| Review Article | ||
Open Vet. J.. 2025; 15(1): 1-7 Open Veterinary Journal, (2024), Vol. 15(1): 1-7 Review Article Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategiesMohamed Tharwat1*, Haytham Ali2 and Abdulrahman A. Alkheraif31Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Animal and Veterinary Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Oman 3Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia *Corresponding Author: Mohamed Tharwat. Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email: atieh [at] qu.edu.sa Submitted: 03/10/2024 Accepted: 25/11/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
AbstractParatuberculosis (PTB), also known as Johne’s disease, is a chronic contagious granulomatous disease of a wide variety of animals. It is caused by Mycobacterium avium subsp. paratuberculosis. This review summarizes the pathogenesis, clinical, hematobiochemical, sonographic, pathologic findings, and approaches to treatment and control of PTB in sheep and goats. Decreased appetite, weight loss, and changed fecal consistency were reported in sheep and goats with PTB. Clinical disease in sheep and goats is generally observed in those 2–4 years old. Other findings may include anorexia, coat roughness, and depression. The recorded hematological changes included neutropenia, leukocytosis, erythrocytosis, and increased hematocrit percent and hemoglobin concentration. Serum changes included hypocalcemia, hypoalbuminemia, hypomagnesemia, hypoproteinemia, and increased activity of creatine kinase. An overall increase in the intestinal mucosa thickness was detected on sonography in goats with PTB. Corrugation and folding of the intestinal mucosa may be also imaged in goats with PTB. However, the remarkable enlargement of the mesenteric lymph nodes is the most important finding. In sheep, postmortem findings included thickened intestinal walls, folding and corrugation of intestinal mucosa especially close to the ileocecal junction. Edematous and enlarged ileocecal and mesenteric lymph nodes may be observed. In goats, necropsy findings included enlargement of the mesenteric lymph nodes and thickened walls of the small intestines with folded and corrugated mucosa. No successful therapy of PTB in sheep and goats has been reported. The control of PTB in sheep and goats can be achieved by vaccination. In conclusion, early detection and eradication programs of PTB should be implemented more effectively for the control of PTB in sheep and goats. More research should be directed toward a vaccination program of PTB in these species. Keywords: Goat, Johne’s disease, Pathology, Paratuberculosis, Sheep. IntroductionParatuberculosis (PTB) or Johne’s disease (JD) is a worldwide chronic contagious granulomatous disease of the intestines affecting ruminant animals (Al-Swailem et al., 2011; Tharwat et al., 2011; Tharwat et al., 2012a,b; Tharwat et al., 2013; Tharwat et al., 2025a). Non-ruminants such as pigs, rabbits, birds, horses, and carnivores are generally considered incidental hosts for PTB, and sometimes they may harbor Mycobacterium avium subsp. paratuberculosis (MAP) (Singh et al., 2014; Stanitznig et al., 2017; Curlik et al., 2020). The disease is caused by MAP, an acid-fast bacillus linked to the genus Mycobacterium (Al-Swailem et al, 2011). The mycobacterium MAP has been found in humans with Crohn’s disease, Hashimoto’s thyroiditis, rheumatoid arthritis, diabetes type 1, autism, and multiple sclerosis (Garvey, 2018). Thus, PTB may be considered a possible public health hazard (Bharathy et al., 2017). PTB was initially detected by Johne and Frothington in 1895, and it was first reported in sheep in Bosnia in 1908 (West et al., 2009). MAP is a slowly growing organism that multiplies in the macrophage of the lymphoid and gastrointestinal tissues (Garvey, 2018). Contaminated water, feed, soiled udders, and bedding are the primary routes of MAP transmission, with young animals under 6 months of age being highly susceptible through the fecal–oral route. (Singh et al., 2010; Stabel et al., 2002). PTB may be suspected with clinical presentations of weight loss and intermittent diarrhea despite good appetite with the detection of MAP in feces, tissue or fecal culture, serology, and molecular techniques. Subclinically infected animals significantly contribute to disease transmission and environmental contamination (Garvey, 2020). Sheep and goats contribute greatly to the reduction of poverty in developing societies through the provision of milk, meat, and skin (Devendra, 1994). This function would be significantly reduced by chronic disorders such as JD especially in the absence of adequate veterinary services and effective programs for the control of such diseases (Idris et al., 2021). Therefore, infection of sheep and goats with PTB will result in huge economic crises. In countries where small ruminant farming is well established, losses due to JD are documented (Whittington et al., 2019). PTB has a great economic effect on sheep and goat production due to the cost of implementing prophylaxis measures and losses in animals’ productivity (Jiménez-Martín et al., 2022). For example, in England, annual losses due to PTB in sheep were reported to range from 0.5 to 16.5 million GBP (Ashworth and Gunn, 2001). The case fatality rate was estimated to be 1.2%–2.7% in Marino sheep in New Zealand with a loss of 1.5 USD/ewe (Whittington et al., 2019). In Australia, annual mortalities due to JD in 12 sheep herds extend between 6.2% and 7.8% (Bush et al., 2006); this percentage increased to 20% in some flocks (Windsor, 2014). It was reported in Italy that a decline in the profit efficiency due to PTB was reduced from 84% to 64% in sheep and goat flocks (Sardaro et al., 2017). This review summarizes the pathogenesis, clinical, hematobiochemical, sonographic, pathologic findings, and approaches to the treatment and control of PTB in sheep and goats. PathogenesisNewborn animals are usually infected orally through the consumption of infected milk and colostrum from diseased dams (Lambeth et al., 2004). MAP invades the gastrointestinal mucosa and replicates within sub-epithelial macrophages, leading to the formation of granulomas and gradual activation of a cell-mediated immune response (Henderson et al., 2000). In the advanced clinical form of the disease, strong humoral antibody response is produced when the mycobacteria are liberated from the macrophages (Milner et al., 1990). Clinical findingsDecreased appetite, weight loss, and changed fecal consistency were reported in sheep and goats with PTB (Wehrle et al., 2024). The body condition score declined to 1.4 ± 0.5 units compared to a value of 3.7 ± 0.3 units in healthy animals. Surprisingly, few goats (7%) suffered from chronic watery diarrhea and most diseased goats had unchanged feces. Infected sheep may also be presented with or without diarrhea, and severely and chronically affected sheep and goats may be admitted in the lateral recumbency position (Fig. 1). Unlike cattle, infected small ruminants may be present without a history of diarrhea. In a previous study involving 54 goats diagnosed with PTB, 29 of the affected animals presented without diarrhea upon admission, while 13 had soft feces, 8 exhibited intermittent diarrhea, and only 4 showed symptoms of chronic watery diarrhea (Tharwat et al., 2012b). It was found also that diarrhea or softening of the feces is found only in 20% of the diseased cases at the final stages of the infection (Carrigan and Seaman, 1990).
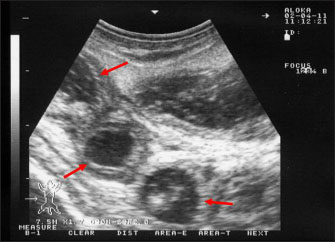
Fig. 1. Clinical presentation of a goat and a sheep with paratuberculosis. The goat (A) was presented with a history of chronic watery diarrhea, while the sheep (B) was presented without a history of diarrhea. Small ruminants are usually infected during the first week of age, but infection can occur at any age (Idris et al., 2021). The disease is typically observed in sheep and goats between the ages of 2–4 years old, and the symptoms are sometimes observed in dams immediately following parturition (Clarke, 1997) as stress may enhance the appearance of the clinical form. A remarkable decrease in body weight is the dominant sign in infected ovine and caprine. Other findings may include anorexia, coat roughness, and depression (Bauman et al., 2016). As the disease progresses, infected animals become more emaciated, dehydrated, anemic, depressed, and the skin appears fragile with submandibular edema (Djønne, 2010). Hematobiochemical changesIn goats with PTB, hematological changes included neutropenia, leukocytosis, erythrocytosis, increased hematocrit percent and hemoglobin concentration, and increased erythrocyte indexes, mean corpuscular hemoglobin, and mean corpuscular volume. Serum changes included hypocalcemia, hypoalbuminemia, and hypomagnesemia increased activity of creatine kinase (Tharwat et al., 2012b). Intermandibular edema, hypoproteinemia, and hypocalcemia have also been found in sheep with clinical forms of PTB (Jones, 1996). Ultrasonographic findingsUltrasonography has been proven effective for the early detection of several abdominal and thoracic disorders in small ruminants (Tharwat et al., 2012b; Tharwat and Al-Sobayil, 2017; Tharwat, 2021; Sadan et al., 2023; Tharwat and Al-Hawas, 2024a,b; Tharwat et al., 2024; Tharwat et al., 2025b). Recently, the technique has been found valuable for the diagnosis of various bacterial and parasitic infections in ruminants (Tharwat and Tsuka, 2024). An overall increase in intestinal mucosa thickness was detected in goats with PTB. It was classified as either mild, moderate, or severe (Fig. 2). Corrugation and folding of the intestinal mucosa may be also imaged in goats with PTB (Fig. 3). However, remarkable enlargement of the mesenteric lymph nodes are the most important findings in goats with PTB (Fig. 4). Other sonographic changes included increased hepatic brightness, intestinal edema consolidated lungs, and pleural, peritoneal, and pericardial effusions (Tharwat et al., 2012b). Pathological findingsIn sheep, reported postmortem findings included thickened intestinal walls, folding and corrugation of intestinal mucosa especially close to the ileocecal junction (Sikandar et al., 2013; Srikanth et al., 2017). Cecal and colonic changes are moderate compared to the terminal ileum (Carrigan and Seaman, 1990; Stehman, 1996). Edematous and enlarged ileocecal and mesenteric lymph nodes may be observed (Coelho et al., 2018). Histopathologically, granulomatous enteritis accompanied by severe cellular infiltrate consisting mainly of lymphocyte, epithelioid, and giant cells and macrophages with acid-fast organisms are detected (Hamid et al., 2018). Peyer’s patches hyperplasia, necrosis, and villous atrophy were also reported. Histopathology of enlarged lymph nodes included macrophages and epithelioid cell infiltration with acid-fast MAP bacilli with occasional foci of caseous necrosis and giant cells (Coelho et al., 2018).
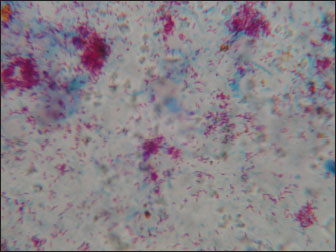
Fig. 2. Ultrasonography of the thickened intestinal walls in a goat with paratuberculosis. Thickening of the intestinal mucosa is apparent cross sectionally (red arrows). In goats with PTB, postmortem findings included enlargement of the mesenteric lymph nodes and thickened walls of the small intestines with folded and corrugated mucosa (Fig. 5). Other findings include pleura and pericardial effusion, intestinal edema, ascites, consolidated lungs, and fatty liver (Tharwat et al., 2012b). Histopathologically, in the early stages, the disease infiltration of plasma cells, lymphocytes, and macrophages was observed in the lamina propria; however, in the advanced progressive stages, giant cells and macrophages can be detected in the muscle layers and submucosa. Acid-fast organisms may be visualized in huge numbers (Greig, 2000). Diagnostic techniquesGenerally, microscopy, tissue and fecal culture, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR) are used for the detection of MAP (Arsenault et al., 2019; O’Brien et al., 2013). Fecal culture is reported to be the gold-standard method to detect MAP in animals (Singh et al., 2007); however, its efficacy is limited due to the low excretion of MAP in feces and the time required for the organism to appear (Mathevon et al., 2017; Schwalm et al., 2018). The PCR assay is sensitive; however, it relies on the stage of infection and quantity and quality of collected DNA (Al-Swailem et al., 2011; Tharwat et al., 2012a,b; Keller et al., 2014). For economics as well as quick monitoring, ELISA is the most used test (McKenna et al., 2005; Hemati et al., 2022). By Ziehl–Neelsen staining of rectal scraping, acid-fact bacilli can be easily visualized (Fig. 6).
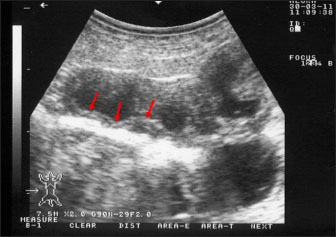
Fig. 3. Ultrasonography of the corrugated intestinal mucosa in a goat with paratuberculosis. The lesion (red arrows) was best imaged longitudinally
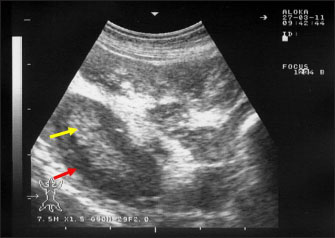
Fig. 4. Ultrasonography of the enlarged mesenteric lymph nodes in a goat with paratuberculosis. The enlarged lymph node showed a hypoechoic cortex (red arrow) and a hyperechoic medulla (yellow arrow).
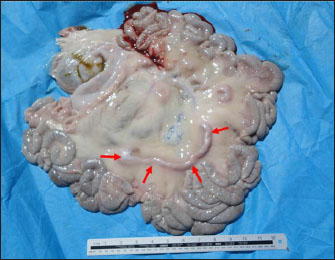
Fig. 5. Postmortem findings in a goat with paratuberculosis. Image shows enlargement of the mesenteric lymph nodes (red arrows). Treatment and controlDepending on the fact that MAP organisms are in vitro resistant to chemotherapeutic agents, therefore, successful response of PTB in sheep and goats has not been documented in diseased animals (Maroudam et al., 2015). The control of PTB in sheep and goats can be easily performed by vaccination that prevents clinical cases and increases productivity. Since bacterial excretion is significantly reduced, vaccination can help mitigate the risk of environmental contamination (Juste and Perez, 2011). Risk factors contributing to the pathogenesis of PTB included animal trading, flock size, and whether other animals such as cattle were reared within the flock (Schrott et al., 2023).
Fig. 6. Ziehl–Neelsen staining revealing Mycobacterium avium subsp. paratuberculosis obtained from a goat with Johne’s disease. Clusters of acid-fast bacilli were identified from an enlarged lymph node at necropsy. Successful eradication and posteradication surveillance programs have been reported in sheep and goats (Gavin et al., 2018; Benedictus and Kalis, 2003). They included frequent serological testing of all adult animals using ELISA and agar gel immunodiffusion assays. Fecal bacterial cultures were performed on positive or suspect goats and for all animals found euthanized or dead, and tissues were delivered for acid-fast staining and histopathology. Additional procedures included minimizing fecal–oral transmission of MAP and maintaining a closed herd (Gavin et al., 2018). The vaccination of young sheep and goats seems to be a useful measure in the prevention of clinical PTB, although changes in the management and hygiene practices are also effective (Benedictus and Kalis, 2003). Because of the health effects and the financial consequences of PTB, suitable surveillance and prophylaxis programs are warranted to decrease the risk of JD in sheep and goat flocks (Jiménez-Martín et al., 2022). Although there have been advancements in controlling PTB in small ruminants, further measures are necessary, particularly in dairy species. There is an urgent need for improvement of diagnostic methods especially those detecting latent or subclinical infections and a better understanding of the possible effects of vaccination. Disease eradication programs should depend on the flock levels as well as the national levels to restrict disease entrance from trade (Windsor, 2015). ConclusionIn conclusion, PTB is a life-threatening, grave prognostic, and possible zoonotic disease in small ruminants. In addition, it has huge economic crises on sheep and goat industry due to the implementation of prophylaxis measures alongside the losses in animals’ productivity. Therefore, early detection and eradication procedures should be implemented more for the control and prevention programs. Initially, animals suspected to be infected with PTB should be clinically evaluated by abdominal sonography and immediately isolated if intestinal sonography results point to infection by PTB until confirmatory laboratory results, such as ELISA or PCR arrive. Finally, in sheep and goats, more research should be directed toward vaccination programs in these species. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Author contributionMT: conceptualization, data collection, and writing the original manuscript draft. HA: sharing in writing of manuscript draft and editing. AA: review and editing. All authors approved the final manuscript for publication. Data availabilityAll data supporting the findings of this study are available within the manuscript, and no additional data sources are required. ReferencesAl-Swailem, A., Al-Dubaib, M.A., Abo El-Hassan, D.G., Tharwat, M., Al-Yamani, E., Shehata, M., Hashad, M. and Mahmoud, O.M. 2011. Treatment of paratuberculosis in camels by rifampin and streptomycin. J. Camel Pract. Res. 18, 225–229. Arsenault, J., Singh Sohal, J., Leboeuf, A., Hélie, P., Fecteau, G., Robinson, Y. and L’Homme, Y. 2019. Validation of an in-house real-time PCR fecal assay and comparison with two commercial assays for the antemortem detection of Mycobacterium avium subsp. paratuberculosis infection in culled sheep. J Vet. Diagn. Investig. 31, 58–68. Ashworth, S. and Gunn, G.J. 2001. Losses associated with paratuberculosis in sheep. In assessment of surveillance and control of Johne’s disease in farm animals in Gb. Eds., Galdow, G. and Gunn, G.J. Edinburgh, Uk: SAC Veterinary Division, pp: 103–115. Bauman, C.A., Jones-Bitton, A., Menzies, P., Toft, N., Jansen, J. and Kelton, D. 2016. Prevalence of paratuberculosis in the dairy goat and dairy sheep industries in ontario. Can. Vet. J. 57, 169–175. Benedictus, G. and Kalis, C.J. 2003. Paratuberculosis: eradication, control and diagnostic methods. Acta Vet. Scand. 44, 231–241. Bharathy, S., Gunaseelan, L. and Porteen, K. 2017. Exploring the potential hazard of Mycobacterium avium subspecies paratuberculosis as a cause for Crohn’s disease. Vet. World 10, 457–460. Bush, R.D., Windsor, P.A. and Toribio, J.A. 2006. Losses of adult sheep due to ovine Johne’s disease in 12 infected flocks over a 3-year period. Aust. Vet. J. 84, 246–253. Carrigan, M.J. and Seaman, J.T. 1990. The pathology of Johne’s disease in sheep. Aust. Vet. J. 67, 47–50. Clarke, C.J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116, 217–261. Coelho, A.C., Coelho, A.M., GarcÍA-Diez, J., Pires, M.A. and Pinto, M.L. 2018. Detection of Mycobacterium avium Subsp. paratuberculosis by several diagnostics techniques in clinical suspected sheep. J. Hell. Vet. Med. Soc. 68, 167. Curlik, J.,Lazar, P., Iglodyova, A., Barbusinova, E., Smiga, L., Novotny, J., Mojzisova, J., Ondrejkova, A., Hromada, R. and Konjevic, D. 2020. Detection of Mycobacterium avium Subsp. paratuberculosis in Slovakian wildlife. Pol. J. Vet. Sci. 23, 529–535. Devendra, C. 1994. Small ruminants: potential value and ccontribution to sustainable development. Outloo Agric. 23, 97–103. Djønne, B. 2010. Paratuberculosis in goats. In Paratuberculosis: organism, disease, control. Eds., Behr, M.A., Collins, D.M. Oxfordshire, UK: CAB International, pp: 169–178. Garvey, M. 2020. Mycobacterium avium paratuberculosis: a disease burden on the dairy industry. Animals 10, 1773. Garvey, M. 2018. Mycobacterium avium subspecies Paratuberculosis: a possible causative agent in human morbidity and risk to public health safety. Open Vet. J. 8, 172–181. Gavin, W.G., Porter, C.A., Hawkins, N., Schofield, M.J. and Pollock, J.M. 2018. Johne’s disease: a successful eradication programme in a dairy goat herd. Vet. Rec. 182, 483. Greig, A. 2000. Johne’s disease in sheep and goats. Practice 22, 146–151. Hamid, M.A., Mohammed, G.E.E., Bakheit, A.O. and Saeed, E.M.A. 2018. Histopathological and RT-PCR detection of mycobacterium paratuberculosis in tissues of clinically suspected small ruminants. Int. J. Life Sci. Sci. Res. 4, 2012–2018. Hemati, Z., Meletis, E., Derakhshandeh, A., Haghkhah, M., Kostoulas, P., Singh, S.V., Chaubey, K.K. and Gupta, S. 2022. Application of Bayesian modeling for diagnostic assays of Mycobacterium avium subsp. paratuberculosis in sheep and goats flocks. BMC Vet. Res. 18, 47. Henderson, D.C., Caldow, G. and Low, C.J. 2000. Paratuberculosis in cattle: pathology and clinical disease. In Assessment of Surveillance and Control of Johne’s Disease in Farm Animals in GB; Scottish Agricultural College, Veterinary Science Division. Edinburgh, UK: pp: 15–19. Idris, S.M., Eltom, K.H., Okuni, J.B., Ojok, L., Elmagzoub, W.A., El Wahed, A.A., Eltayeb, E. and Gameel, A.A. 2021. Paratuberculosis: the hidden killer of small ruminants. Animals 12, 12. Jiménez-Martín, D., García-Bocanegra, I., Risalde, M.A., Fernández-Molera, V., Jiménez-Ruiz, S., Isla, J. and Cano-Terriza, D. 2022. Epidemiology of paratuberculosis in sheep and goats in southern Spain. Prev. Vet. Med. 202, 105637. Jones, D.G. and Kay, J.M. 1996. Serum biochemistry and the diagnosis of Johne’s disease (Paratuberculosis) in Sheep. Vet. Rec. 139, 498–499. Juste, R.A. and Perez, V. 2011. Control of paratuberculosis in sheep and goats. Vet. Clin. North Am. Food Anim. Pract. 27, 127–138. Keller, S.M., Stephan, R., Kuenzler, R., Meylan, M. and Wittenbrink, M.M. 2014. Comparison of fecal culture and F57 real-time polymerase chain reaction for the detection of Mycobacterium avium subspecies paratuberculosis in Swiss cattle herds with a history of paratuberculosis. Acta Vet. Scand. 56, 68. Lambeth, C., Reddacliff, L.A., Windsor, P., Abbott, K.A., McGregor, H. and Whittington, R.J. 2004. Intrauterine and Transmammary Transmission of Mycobacterium avium Subsp Paratuberculosis in sheep. Aust. Vet. J. 82, 504–508. Maroudam, V., Subramanian, B.M., Kumar, P.P. and Raj, G.D. 2015. Paratuberculosis: diagnostic methods and their constraints. J. Vet. Sci. Technol. 6, 4172 Mathevon, Y., Foucras, G., Falguières, R. and Corbiere, F. 2017. Estimation of the sensitivity and specificity of two serum ELISAs and one fecal qPCR for diagnosis of paratuberculosis in sub-clinically infected young adult French sheep using latent class Bayesian modeling. BMC Vet. Res. 13, 230. McKenna, S.L., Keefe, G.P., Barkema, H.W. and Sockett, D.C. 2005. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 110, 105–111. Milner, A.R., Wendy, N., Mack, K.J., Coates, J.H., Ian, G. and Sheldrick, P. 1990. The sensitivity and specificity of a modified Elisa for the diagnosis of Johne’s disease from a field trial in cattle. Vet. Microbiol. 25, 193–198. O’Brien, D.P., Robson, M., Friedman, N.D., Walton, A., McDonald, A., Callan, P., Hughes, A., Rahdon, R. and Athan, E. 2013. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of mycobacterium ulcerans infections. BMC Infect. Dis. 13, 416. Sardaro, R., Pieragostini, E., Rubino, G. and Petazzi, F. 2017. Impact of Mycobacterium avium subspecies paratuberculosis on profit efficiency in semi-extensive dairy sheep and goat farms of Apulia, Southern Italy. Prev. Vet. Med. 136, 56–64. Schrott, J., Sodoma, E., Dünser, M., Tichy, A. and Khol., J.L. 2023. Mycobacterium avium subsp. paratuberculosis in sheep and goats in Austria: seroprevalence, risk factors and detection from boot swab samples. Animals 13, 1517. Schwalm, A.K., Obiegala, A., Pfeffer, M. and Sting, R. 2018. Enhanced sensitivity and fast turnaround time in laboratory diagnosis for bovine paratuberculosis in fecal samples. J. Microbiol. Methods. 152, 39–47. Sikandar, A., Cheema, A.H., Adil, M., Younus, M., Zaneb, H., Zaman, M.A. and Masood, S. 2013. Ovine paratuberculosis-a histopathological study from Pakistan. J. Anim. Plant Sci. 23, 749–753. Singh, A.V., Singh, S.V., Singh, P.K. and Sohal, J.S. 2010. Is Mycobacterium avium subsp. paratuberculosis, the cause of Johne’s disease in animals, a good candidate for Crohn’s disease in man? Indian J. Gastroenterol. 29, 53–8. Singh, S., Sohal, J.S., Kumar, N., Gupta, S., Chaubey, K. and Rawat, K.D. 2014. Recent approaches in diagnosis and control of Mycobacterial infections with special reference to Mycobacterium avium subspecies paratuberculosis. Adv. Anim. Vet. Sci. 1, 1–11. Singh, S.V., Singh, A.V., Singh, R., Sandhu, K.S., Singh, P.K., Sohal, J.S., Gupta, V.K. and Vihan, V.S. 2007. Evaluation of highly sensitive indigenous milk ELISA kit with fecal culture, milk culture and fecal-PCR for the diagnosis of bovine Johne’s disease (BJD) in India. Comp. Immunol. Microbiol. Infect. Dis. 30, 175. Srikanth, M., Narayanaswamy, H.D., Satyanarayana, M.L., Suguna, R.A.O., Rathnamma, D., Ranganath, L., Mukurtal, S.Y., Sarvesha, K. and Manjunatha, S.S. 2017. Pathomorphological studies on ovine paratuberculosis in an organised sheep farm in Karnataka. J. Cell Tissue Res. 17, 6067–6072. Stabel, J.R., Wells, S.J. and Wagner, B.A. 2002. Relationships between fecal culture, ELISA, and bulk tank milk test results for Johne’s disease in US dairy herds. J Dairy Sci. 85, 525–31. Stanitznig, A., Khol, J.L., Lambacher, B., Franz, S., Wittek, T., Kralik, P., Slana, I. and Vasickova, P. 2017. Prevalence of Mycobacterium avium subspecies paratuberculosis and hepatitis E in new world camelids in Austria. Vet. Rec. 181, 46. Stehman, S.M. 1996. Paratuberculosis in small ruminants, deer, and South American Camelids. Vet. Clin. N. Am. Food Anim. Pract. 12, 441–455. Sadan, M., Tharwat, M. and El-Deeb, W, 2023. Deep swellings in sheep and goats: clinical, ultrasonographic and post-mortem findings. Int. J. Vet. Sci. 12, 793–801. Tharwat, M, 2021. Clinical, ultrasonographic, and postmortem findings in sheep and goats with urinary tract disorders. Vet. World 14, 1879–1887. Tharwat, M., Ali, H. and Alkheraif, A.A. 2025a. Clinical insights on paratuberculosis in Arabian camels (Camelus dromedarius): a review. Open Vet. In press. 15(1). Tharwat, M., Al-Sobayil, F., El-Magawry, S. 2013. Clinicobiochemical and postmortem investigations in 60 Camels (Camelus dromedarius) with Johne’s disease. J. Camel Pract. Res. 20, 145–149. Tharwat, M., Alkheraif, A.A. and Marzok, M. 2025b. Pregnancy toxemia in small ruminants: clinical, sonographic, hematobiochemical and pathologic findings. Int. J. Vet. Sci. 14, 204–211. Tharwat, M. and Al-Hawas, A. 2024a. Liver diseases in sheep and goats: parallel sonographic and pathologic findings. Int. J. Vet. Sci. 13, 284–290. Tharwat, M. and Al-Hawas, A. 2024b. Suppurative pyelonephritis in a caprine buck: clinical, laboratory, ultrasonographic and pathologic findings. Int. J. Vet. Sci. 13, 479–483. Tharwat, M., Hegazy, Y. and Alkheraif, A.A. 2024. Discolored urine in sheep and goats: clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Vet. J. 14, 1059–1071. Tharwat, M., Al-Sobayil, F., Ali, A., Hashad, M. and Buczinski, S. 2012a. Clinical, ultrasonographic, and pathologic findings in 70 camels (Camelus dromedarius) with Johne’s disease. Can. Vet. J. 53, 543–548. Tharwat, M., Al-Sobayil F. and El-Magawry, S. 2011. Clinicobiochemical and postmortem investigations in 60 camels (Camelus dromedarius) with Johne’s disease. J. Camel Pract. Res. 20, 145–149. Tharwat, M., Al-Sobayil, F., Hashad, M. and Buczinski, S. 2012b. Transabdominal ultrasonographic findings in goats with paratuberculosis. Can. Vet. J. 53, 1063–1070. Tharwat, M. and Al-Sobayil, F. 2017. Ultrasonographic findings in goats with contagious caprine pleuropneumonia caused by Mycoplasma capricolum subsp. capripneumoniae. BMC Vet. Res. 13, 263. Tharwat, M. and Tsuka, T. 2024. Diagnostic utility of ultrasonography for thoracic and abdominal bacterial and parasitic diseases in ruminants: a comprehensive overview. Front. Vet. Sci. 11, 1435395. Wehrle, F., Moog, U., Donat, K., Köhler, H. and Klassen, A. 2024. Verbreitung der Paratuberkulose in Thüringer Schaf- und Ziegenherden [Prevalence of paratuberculosis in Thuringian sheep and goat flocks]. Tierarztl Prax Ausg G Grosstiere Nutztiere. 52, 25–32. West, D.M., Bruère, A.N. and Ridler, A.L. 2009. The sheep: health, disease and production, 3rd ed. Palmerston North, New Zealnd: Veterinary Continuing Education, Massey University. Whittington, R., Donat, K., Weber, M.F., Kelton, D., Nielsen, S.S., Eisenberg, S., Arrigoni, N., Juste, R., Saez, J.L. and Dhand, N. 2019. Control of paratuberculosis: who, why and how. a review of 48 countries. BMC Vet. Res. 15, 198. Windsor, P.A. 2015. Paratuberculosis in sheep and goats. Vet. Microbiol. 181, 161–9. Windsor, P.A. 2014. Managing control programs for ovine caseous lymphadenitis and paratuberculosis in Australia, and the need for persistent vaccination. Vet. Med. 5, 11–22. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Ali H, Alkheraif AA. Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. Open Vet. J.. 2025; 15(1): 1-7. doi:10.5455/OVJ.2025.v15.i1.1 Web Style Tharwat M, Ali H, Alkheraif AA. Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. https://www.openveterinaryjournal.com/?mno=223095 [Access: January 08, 2026]. doi:10.5455/OVJ.2025.v15.i1.1 AMA (American Medical Association) Style Tharwat M, Ali H, Alkheraif AA. Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. Open Vet. J.. 2025; 15(1): 1-7. doi:10.5455/OVJ.2025.v15.i1.1 Vancouver/ICMJE Style Tharwat M, Ali H, Alkheraif AA. Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. Open Vet. J.. (2025), [cited January 08, 2026]; 15(1): 1-7. doi:10.5455/OVJ.2025.v15.i1.1 Harvard Style Tharwat, M., Ali, . H. & Alkheraif, . A. A. (2025) Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. Open Vet. J., 15 (1), 1-7. doi:10.5455/OVJ.2025.v15.i1.1 Turabian Style Tharwat, Mohamed, Haytham Ali, and Abdulrahman A. Alkheraif. 2025. Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. Open Veterinary Journal, 15 (1), 1-7. doi:10.5455/OVJ.2025.v15.i1.1 Chicago Style Tharwat, Mohamed, Haytham Ali, and Abdulrahman A. Alkheraif. "Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies." Open Veterinary Journal 15 (2025), 1-7. doi:10.5455/OVJ.2025.v15.i1.1 MLA (The Modern Language Association) Style Tharwat, Mohamed, Haytham Ali, and Abdulrahman A. Alkheraif. "Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies." Open Veterinary Journal 15.1 (2025), 1-7. Print. doi:10.5455/OVJ.2025.v15.i1.1 APA (American Psychological Association) Style Tharwat, M., Ali, . H. & Alkheraif, . A. A. (2025) Paratuberculosis in sheep and goats: Pathogenesis, diagnostic findings, and control strategies. Open Veterinary Journal, 15 (1), 1-7. doi:10.5455/OVJ.2025.v15.i1.1 |