current antibiotics and future therapies. Lung India 36(6), 525–533. Ng, A., Tam, W.W., Zhang, M.W., Ho, C.S., Husain, S.F., McIntyre, R.S. and Ho, R.C. 2018. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci. Rep. 8(1), 12050. Ngwa, D.N., Pathak, A. and Agrawal, A. 2022. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Front. Immunol. 146, 50–56. O’Byrne, S.M. and Blaner, W.S. 2013. Retinol and retinyl esters: biochemistry and physiology. J. Lipid Res. 54(7), 1731–1743. Odeny, T.A., Lurain, K., Strauss, J., Fling, S.P., Sharon, E., Wright, A., Martinez-Picado, J., Moran, T., Gulley, J.L., Gonzalez-Cao, M., Uldrick, T.S., Yarchoan, R. and Ramaswami, R. 2022. Effect of CD4+ T cell count on treatment-emergent adverse events among patients with and without HIV receiving immunotherapy for advanced cancer. J. Immunother. Cancer 10(9), e005128. Oliveira, L.M., Teixeira, F.M.E. and Sato, M.N. 2018. Impact of retinoic acid on immune cells and inflammatory diseases. Mol. Cancer 2018, 3067126. Öztürk, İ., Eraç, Y., Ballar Kırmızibayrak, P. and Ermertcan, Ş. 2021. Nonsteroidal antiinflammatory drugs alter antibiotic susceptibility and expression of virulence-related genes and protein A of Staphylococcus aureus. Turk. J. Med. Sci. 51(2), 835–847. Patel, H., Patel, N., Patel, H. and Chow, R.D. 2021. A bite difficult to heal: Pasteurella multocida induced decompensated hepatic cirrhosis. J. Commun. Hosp. Intern. Med. Perspect. 11(3), 379–383. Peiris, J.S., Hui, K.P. and Yen, H.L. 2010. Host response to influenza virus: protection versus immunopathology. J. Virol. 22(4), 475–481. Piorunek, M., Brajer-Luftmann, B. and Walkowiak, J. 2023. Pasteurella multocida infection in humans. Pathogens 12(10), 1210. Popa, D.S., Bigman, G. and Rusu, M.E. 2021. The role of Vitamin K in humans: implication in aging and age-associated diseases. Antioxidants 10(4), 566. Prakoso, Y.A., Rini, C.S., Rahayu, A., Sigit, M. and Widhowati, D. 2020. Celery (Apium graveolens) as a potential antibacterial agent and its effect on cytokeratin-17 and other healing promoters in skin wounds infected with methicillin-resistant Staphylococcus aureus. Vet. World 13(5), 865–871. Qin, K., Xu, B., Pang, M., Wang, H. and Yu, B. 2022. The functions of CD4 T-helper lymphocytes in chronic obstructive pulmonary disease. Acta Biochim. Biophys. Sin. 54(2), 173–178. Saeed, M., Khan, M.S., Amir, K., Bi, J.B., Asif, M., Madni, A., Kamboh, A.A., Manzoor, Z., Younas, U. and Chao, S. 2022. Lagenaria siceraria fruit: a review of its phytochemistry, pharmacology, and promising traditional uses. Front. Nutr. 9, 927361. Samsudin, I. and Vasikaran, S.D. 2017. Clinical utility and measurement of procalcitonin. Clin. Biochem. Rev. 38(2), 59–68. Schlichthaar, H., Rohrer, T., Schuster, G. and Lehnert, H. 1995. Interstitielle Pneumonie und Sepsis durch Pasteurella-multocida-Infektion [Interstitial pneumonia and sepsis due to a Pasteurella multocida infection]. Dtsch. Med. Wochenschr. 120(46), 1582–1586. Sengupta, S., Bhattacharya, G., Mohanty, S., Shaw, S.K., Jogdand, G.M., Jha, R., Barik, P.K., Parida, J.R. and Devadas, S. 2023. IL-21, inflammatory cytokines and hyperpolarized CD8+ T cells are central players in lupus immune pathology. Antioxidants 12(1), 181. Shenoy, A.T., Wasserman, G.A., Arafa, E.I., Wooten, A.K., Smith, N.M.S., Martin, I.M.C., Jones, M.R., Quinton, L.J. and Mizgerd, J.P. 2020. Lung CD4+ resident memory T cells remodel epithelial responses to accelerate neutrophil recruitment during pneumonia. Mucosal. Immunol. 13(2), 334–343. Shioi, A., Morioka, T., Shoji, T. and Emoto, M. 2020. The inhibitory roles of Vitamin K in progression of vascular calcification. Nutrients 12(2), 583. Simes, D.C., Viegas, C.S.B., Araújo, N. and Marreiros, C. 2019. Vitamin K as a powerful micronutrient in aging and age-related diseases: pros and cons from clinical studies. Int. J. Mol. Sci. 20(17), 4150. Simes, D.C., Viegas, C.S.B., Araújo, N. and Marreiros, C. 2020. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients 12(1), 138. Sohail, R., Mathew, M., Patel, K.K., Reddy, S.A., Haider, Z., Naria, M., Habib, A., Abdin, Z.U., Razzaq Chaudhry, W. and Akbar, A. 2023. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: a narrative review. Cureus 15(4), e37080. Stanimirovic, J., Radovanovic, J., Banjac, K., Obradovic, M., Essack, M., Zafirovic, S., Gluvic, Z., Gojobori, T. and Isenovic, E.R. 2022. Role of C-reactive protein in diabetic inflammation. Mediat. Inflamm. 2022, 3706508. Stone, C.A., Jr, McEvoy, C.T., Aschner, J.L., Kirk, A., Rosas-Salazar, C., Cook-Mills, J.M., Moore, P.E., Walsh, W.F. and Hartert, T.V. 2018. Update on vitamin E and its potential role in preventing or treating bronchopulmonary dysplasia. Neonatology 113(4), 366–378. Suri, D.J., Wirth, J.P., Adu-Afarwuah, S., Petry, N., Rohner, F., Sheftel, J. and Tanumihardjo, S.A. 2021. inflammation adjustments to serum retinol and retinol-binding protein improve specificity but reduce sensitivity when estimating vitamin A deficiency compared with the modified relative dose-response test in ghanaian children. Curr. Dev. Nutr. 5(8), nzab098. Voiriot, G., Philippot, Q., Elabbadi, A., Elbim, C., Chalumeau, M. and Fartoukh, M. 2019. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J. Clin. Med. 8(6), 786. Williams, M., Todd, I. and Fairclough, L.C. 2021. The role of CD8+ T lymphocytes in chronic obstructive pulmonary disease: a systematic review. Inflamm. Res. 70(1), 11–18. Wilujeng, S., Prakoso, Y.A. and Wirjaatmadja, R. 2023. Effects of extraction, fermentation, and storage processes on the level of choline derived from calabash fruit (Crescentia cujete L.). J. Res. Pharm. 27(2), 620–626. Xie, Y., Li, S., Wu, D., Wang, Y., Chen, J., Duan, L., Li, S. and Li, Y. 2024. Vitamin K: infection, inflammation, and auto-immunity. J. Inflamm. Res. 17, 1147–1160. Yang, W., Li, M., Zhang, C., Zhang, X., Guo, M. and Wu, Y. 2022. Pathogenicity, colonization, and innate immune response to Pasteurella multocida in rabbits. BMC Vet. Res. 18(1), 416. Yuan, R., Yu, J., Jiao, Z., Li, J., Wu, F., Yan, R., Huang, X. and Chen, C. 2021. The roles of tissue-resident memory T cells in lung diseases. Front. Immunol. 12, 710375. | ||
| How to Cite this Article |
| Pubmed Style Prakoso YA, Susilo A, Widyarini S. The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. Open Vet. J.. 2024; 14(12): 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 Web Style Prakoso YA, Susilo A, Widyarini S. The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. https://www.openveterinaryjournal.com/?mno=222489 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.25 AMA (American Medical Association) Style Prakoso YA, Susilo A, Widyarini S. The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. Open Vet. J.. 2024; 14(12): 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 Vancouver/ICMJE Style Prakoso YA, Susilo A, Widyarini S. The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 Harvard Style Prakoso, Y. A., Susilo, . A. & Widyarini, . S. (2024) The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. Open Vet. J., 14 (12), 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 Turabian Style Prakoso, Yos Adi, Achmadi Susilo, and Sitarina Widyarini. 2024. The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. Open Veterinary Journal, 14 (12), 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 Chicago Style Prakoso, Yos Adi, Achmadi Susilo, and Sitarina Widyarini. "The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models." Open Veterinary Journal 14 (2024), 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 MLA (The Modern Language Association) Style Prakoso, Yos Adi, Achmadi Susilo, and Sitarina Widyarini. "The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models." Open Veterinary Journal 14.12 (2024), 3404-3416. Print. doi:10.5455/OVJ.2024.v14.i12.25 APA (American Psychological Association) Style Prakoso, Y. A., Susilo, . A. & Widyarini, . S. (2024) The standardization and efficacy of fermented Crescentia cujete (L.) in combination with enrofloxacin against artificially induced pneumonic pasteurellosis in rat models. Open Veterinary Journal, 14 (12), 3404-3416. doi:10.5455/OVJ.2024.v14.i12.25 |