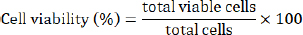| Research Article | ||
Open Vet. J.. 2024; 14(12): 3428-3439 Open Veterinary Journal, (2024), Vol. 14(12): 3428-3439 Research Article Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skinMelpa Susanti Purba1,2, Dito Anggoro1,2,3, Naohiro Yamamoto4, Sota Yoshimine4, Junichi Murakami4, Toshiki Tanaka4, Kimikazu Hamano4, Harumichi Itoh5, Kazuhito Itamoto5, Yuki Nemoto6, Munekazu Nakaichi6, Hiroshi Sunahara2 and Kenji Tani2*1Joint Graduate School of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan 2Laboratory of Veterinary Surgery, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan 3Department of Surgery and Radiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Department of Surgery and Clinical Science, Graduate School of Medicine, Yamaguchi University, Ube, Yamaguchi, Japan 5Laboratory of Small Animal Clinical Science, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan 6Laboratory of Veterinary Radiology, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan *Corresponding Author: Kenji Tani. Laboratory of Veterinary Surgery, Joint Faculty of Veterinary Medicine, Yamaguchi University, Yamaguchi, Japan. Email: ktani [at] yamaguchi-u.ac.jp Submitted: 01/10/2024 Accepted: 26/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
AbstractBackground: Cell sheet therapy has been developed as an effective regenerative medicine to improve wound repair treatment and prevent postoperative complications. Fibroblasts are widely used to create cell sheet engineering because of their essential role in tissue regeneration and the acceleration of the wound healing process. Aim: The study aimed to establish the multilayered fibroblast sheets from canine fibroblast. Methods: The study used fibroblasts from three origin canine tissues, including oral mucosa, skin, and tail skin. A canine fibroblast sheet was produced using the large-numbers cell seeding method with Rho kinase inhibitor. The assessment included viability cells, histological evaluation of the sheet, and secretion of vascular endothelial growth factor (VEGF) and monocyte chemoattractant protein-1 (MCP-1) of the fibroblast sheet. Results: The three fibroblast groups successfully established a multilayered fibroblast sheet. Hematoxylin and eosin staining examination showed the thickest sheet in the oral mucosa fibroblast sheet. Immunohistochemical examination revealed that the multilayered cell sheet comprised fibroblasts expressed by vimentin. All multilayered fibroblast sheet groups secreted the VEGF and MCP-1. No differences were observed in the viability cells of each origin cell. Conclusion: The multilayered fibroblast sheets were successfully established from canine fibroblast. This finding provides the primary data to develop regenerative medicine in the veterinary field. Keywords: Angiogenesis, Canine, Regenerative medicine, Rho kinase inhibitor. IntroductionCell sheet therapy can be a promising method for promoting wound healing via tissue regeneration and accelerated wound healing process. Various cell types, including mesenchymal stem cells, keratinocytes, and fibroblasts, fabricate cell sheets for wound treatment (Kirby et al., 2018; Li et al., 2019; Farabi et al., 2024). These cell sheets can be detached as intact sheets and transplanted onto the wound site, where they promote the reattachment, migration, and proliferation of cells, leading to faster wound closure and reduced scarring (Benchaprathanphorn et al., 2020; Kim et al., 2023). In addition, they can synthesize and release essential cytokines and growth factors involved in wound healing, further enhancing tissue repair and regeneration (Mizoguchi et al., 2018; Nagase et al., 2020; Ike et al., 2022; Matsuno et al., 2022; Yoshimine et al., 2022). Cell sheet transplantation is effective, i.e., cell sheets can be grafted to a target site to repair damaged tissues, including those in the articular cartilage, bone, periodontal ligaments, corneas, blood vessels, skin, and myocardium (Chen et al., 2015; Shirasaka et al., 2016; Tsumanuma et al., 2016; Lee et al., 2019; Pakshir et al., 2020; Matsuno et al., 2022). Furthermore, they are beneficial in preventing postoperative complications (Kim et al., 2018; Yoshimine et al., 2022; Fujino et al., 2023). Thus, the transplantation cell sheets on the canine may enhance wound healing and address postoperative complications such as anastomotic leakage after esophagectomy or bronchopleural fistula. Fibroblasts are involved in critical processes such as the breakdown of fibrin clots, angiogenesis, and the production of new extracellular matrix and collagen structures, which support other cells that are associated with effective wound healing and wound contraction (Bainbridge, 2013; Cialdai et al., 2022; Roman, 2023). Fibroblast sheets can have a significant potential in tissue engineering and wound healing (Benchaprathanphorn et al., 2020; Ike et al., 2022; Matsuno et al., 2022; Yoshimine et al., 2022). Fibroblast sheet therapy is widely developed using fibroblast from humans (Matsushima et al., 2016; Imamura et al., 2018; Kim et al., 2021), rats (Kanzaki et al., 2007; Yoshimine et al., 2022; Yamamoto et al., 2023), and mice (Ike et al., 2022; Matsuno et al., 2022) with various origin cells including skin, tail, and oral mucosa. Therefore, the canine fibroblast sheet was expected to have significant potential in treating various canine medical conditions. Vascular endothelial growth factor (VEGF) is an important angiogenic factor that plays a significant role in angiogenesis (Johnson and Wilgus, 2014; Kendall and Feghali-Bostwick, 2014; Apte et al., 2019). Monocyte chemoattractant protein-1 (MCP-1) chemokines mainly regulate the migration and infiltration of monocytes/macrophages (Deshmane et al., 2009). VEGF and MCP-1 were found in rats and mice fibroblast sheets, promoting wound healing (Nagase et al., 2020; Ike et al., 2022; Yoshimine et al., 2022). Various methods to produce cell sheets already published include those cultured on a dish with thermo-responsive polymeric surfaces (Kanzaki et al., 2007; Flores et al., 2008; Nishimura et al., 2019) and those cultured using cell sheet engineering methods with thin biodegradable polymerized fibrin-coated dishes (Itabashi et al., 2005). A previous study (Yoshimine et al., 2022) developed multilayered fibroblast sheets by seeding large numbers of fibroblast. This cell sheet method is relatively simple and cost-effective compared with other cumbersome methods. Previous studies have reported in vitro and in vivo investigations in mice using multilayered cell sheets (Ike et al., 2022; Matsuno et al., 2022; Yoshimine et al., 2022). The multilayered cell sheets could be transplanted and engrafted more easily with favorable handling than monolayered cell sheets (Sakai et al., 2013; Ike et al., 2022). Rho kinase (ROCK) inhibitors have shown beneficial effects in various cell cultures, including the promotion of cell aggregation by suppressing apoptosis (Watanabe et al., 2007; Gao et al., 2019), promotes cell adhesion (Gao et al., 2019), and cell attachment, migration, and proliferation (Piltti et al., 2015; Sun et al., 2015; Croze et al., 2016; McGifford et al., 2022). Matsumoto et al. (2022) reported that ROCK inhibitors inhibited the cell aggregate’s collapse and formed the spherical cell aggregates. Studies in cell sheets are still unclear on whether ROCK inhibitors affect cell sheet formation. To the best of our knowledge, there are no reports on the production and therapeutic potential of the canine fibroblast sheet. Therefore, this study aimed to investigate and establish the multilayered fibroblast sheets from canine fibroblast using oral mucosa, skin, and tail tissues with and without ROCK inhibitors. In addition, we evaluated the proliferation and viability of the three canine fibroblast groups. The result of this study may provide essential data for developing regenerative medicine using fibroblast sheets in the veterinary field. We hypothesize that the fibroblast sheet can be produced using the oral mucosa, skin, and tail of canine fibroblasts. Materials and MethodsSamples and culture cellsThis study used four healthy dogs (beagles) with a mean body weight of 13.79 ± 0.5 kg, with the median age of the dogs was 7.7 (range: 6.2–8.9) years. The dogs were housed in cages with free food and water access. The oral mucosa, dorsal skin of the abdomen, and tail skin tissues of dogs were collected using a 6-mm biopsy trepan (Biopsy Punch, Kai Industries Co., Ltd., Gifu, Japan) with a tissue thickness of 3 mm under general anesthesia. The collected tissues were washed with phosphate-buffered saline (PBS) and penicillin/streptomycin (Fujifilm Wako Pure Chemical Industries, Ltd., Osaka, Japan) five times and then aseptically cut into 2-mm pieces or less with a Metzenbaum Scissor. The cells were isolated using 5,000 units of collagenase type I (Collagenase, Fuji Film Wako Pure Chemical Co., Ltd., Osaka, Japan) and cultured in DMEM (DMEM®, Life Technology Corporation, Grand Island, NY) with 10% fetal bovine serum (Fetal Bovine Serum, Thermo Li Scientific Group, Tokyo, Japan), 100 U/ml penicillin, and 100 μg/ml streptomycin. The collagenase medium was filtered using a 70-µm cell strainer (FALCON®, Corning International Inc., Tokyo, Japan). After 8 hours, the cells were collected and seeded in the DMEM medium (DMEM, 10% fetal bovine serum, and penicillin/streptomycin). The cells were cultured in an incubator for 3–5 days until fibroblast proliferation and adhesion were confirmed. After the cells were confirmed, the primary fibroblasts were seeded in a new DMEM medium with a cell density of 2.1 × 106 cells in an uncoated T-75 flask (FALCON®, Corning International Inc., Tokyo, Japan). Then, the cells were cultured until they occupied the 70%–80% confluency. Trypan blue exclusion assayThe trypan blue exclusion assay assessed the proliferation cell and viability in the second passage fibroblast. Initially, 1 × 105 cells/well for each cell were seeded in an uncoated six-well plate (FALCON®, Corning International Inc., Tokyo, Japan). For each fibroblast group, a series of five cultures were set up. One plate of the cells was collected every 24 hours for 5 days, and the cells were counted using a Burker Turk cytometer. Cell viability was calculated by dividing the viable cells by the total cell count and multiplying the ratio by 100.
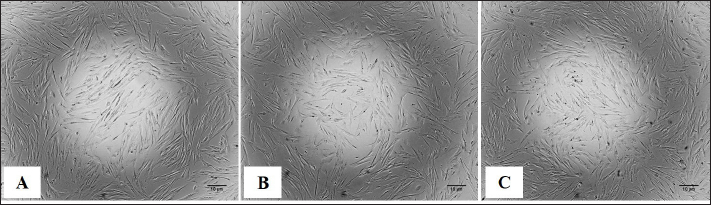
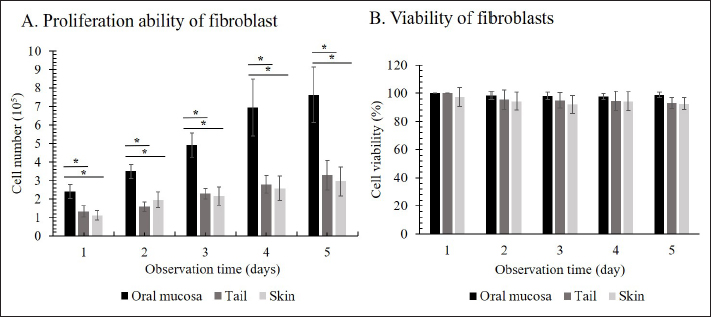
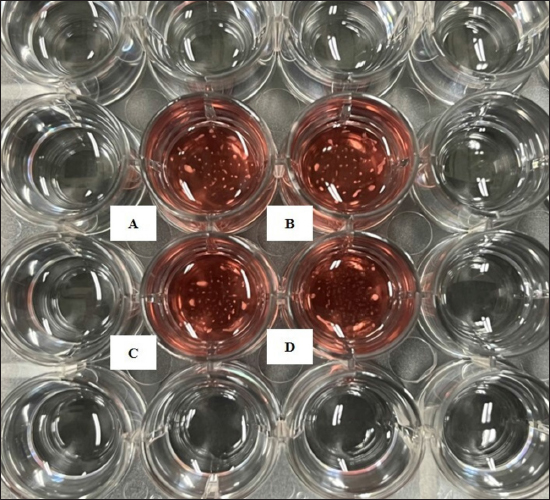
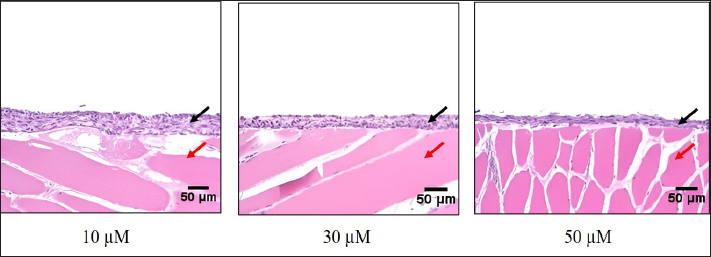
Preparation of fibroblast sheetsFor the cell sheet preparation method, this study referred to the multilayered sheet preparation method using rat-derived fibroblasts (Yoshimine et al., 2022). Fibroblasts at the second passage were used to produce the sheet. The cells were detached from the cell dish using TrypLE Express (TrypLE Express®, Life Technology Corporation, Grand Island, NY). Primary fibroblast cells were seeded in a 24-well plate (5 × 105 cells/well) using a 2 ml medium consisting of DMEM and HFDM-1 (+) (Cell Science and Technology Institute, Miyagi, Japan) supplemented with 5% FBS. The ROCK inhibitor (Culture Sure® Y-27632, Fujifilm Wako Pure Chemical Industries, Ltd., Osaka, Japan) was evaluated by adding 2.5, 5, 10, 30, and 50 µM to the oral mucosa fibroblast culture. The surrounding wells without suspension cells were filled with 2 ml of the PBS to create uniform temperature around the suspension cells and cultured for 3 days at 37°C and 5% CO2. Enzyme-linked immunosorbent assayThe VEGF and MCP-1 concentrations were assessed using the supernatant fibroblast sheet after 3 days incubation. The enzyme-linked immunosorbent assay (ELISA) method following the manufacturer’s instructions, VEGF (Canine VEGF, Funakoshi Co., Ltd., Osaka, Japan) and MCP-1 (Canine MCP-1, Funakoshi Co., Ltd., Osaka, Japan) and were measured using an Epoch spectrophotometer (BioTek, Winooski, VT). All samples were assayed in triplicate. Histological and immunohistochemical analyses of fibroblast sheetThe prepared cell sheet was peeled off by pipetting and fixed with formalin. The fabricated cell sheets were placed on a raw ham to layer the sheet and embedded in paraffin. Sections with a thickness of 3 µm were cut and mounted on the glass slides, deparaffinized in xylene, and rehydrated in a graded ethanol series (100%, 90%, and 70%). The glass slide was stained with hematoxylin and eosin (H&E). For immunostaining, heat-induced antigen retrieval was performed using the target retrieval solution with 10 times dilution (S1699; DakoCytomation A/S, Copenhagen, Denmark) at 95°C for 20 minutes, cooling for 20 minutes, and incubated with blocking buffer (X0909, DakoCytomation A/S, Copenhagen, Denmark) for 30 minutes at room temperature. The primary antibody was added and incubated for 60 minutes at room temperature. After washing, the secondary antibody was added and incubated for 60 minutes at room temperature. The primary and secondary antibodies were diluted with Dako anti-diluent with background. Immunostaining was performed using mouse monoclonal anti-vimentin antibody (1:500, ab8069, Abcam, Cambridge, UK) and secondary antibodies goat anti-mouse IgG (1:200, Alexa FluorTM488, A11029, Life Technologies, Waltham, MA). The nuclei were stained using 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (PureBlu™ DAPI, Bio-Rad, Hercules, CA) for 20 min at room temperature. All histological images were captured using a BZ-X710 microscope (Keyence, Osaka, Japan). The thickness of the fibroblast sheet based on H&E staining was measured using ImageJ (National Institutes of Health, Bethesda, MD) in four parts randomly for four figures in each sheet. Data analysisBody weight was expressed as means ± SDs and age as median with range. The proliferation cell and viability of the fibroblast, ELISA results, and fibroblast sheet thickness were analyzed using a one-way analysis of variance, followed by the Tukey–Kramer Honest Significant Difference (HSD) test for multiple comparisons test. These calculations were performed using the JMP software, version 9.0 (SAS Institute Japan, Tokyo, Japan). A p value of <0.05 was considered statistically significant. Ethical approvalThe samples were collected under the authorization of the Ethics Committee of Animal Experiments of Yamaguchi University, Japan (protocol number: 484). ResultsMorphological characteristics of the fibroblastFigure 1 shows the morphological features of fibroblasts in the first passage from three tissues of origin. The fibroblast had a spindle-shaped form. The three tissue groups did not show a difference in terms of cell morphology. Trypan blue exclusion assayThe oral mucosa fibroblast had the highest proliferation cells among the three tissue samples at all observation times (1–5 days). The proliferation cell of the fibroblast significantly differed between the oral mucosa group and the skin and tail skin groups (p < 0.05) (Fig. 2a). The cell viability of the three groups in all observation days was >90% (Fig. b). The viability of the three cell groups did not significantly differ. Canine fibroblast sheetUsing the following methods in rats, the cell sheet shrunk, the marginal sheet collapsed, and the sheet formation was difficult (Fig. 3). The ROCK inhibitor was evaluated in oral mucosa fibroblast to prevent shrinking fibroblast sheets. The results showed that adding 2.5 and 5 µM of ROCK inhibitor did not form a sheet, while adding 10, 30, and 50 µM of ROCK inhibitor successfully made a sheet. Histological evaluation showed that the thickest layered sheet was established by adding 10 µM of ROCK inhibitor (Fig. 4). Therefore, 10 µM of ROCK inhibitor was added to each fibroblast group. A fibroblast sheet was produced (Fig. 5). Macroscopic observation of the oral mucosa fibroblast sheet showed circular shapes and was easy to detach from the 24-well plates. The tail fibroblast became a cell sheet, and the circular shape was maintained. Nevertheless, it was hard to detach from the culture plate. Meanwhile, the skin fibroblast became a cell sheet. However, its circular shape was not maintained and collapsed during detachment.
Fig. 1. The morphology of the canine fibroblast presented with a spindle-shaped single cell. (A) Oral mucosa. (B) Tail skin. (C) Skin. Scale bars=10 µm.
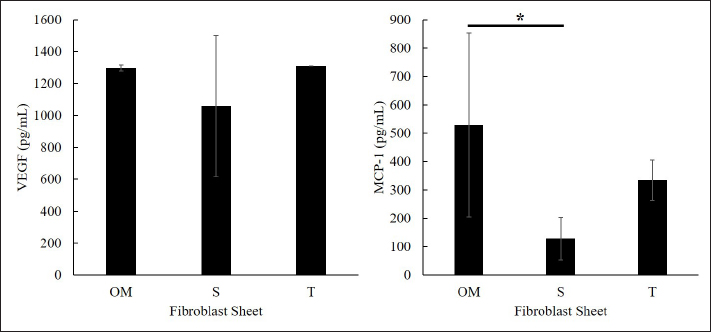
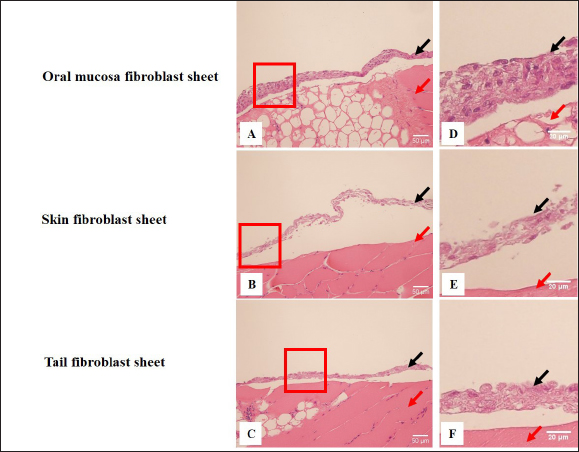
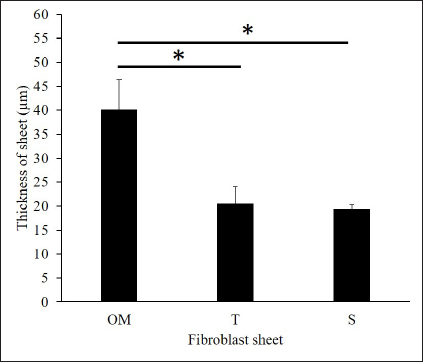
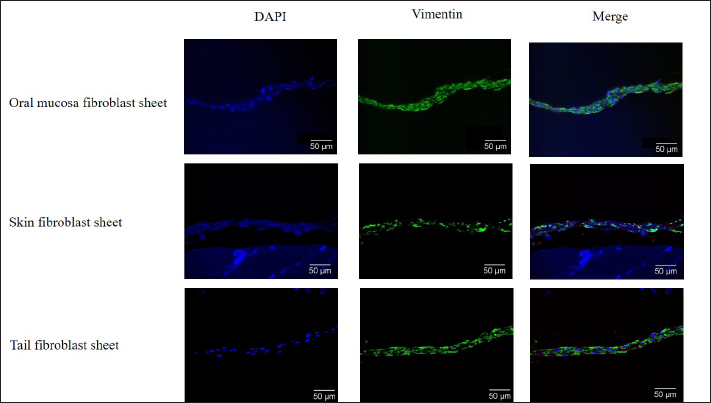
Fig. 2. The proliferation and viability of canine fibroblasts. (A) The oral mucosa fibroblast had the highest proliferation. Fibroblast proliferation significantly differed between the oral mucosa fibroblast group and other groups. (B) The fibroblast viability of all groups was >90% during the 5 days of observation. The fibroblast viability did not show a significant difference between the three groups. The fibroblasts were counted every 24 hours during 5 days of observation. *p < 0.05. Enzyme-linked immunosorbent assayThe VEGF and MCP-1 concentrations were measured and summarized via graphs (Fig. 6). All fibroblast sheet groups secreted VEGF, and there were no significant differences between the three sheet groups. All groups produced MCP-1. The MCP-1 levels of the oral mucosa fibroblast significantly differed from the skin fibroblast. There were no significant differences between the oral mucosal group and the tail group. Histological analysis of canine fibroblast sheetsFigure 7 shows each sheet stained with H&E. All sheets are presented with multilayered cell sheets. The thickness values of the oral mucosa, skin, and tail fibroblast sheet were 40.10 ± 6.31, 19.44 ± 0.81, and 20.54 ± 3.47 µm, respectively (Fig. 8). The oral mucosa fibroblast sheet was thicker than the skin and tail fibroblast sheet. All fibroblast sheet groups tested positive for DAPI and vimentin (Fig. 9). Discussion
Fig. 3. Macroscopic image of the fibroblast sheet peeled from the flask without ROCK inhibitor. The fibroblast sheet shrunk, and the margin collapsed. (A, B) Oral mucosa fibroblast sheet. (C) Skin fibroblast sheet. (D) Tail skin fibroblast sheet. The surrounding wells around the fibroblast sheet were filled with 2 ml of PBS to create a uniform temperature.
Fig. 4. H&E staining of the canine oral mucosa fibroblast sheets according to the amount of ROCK inhibitor (10, 30, and 50 µM). Histological staining was performed on the raw ham. Black arrow: a marker for the fibroblast sheet. Red arrow: a marker for the raw ham. Scale bars=50 µm. In the current study, the canine multilayered fibroblast sheets were successfully established using fibroblast from the oral mucosa, skin, and tail skin by seeding large numbers of cells (Yoshimine et al., 2022) with ROCK inhibitor. VEGF and MCP-1 were secreted from the three fibroblast sheet groups, showing the potential of the sheet to stimulate healing processes. This study showed that the oral mucosa fibroblast tended to have a higher potential to create a favorable canine multilayered sheet than fibroblast from the skin and tail.
Fig. 5. Macroscopic image of the shape of the canine fibroblast sheet peeled from the dishes with ROCK inhibitor. Oral mucosa fibroblast sheet (left). Tail skin fibroblast sheet (middle). Skin fibroblast sheet (right). The skin fibroblast sheet did not retain its circular shape and collapsed during detachment. Scale bars=5 mm.
Fig. 6. VEGF (left) and MCP-1 (right) concentration in the culture supernatants of the canine fibroblast sheet that were cultured for 3 days (each bar, n=4). All fibroblast sheet groups secreted VEGF and MCP-1. There were no significant VEGF values between the three groups. Significant differences in MCP-1 concentration were observed between oral mucosa and skin. OM=oral mucosa, S=skin, and T=tail skin. * p < 0.05. Fibroblasts are the most common cell type represented in connective tissues. The morphology of the cells remains the same even when it is present in multiple organ systems (Rinn et al., 2006). In terms of morphology, the fibroblasts are spindle-shaped with a centrally placed oval or round nucleus (Rinn et al., 2006; Ravikanth et al., 2011; Kalluri, 2016). This study showed the fibroblasts from the three origins presented with a spindle-shaped form under light microscope observation. No morphological differences were observed between the oral mucosa, skin, and tail groups. The current study produced canine fibroblast sheets using the methods in rats (Yoshimine et al., 2022). On the first day of observation after seeding, the fibroblast did not retain its circular shape, and the margin of the sheet collapsed. This condition may occur due to the activity of myosin phosphorylation during sheet formation. Myosin phosphorylation regulated by ROCK plays an essential role in smooth muscle contraction and cell migration (Niggli, 1999; Matsumura et al., 2001; Seaholtz, 2003; Álvarez-Santos et al., 2020; Sun et al., 2020). Therefore, ROCK inhibitor was required to prevent smooth muscle contraction by suppressing the myosin phosphorylation activation (Nakayama et al., 2005; Wirth, 2010; Kaneko-Kawano et al., 2012). Previous studies on human nasal epithelial cell sheets have shown that ROCK inhibitors affect myosin regulation by inducing the downregulation of myosin light chain phosphorylation (Kasai et al., 2020). Moreover, Okumura et al. (2017) have found that ROCK inhibitor enhances cell adhesion and engraftment by suppressing myosin phosphorylation in cultured corneal endothelial cell transplantation in primate models. In addition, several studies have reported that the inhibition of the ROCK signaling pathway significantly promotes cell aggregation, cell adhesion, and proliferation, suppresses T cell proliferation, and inhibits apoptosis/necrosis (Peh et al., 2015; Croze et al., 2016; Okumura et al., 2017; Kim et al., 2022; Matsumoto et al., 2022; McGifford et al., 2022). The current study successfully produced the multilayered canine fibroblast sheet from the oral mucosa, skin, and tail skin fibroblasts by adding 10 µM of ROCK inhibitor.
Fig. 7. H&E staining of the canine fibroblast sheets peeled from the dishes. Oral mucosa fibroblast sheet (A), skin fibroblast sheet (B), and tail skin fibroblast sheet (C). Observation at higher magnification of oral mucosa fibroblast sheet (D), skin fibroblast sheet (E), and tail skin fibroblast sheet (F). Histological staining was performed on the raw ham. Black arrow: a marker for the fibroblast sheet. Red arrow: a marker for the raw ham. Scale bars (A, B, C)=50 µm. Scale bars (D, E, F)=20µm.
Fig. 8. The thickness of the fibroblast sheet according to the addition of 10 µM of ROCK inhibitor. The thickest sheet was found on the oral mucosa fibroblast sheet. There were significant differences in thickness between the oral mucosa group and the skin and tail skin groups. OM=oral mucosa, S=skin, and T=tail skin. * p < 0.05.
Fig. 9. Immunohistochemical fluorescence staining of the canine fibroblast sheets peeled from the dishes. Oral mucosa fibroblast sheet (upper), skin fibroblast sheet (middle), and tail skin fibroblast sheet (lower). All groups tested positive for DAPI and vimentin. Scale bars=50 µm. H&E staining was performed to evaluate the canine-layered cell sheets. Histological analysis showed that the oral mucosa, skin, and tail skin fibroblast sheet groups presented with multilayered cells. The oral mucosa fibroblast sheet was significantly thicker than the skin and tail skin fibroblast sheets. Immunohistochemistry staining showed that the oral mucosa, skin, and tail skin fibroblast sheet groups tested positive for vimentin. Vimentin, which is a marker of fibroblast, is a type III intermediate filament protein that plays a role in wound healing progress, which is essential in promoting fibroblast proliferation, collagen accumulation, and epithelial-mesenchymal transition signals (Cheng et al., 2016; Ridge et al., 2022; Coelho-Rato et al., 2024). The presence of vimentin in all the fibroblast sheet groups indicated that applying a fibroblast sheet in the wound can promote fibroblast proliferation and stimulate wound healing. VEGF is an endothelial cell-specific mitogen, an angiogenic inducer, and a vascular permeability mediator promoting neovascularization (Ferrara, 2004). Moreover, it contributes to endothelial cell migration, proliferation, and tube formation (Ferrara, 2004; Apte et al., 2019;). MCP-1 is a member of the C-C family of chemokines, which is a potent chemotactic factor of monocytes that plays a vital role in wound healing processes by influencing inflammation, immune responses, tissue remodeling, fibroblast chemotaxis, and collagen synthesis (Deshmane et al., 2009; Yadav et al., 2010; Arefieva et al., 2012). A previous study showed that the proinflammatory MCP-1 promotes healing by restoring the macrophage response in mice with diabetic wounds (Wood, 2014). The current study showed that the three fibroblast sheet groups secreted VEGF and MCP-1. The VEGF result revealed that the oral mucosa and tail fibroblast sheet groups tended to be higher than the skin group. On the other hand, the oral mucosa groups showed a high tendency compared to other groups for MCP-1 assessment. The role of VEGF and MCP-1 was in accordance with the previous studies in mice and rats, which showed the fibroblast sheets produced various growth factors and cytokines including VEGF and MCP-1, which are essential in promoting the wound healing process (Mizoguchi et al., 2018; Nagase et al., 2020; Ike et al., 2022; Matsuno et al., 2022; Yoshimine et al., 2022). Furthermore, the current study used large numbers of fibroblasts to make the sheet. Cell proliferation and viability are considered when selecting the origin of fibroblasts. The trypan blue exclusion assay is a standard method to assess cell viability by staining dead cells with compromised membranes, allowing the dye to enter while excluding the dye for live cells (Mani and Swargiary, 2023). In this study, the number of viable cells in the oral mucosa fibroblasts significantly increased compared with skin and tail fibroblasts. This result follows a previous study that showed oral mucosa fibroblasts proliferated more than dermal fibroblasts (Alfonso García et al., 2020; Waasdorp et al., 2021). Previous studies have revealed that oral mucosa fibroblasts in mice exhibit a higher viability and promote faster healing with less scarring compared with dermal fibroblasts (Sezgin et al., 2021; Vijayashree and Sivapathasundharam, 2022). Interestingly, in this study, the cell viability of the oral mucosa, tail, and tail skin groups was >90%. Moreover, there was no significant difference between the three groups. Based on this result, the oral mucosa has a strong potential when it is used as a source of fibroblasts to develop canine regenerative medicine. The current study had several limitations. First, the effect of ROCK inhibitor on myosin phosphorylation regulation, which can produce the canine fibroblast sheet, was not investigated. Second, various growth factors and cytokines, which are essential in promoting the wound-healing process, were not examined. Finally, this study was performed regardless of age and sex in dogs. Hence, further studies should be performed to address these limitations. ConclusionTo the best of our knowledge, this represents the first study conducted to establish a method for creating a canine fibroblast sheet. In conclusion, a method that can produce a canine multilayered fibroblast sheet was successfully established by using large numbers of fibroblast and ROCK inhibitors. According to fibroblast origin, canine oral mucosa fibroblasts are better than canine skin and tail fibroblasts. Our results may contribute to providing primary data for using canine fibroblasts to support the development of regenerative medicine in the veterinary field. AcknowledgmentsThe authors would like to thank the supporter of this study. Conflict of interestThere are no conflicts of interest associated with this publication. FundingThis work was partially supported by JSPS KAKENHI Grant Number JP20K06394. Authors’ contributionsConceptualization: M.S.P., N.Y., S.Y., and K.T. Investigation: M.S.P, D.A, J. M., T.T., H.S., K.I., and K.T. Methodology: M.S.P., N.Y., K.H., H.I., and K.T. Analysis data: Y.N., and M.N. Supervision: N.Y and K.T. Writing, review, and editing: M.S.P and K.T. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAlfonso García, S.L., Parada-Sanchez, M.T. and Arboleda Toro, D. 2020. The phenotype of gingival fibroblasts and their potential use in advanced therapies. Eur. J. Cell Biol. 99(7), 151123. Álvarez-Santos, M.D., Álvarez-González, M., Estrada-Soto, S. and Bazán-Perkins, B. 2020. Regulation of myosin light-chain phosphatase activity to generate airway smooth muscle hypercontractility. Front. Physiol. 11, 701. Apte, R.S., Chen, D.S. and Ferrara, N. 2019. VEGF in signaling and disease: beyond discovery and development. Cell 176(6), 1248–1264. Arefieva, T.I., Sokolov, V.O., Pylaeva, E.A., Kukhtina, N.B., Potekhina, A.V., Ruleva, N.Y., Sidorova, M.V., Bespalova, Z.D., Azmuko, A.A. and Krasnikova, T.L. 2012. Peptide fragments 29-40 of an amino acid sequence of monocyte chemoattractant protein-1 (MCP-1) stimulate monocyte migration in vivo and facilitate wound healing. Dokl. Biol. Sci. 446, 327–330. Bainbridge, P. 2013. Wound healing and the role of fibroblasts. J. Wound. Care. 22, 407–412. Benchaprathanphorn, K., Sakulaue, P., Siriwatwechakul, W., Muangman, P., Chinaroonchai, K. and Viravaidya-Pasuwat, K. 2020. Preparation and characterization of human keratinocyte-fibroblasts cell sheets constructed using PNIAM-co-AM grafted surfaces for burn wound healing. J. Mater. Sci. Mater. Med. 31(12), 126. Chen, G., Qi, Y., Niu, L., Di, T., Zhong, J., Fang, T. and Yan, W. 2015. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 3(6), 749–757. Cheng, F., Shen, Y., Mohanasundaram, P., Lindström, M., Ivaska, J., Ny, T. and Eriksson, J.E. 2016. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. U. S. A. 113(30), 34320–34327. Cialdai, F., Risaliti, C. and Monici, M. 2022. Role of fibroblasts in wound healing and tissue remodeling on earth and in space. Front. Bioeng. Biotechnol. 10, 958381. Coelho-Rato, L.S., Parvanian, S., Modi, M.K. and Eriksson, J.E. 2024. Vimentin at the core of wound healing. Trends. Cell. Biol. 34, 239–254. Croze, R.H., Thi, W.J. and Clegg, D.O. 2016. ROCK inhibition promotes attachment, proliferation and wound closure in human embryonic stem cell-derived retinal pigmented epithelium. Trans. Vis. Sci. Tech. 5(6), 7. Deshmane, S.L., Kremlev, S., Amini, S. and Sawaya, B.E. 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon. Cytokine. Res. 29(6), 313–326. Farabi, B., Roster, K., Hirani, R., Tepper, K., Atak, M.F. and Safai, B. 2024. The efficacy of stem cells in wound healing: a systematic review. Int. J. Mol. Sci. 25(5), 3006. Ferrara N. 2004. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 25(4), 581–611. Flores, M.G., Hasegawa, M., Yamato, M., Takagi, R., Okano, T. and Ishikawa, I. 2008. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J. Periodont. Res. 43(3), 364–371. Fujino, A., Fuchimoto, Y., Mori, T., Kano, M., Yamada, Y., Ohno, M., Baba, Y., Isogawa, N., Arai, K., Yoshioka, T., Abe, M., Kanai, N., Takagi, R., Maeda, M. and Umezawa, A. 2023. Evaluation of safety and efficacy of autologous oral mucosa-derived epithelial cell sheet transplantation for preventing anastomotic restenosis in congenital esophageal atresia and congenital esophageal stenosis. Stem. Cell. Res. Ther. 14(1), 86. Gao, L., Nath, S.C., Jiao, X., Zhou, R., Nishikawa, S., Krawetz, R., Li, X. and Rancourt, D.E. 2019. Post-passage rock inhibition induces cytoskeletal aberrations and apoptosis in human embryonic stem cells. Stem. Cell. Res. 41, 101641. Ike, S., Ueno, K., Yanagihara, M., Mizoguchi, T., Harada, T., Suehiro, K., Kurazumi, H., Suzuki, R., Kondo, T., Murata, T., Shirasawa, B., Morikage, N. and Hamano, K. 2022. Cryopreserved allogenic fibroblast sheets: development of a promising treatment for refractory skin ulcers. Am. J. Transl. Res. 14(6), 3879–3892. Imamura, H., Adachi, T., Kin, T., Ono, S., Sakai, Y., Adachi, T., Soyama, A., Hidaka, M., Takatsuki, M., Shapiro, A.M.J. and Eguchi, S. 2018. An engineered cell sheet composed of human islets and human fibroblast, bone marrow-derived mesenchymal stem cells, or adipose-derived mesenchymal stem cells: an in vitro comparison study. Islets 10(3), 95–105. Itabashi, Y., Miyoshi, S., Kawaguchi, H., Yuasa, S., Tanimoto, K., Furuta, A., Shimizu, T., Okano, T., Fukuda, K. and Ogawa, S. 2005. A new method for manufacturing cardiac cell sheets using fibrin-coated dishes and its electrophysiological studies by optical mapping. Artif. Organs 29(2), 95–103. Johnson, K.E. and Wilgus, T.A. 2014. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound. Care 3(10), 647–661. Kalluri, R. 2016. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16(9), 582–598. Kaneko-Kawano, T., Takasu, F., Naoki, H., Sakumura, Y., Ishii, S., Ueba, T., Eiyama, A., Okada, A., Kawano, Y. and Suzuki, K. 2012. Dynamic regulation of myosin light chain phosphorylation by rho-kinase. PLoS One 7(6), e39269. Kanzaki, M., Yamato, M., Yang, J., Sekine, H., Kohno, C., Takagi, R., Hatakeyama, H., Isaka, T., Okano, T. and Onuki, T. 2007. Dynamic sealing of lung air leaks by the transplantation of tissue-engineered cell sheets. Biomaterials 28(29), 4298–4302. Kasai, Y., Morino, T., Mori, E., Yamamoto, K. and Kojima, H. 2020. ROCK inhibitor combined with Ca2+ controls the myosin II activation and optimizes human nasal epithelial cell sheets. Sci. Rep. 10(1), 16853. Kendall, R.T. and Feghali-Bostwick, C.A. 2014. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 5, 123. Kim, K.W., Shin, Y.J. and Lee, S.C.S. 2022. Novel ROCK inhibitors, sovesudil and php-0961, enhance proliferation, adhesion and migration of corneal endothelial cells. Int. J. Mol. Sci. 23(23), 14690. Kim, S.R., Yi, H.J., Lee, Y.N., Park, J.Y., Hoffman, R.M., Okano, T., Shim, I.K. and Kim, S.C. 2018. Engineered mesenchymal stem-cell-sheets patches prevents postoperative pancreatic leakage in a rat model. Sci. Rep. 8(1), 360. Kim, S.W., Im, G.B., Jeong, G.J., Baik, S., Hyun, J., Kim, Y.J., Pang, C., Jang, Y.C. and Bhang, S.H. 2021. Delivery of a spheroids-incorporated human dermal fibroblast sheet increases angiogenesis and M2 polarization for wound healing. Biomaterials 275, 120954. Kim, S.W., Seo, I., Hyun, J., Eom, J., Um, S.H. and Bhang, S.H. 2023. Fibronectin-enriched interface using a spheroid-converged cell sheet for effective wound healing. ACS Appl. Mater. Interfaces 15(9), 11536–11548. Kirby, G.T.S., Michelmore, A., Smith, L.E., Whittle, J.D. and Short, R.D. 2018. Cell sheets in cell therapies. Cytotherapy 20(2), 169–180. Lee, J., Shin, D. and Roh, J.L. 2019. Promotion of skin wound healing using pre vascularized oral mucosa cell sheet. Head Neck 41(3), 774–779. Li, M., Ma, J., Gao, Y. and Yang, L. 2019. Cell sheet technology: a promising strategy in regenerative medicine. Cytotherapy 21(1), 3–16. Mani, S. and Swargiary, G. 2023. In vitro cytotoxicity analysis: mtt/xtt, trypan blue exclusion. In Animal cell culture: principles and practice. Techniques in life science and biomedicine for the non-expert. Eds., Mani, S., Singh, M. and Kumar, A. Springer, Cham, Switzerland. pp: 267–284. Matsumoto, T., Kim, M.H. and Kino-Oka, M. 2022. Effect of rho-associated kinase inhibitor on growth behaviors of human induced pluripotent stem cells in suspension culture. Bioengineering 9(11), 613. Matsumura, F., Totsukawa, G., Yamakita, Y. and Yamashiro, S. 2001. Role of myosin chain phosphorylation in the regulation of cytokinesis. Cell. Struct. Funct. 26(6), 639–644. Matsuno, Y., Yanagihara, M., Ueno, K., Saito, T., Kurazumi, H., Suzuki, R., Katsura, S., Oga, A. and Hamano, K. 2022. Dry preserved multilayered fibroblast cell sheets are a new manageable tool for regenerative medicine to promote wound healing. Sci. Rep. 12(1), 12519. Matsushima, H., Kuroki, T., Adachi, T., Kitasato, A., Ono, S., Tanaka, T., Hirabaru, M., Kuroshima, N., Hirayama, T., Sakai, Y., Soyama, A., Hidaka, M., Takatsuki, M., Kin, T., Shapiro, J. and Eguchi, S. 2016. Human fibroblast sheet promotes human pancreatic islet survival and function in vitro. Cell. Transplant. 25(8), 1525–1537. McGifford, O.J., Harkin, D.G. and Cuttle, L. 2022. Effect of Rho-associated protein kinase inhibitors on epidermal keratinocytes: a proposed application for burn wound healing. Tissue. Eng. Part. B. Rev. 28(3), 555–568. Mizoguchi, T., Ueno, K., Takeuchi, Y., Samura, M., Suzuki, R., Murata, T., Hosoyama, T., Morikage, N. and Hamano, K. 2018. Treatment of cutaneous ulcers with multilayered mixed sheets of autologous fibroblasts and peripheral blood mononuclear cells. Cell. Physiol. Biochem. 47(1), 201–211. Nagase, T., Ueno, K., Mizoguchi, T., Samura, M., Harada, T., Suehiro, K., Shirasawa, B., Morikage, N. and Hamano, K. 2020. Allogeneic fibroblast sheets accelerate cutaneous wound healing equivalent to autologous fibroblast sheets in mice. Am. J. Transl. Res. 12(6), 2652–2663. Nakayama, M., Amano, M., Katsumi, A., Kaneko, T., Kawabata, S., Takefuji, M. and Kaibuchi, K. 2005. Rho-kinase and myosin II activities are required for cell type and environment specific migration. Genes Cells. 10(2), 107–117. Niggli V. 1999. Rho-kinase in human neutrophils: a role in signalling for myosin light chain phosphorylation and cell migration. FEBS Lett. 445(1), 69–72. Nishimura, A., Nakajima, R., Takagi, R., Zhou, G., Suzuki, D., Kiyama, M., Nozaki, T., Owaki, T., Takahara, T., Nagai, S., Nakamura, T., Sugaya, M., Terada, K., Igarashi, Y., Hanzawa, H., Okano, T., Shimizu, T., Yamato, M. and Takeda, S. 2019. Fabrication of tissue-engineered cell sheets by automatic cell culture equipment. J. Tissue. Eng. Regen. Med. 13(12), 2246–2255. Okumura, N., Kinoshita, S. and Koizumi, N. 2017. Application of Rho kinase inhibitors for the treatment of corneal endothelial diseases. J. Ophthalmol. 2017, 2646904. Pakshir, P., Noskovicova, N., Lodyga, M., Son, D.O., Schuster, R., Goodwin, A., Karvonen, H. and Hinz, B. 2020. The myofibroblast at a glance. J. Cell. Sci. 133(13), jcs227900. Peh, G.S., Adnan, K., George, B.L., Ang, H.P., Seah, X.Y., Tan, D.T. and Mehta, J.S. 2015. The effects of Rho-associated kinase inhibitor Y-27632 on primary human corneal endothelial cells propagated using a dual media approach. Sci. Rep. 5, 9167. Piltti, J., Varjosalo, M., Qu, C., Häyrinen, J. and Lammi, M.J. 2015. Rho-kinase inhibitor Y-27632 increases cellular proliferation and migration in human foreskin fibroblast cells. Proteomics. 15(17), 2953–2965. Ravikanth, M., Soujanya, P., Manjunath, K., Saraswathi, T.R. and Ramachandran, C.R. 2011. Heterogenecity of fibroblasts. J. Oral. Maxillofac. Pathol. 15(2), 247–250. Ridge, K.M., Eriksson, J.E., Pekny, M. and Goldman, R.D. 2022. Roles of vimentin in health and disease. Genes Dev. 36(7–8), 391–407. Rinn, J.L., Bondre, C., Gladstone, H.B., Brown, P.O. and Chang, H.Y. 2006. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2(7), e119. Roman, J. 2023. Fibroblast-warriors at the intersection of wound healing and disrepair. Biomolecules 13(6), 945. Sakai, Y., Koike, M., Hasegawa, H., Yamanouchi, K., Soyama, A., Takatsuki, M., Kuroki, T., Ohashi, K., Okano, T. and Eguchi, S. 2013. Rapid fabricating technique for multi-layered human hepatic cell sheets by forceful contraction of the fibroblast monolayer. PLoS One. 8(7): e70970. Seasholtz, T.M. 2003. The RHOad less traveled: the myosin phosphorylation-independent path from Rho kinase to cell contraction. Focus on “Rho kinase mediates serum-induced contraction in fibroblast fibers independent of myosin LC20 phosphorylation”. Am. J. Physiol. Cell. Physiol. 284(3), C596–C598. Sezgin, B., Tatar, S., Karahuseyinoglu, S., Sahin, G.N., Ergun, Y., Meric, G. and Ersoy, K. 2021. The effects of oral mucosa-derived heterotopic fibroblasts on cutaneous wound healing. J. Plast. Reconstr. Aesthet. Surg. 74(10), 2751–2758. Shirasaka, T., Miyagawa, S., Fukushima, S., Kawaguchi, N., Nakatani, S., Daimon, T., Okita, Y. and Sawa, Y. 2016. Skeletal myoblast cell sheet implantation ameliorates both systolic and diastolic cardiac performance in canine dilated cardiomyopathy model. Transplantation 100(2), 295–302. Sun, C.C., Chiu, H.T., Lin, Y.F., Lee, K.Y. and Pang, J.H. 2015. Y-27632, a ROCK inhibitor, promoted limbal epithelial cell proliferation and corneal wound healing. PLoS One 10(12), e0144571. Sun, J., Qiao, Y.N., Tao, T., Zhao, W., Wei, L.S., Li, Y.Q., Wang, W., Wang, Y., Zhou, Y.W., Zheng, Y.Y., Chen, X., Pan, H.C., Zhang, X.N. and Zhu, M.S. 2020. Distinct roles of smooth muscle and non-muscle myosin light chain-mediated smooth muscle contraction. Front. Physiol. 11, 593966. Tsumanuma, Y., Iwata, T., Kinoshita, A., Washio, K., Yoshida, T., Yamada, A., Takagi, R., Yamato, M., Okano, T. and Izumi, Y. 2016. Allogeneic transplantation of periodontal ligament-derived multipotent mesenchymal stromal cell sheets in canine critical-size supra-alveolar periodontal defect model. BioRes. Open Access 5(1), 22–36. Vijayashree, R.J. and Sivapathasundharam, B. 2022. The diverse role of oral fibroblasts in normal and disease. J. Oral. Maxillofac. Pathol. 26(1), 6–13. Waasdorp, M., Krom, B.P., Bikker, F.J., van Zuijlen, P.P.M., Niessen, F.B. and Gibbs, S. 2021. The bigger picture: why oral mucosa heals better than skin. Biomolecules 11(8), 1165. Watanabe, K., Ueno, M., Kamiya, D., Nishiyama, A., Matsumura, M., Wataya, T., Takahashi, J.B., Nishikawa, S., Nishikawa, S., Muguruma, K. and Sasai, Y. 2007. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25(6), 681–686. Wirth, A. 2010. Rho kinase and hypertension. Biochim. Biophys. Acta. 1802(12), 1276–1284. Wood, S., Jayaraman, V., Huelsmann, E.J., Bonish, B., Burgad, D., Sivaramakrishnan, G., Qin, S., DiPietro, L.A., Zloza, A., Zhang, C. and Shafikhani, S.H. 2014. Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PLoS One 9(3), e91574. Yadav, A., Saini, V. and Arora, S. 2010. MCP-1: chemoattractant with a role beyond immunity: a review. Clin. Chim. Acta. 411(21–22), 1570–1579. Yamamoto, N., Ueno, K., Yanagihara, M., Kurazumi, H., Tanaka, Y., Oga, A., Shimokawa, M., Harada, E., Tanaka, T. and Hamano, K. 2023. Allogenic multilayered fibroblast sheets promote anastomotic site healing in a rat model of esophageal reconstruction. Am. J. Transl. Res. 15(5), 3217–3228. Yoshimine, S., Ueno, K., Murakami, J., Saito, T., Suzuki, R., Asai, Y., Ikeda, E., Tanaka, T. and Hamano, K. 2022. Autologous multilayered fibroblast sheets can reinforce bronchial stump in a rat model. Semin. Thoracic. Cardiovasc. Surg. 34(1), 349–358. | ||
| How to Cite this Article |
| Pubmed Style Purba MS, Anggoro D, Yamamoto N, Yoshimine S, Murakami J, Tanaka T, Hamano K, Itoh H, Itamoto K, Nemoto Y, Nakaichi M, Sunahara H, Tani K. Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. Open Vet. J.. 2024; 14(12): 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 Web Style Purba MS, Anggoro D, Yamamoto N, Yoshimine S, Murakami J, Tanaka T, Hamano K, Itoh H, Itamoto K, Nemoto Y, Nakaichi M, Sunahara H, Tani K. Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. https://www.openveterinaryjournal.com/?mno=222546 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i12.27 AMA (American Medical Association) Style Purba MS, Anggoro D, Yamamoto N, Yoshimine S, Murakami J, Tanaka T, Hamano K, Itoh H, Itamoto K, Nemoto Y, Nakaichi M, Sunahara H, Tani K. Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. Open Vet. J.. 2024; 14(12): 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 Vancouver/ICMJE Style Purba MS, Anggoro D, Yamamoto N, Yoshimine S, Murakami J, Tanaka T, Hamano K, Itoh H, Itamoto K, Nemoto Y, Nakaichi M, Sunahara H, Tani K. Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. Open Vet. J.. (2024), [cited January 25, 2026]; 14(12): 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 Harvard Style Purba, M. S., Anggoro, . D., Yamamoto, . N., Yoshimine, . S., Murakami, . J., Tanaka, . T., Hamano, . K., Itoh, . H., Itamoto, . K., Nemoto, . Y., Nakaichi, . M., Sunahara, . H. & Tani, . K. (2024) Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. Open Vet. J., 14 (12), 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 Turabian Style Purba, Melpa Susanti, Dito Anggoro, Naohiro Yamamoto, Sota Yoshimine, Junichi Murakami, Toshiki Tanaka, Kimikazu Hamano, Harumichi Itoh, Kazuhito Itamoto, Yuki Nemoto, Munekazu Nakaichi, Hiroshi Sunahara, and Kenji Tani. 2024. Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. Open Veterinary Journal, 14 (12), 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 Chicago Style Purba, Melpa Susanti, Dito Anggoro, Naohiro Yamamoto, Sota Yoshimine, Junichi Murakami, Toshiki Tanaka, Kimikazu Hamano, Harumichi Itoh, Kazuhito Itamoto, Yuki Nemoto, Munekazu Nakaichi, Hiroshi Sunahara, and Kenji Tani. "Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin." Open Veterinary Journal 14 (2024), 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 MLA (The Modern Language Association) Style Purba, Melpa Susanti, Dito Anggoro, Naohiro Yamamoto, Sota Yoshimine, Junichi Murakami, Toshiki Tanaka, Kimikazu Hamano, Harumichi Itoh, Kazuhito Itamoto, Yuki Nemoto, Munekazu Nakaichi, Hiroshi Sunahara, and Kenji Tani. "Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin." Open Veterinary Journal 14.12 (2024), 3428-3439. Print. doi:10.5455/OVJ.2024.v14.i12.27 APA (American Psychological Association) Style Purba, M. S., Anggoro, . D., Yamamoto, . N., Yoshimine, . S., Murakami, . J., Tanaka, . T., Hamano, . K., Itoh, . H., Itamoto, . K., Nemoto, . Y., Nakaichi, . M., Sunahara, . H. & Tani, . K. (2024) Establishment and characterization of multilayered fibroblast cell sheets from the canine oral mucosa, skin, and tail skin. Open Veterinary Journal, 14 (12), 3428-3439. doi:10.5455/OVJ.2024.v14.i12.27 |