| Case Report | ||
Open Vet. J.. 2025; 15(1): 478-481 Open Veterinary Journal, (2024), Vol. 15(1): 478-481 Case Report MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta)Aistė Gradeckienė*, Ieva Plungytė, Martinas Jankauskas, Dmitrij Kvitka and Dalia JuodžentėDr. L. Kriaučeliūnas Small Animal Clinic, Faculty of Veterinary, Veterinary Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania *Corresponding Author: A. Gradeckienė. Dr. L. Kriaučeliūnas Small Animal Clinic, Faculty of Veterinary, Veterinary Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania. Email: mhelmieffendi [at] gmail.com Submitted: 10/10/2024 Accepted: 24/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
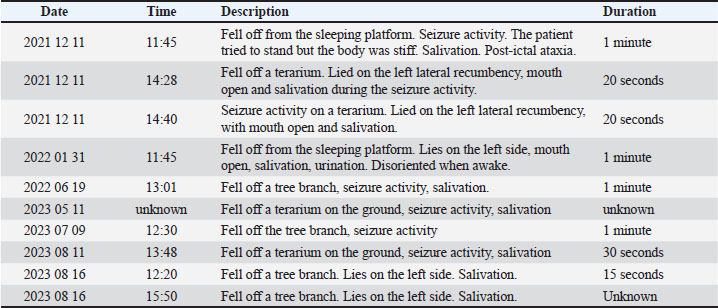
AbstractBackground: Epilepsy is a well-established and extensively studied condition in both human and veterinary medicine. Case Description: A 4-year-old captive, intact male ring-tailed lemur (Lemur catta) presented with a 2-year history of recurrent seizures. History of disease, clinical and neurological examination, blood and immunological examination for Toxoplasma gondi and magnetic resonance imaging were conducted to identify potential underlying causes of the seizures. Magnetic resonance imaging findings were consistent with idiopathic epilepsy, and antiepileptic drug therapy was initiated with oral phenobarbital 10 mg/animal twice a day Serum levels were monitored to ensure therapeutic efficacy. Following the initiation of treatment, the patient experienced successful control of seizures, with no significant adverse effects noted during the follow-up period. Conclusion: This case describes the diagnostic approach and management of seizures in ring-tailed lemur, a species, that has not been described before. Keywords: Idiopathic epilepsy, Ring-tailed lemur, Magnetic resonance imaging. IntroductionEpilepsy is a well-established and extensively studied condition in both human and veterinary medicine. Despite significant research, the underlying mechanisms of abnormal neuronal excitability remain incompletely understood, complicating effective treatment. In zoo animal medicine, epilepsy in non-human primates is particularly underreported, with limited literature and no standardized guidelines available for diagnostic and therapeutic approaches. Here, we present a case demonstrating the successful management of epileptic seizures in a captive ring-tailed lemur, contributing valuable insights into diagnostic and therapeutic options for similar cases in non-human primates. Case DetailsA 4-year-old captive intact male ring-tailed lemur (Lemur Catta) was presented to the Lithuanian University of Health Sciences for a diagnostic work-up due to multiple seizure episodes. The lemur was owned by a private zoo since birth. The first seizure activity was documented 2 years ago. The seizure activity initially presented as a cluster, with three episodes occurring within 24 hours, followed by two isolated episodes a few months later. At this stage, the zoo veterinarian initiated gabapentin therapy at a dose of 12.5 mg per animal, once a day (SID). Following the introduction of gabapentin, a 12-month seizure-free period was documented. However, another cluster of seizures occurred despite ongoing gabapentin therapy, prompting the decision to pursue further diagnostic investigation (Table 1). No additional health issues apart from seizures were documented in the patient. The lemurs live in a small walk-by zoo, they receive their first feeding at 10:30 and no other intense activity is reported. The similar timings of seizure activity were not related to any specific disturbances like cleaning of the environment, higher visitor rate, training, or feeding. All episodes were documented by zookeepers and were described as generalized tonic-clonic seizures lasting less than 1 minute with loss of consciousness, and concurrent autonomous nervous system signs such as salivation and urination. Afterward, the animal was experiencing post-ictal signs, being in-coordinated and confused. However, during inter-ictal period, no neurological deficits, nor predisposing factors were observed by the zoo personnel. Multiple morphological and biochemical blood examinations, during the period of 2022–2024, including: glucose, total protein, globulin, albumin, creatinine, blood urea nitrogen (BUN):creatinine ratio, alanine transaminase (ALT), and alkaline phosphatase (ALP). Gamma glutamyl transferase (GGT), total bilirubin, phosphorus, and calcium were unremarkable. In addition, a blood sample was collected for the rapid Toxoplasma immunoglobulin G and immunoglobulin M (IgG/IgM) antibody test (Toxoplasma IgG/IgM Antibody Rapid Test, J&G Biotech Ltd, London), based on lateral flow immunochromatographic assay. The test was considered negative. Fecal examinations for parasites and ova were all unremarkable. Table 1. Seizure diary.
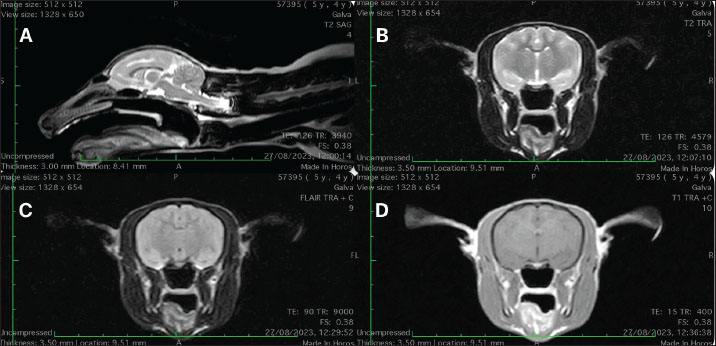
After metabolic causes of seizures were ruled out, an intracranial structural disease or idiopathic epilepsy was suspected and investigated further. The animal was sedated for the MRI examination using intramuscular injection of midazolam 0.2 mg/kg (Midazolam Kalceks 5 mg/ml, AS Kalceks, Riga), buprenorphine 0.01 mg/kg (Bupaq Multidose 0.3 mg/ml, Richter Pharma AG, Wels), and ketamine 5 mg/kg (Ketamidor 10 %, Richter Pharma AG, Wels). A 24G intravenous catheter was placed into the left saphenous vein. Propofol (Propoven 1 %, Fresenius Kabi, Hamburg) was used for the induction. Intubation was performed with a 3.0 endotracheal tube and the maintenance of anesthesia was provided with a 1.5% minimum alveolar concentration of sevoflurane mixed in oxygen 2 l/minute. Intravenous fluid therapy with Ringer lactate at the rate of 5 ml/kg/h was given during general anesthesia. MRI scan was performed using a Hitachi Aperto Lucent 0.4 T (Hitachi Medical Corporation, Tokyo) open-field scanner. The animal was placed in a human knee coil in dorsal recumbency. A standard small animal head protocol was applied that consisted of T2-weighted sequences in sagittal, transversal, and dorsal planes, T1-Weighted Fast spin ech pre- and post-contrast sequences were also acquired in three planes. In addition, fluid-attenuated invertion recovery (FLAIR) was acquired in the transversal plane. Gadobutrol (Gadovist®, Bayer, HealthCare Pharmaceuticals, Ontario) at 0,1 mmol/kg dose, was used as a contrast agent. No structural abnormalities, nor pathological contrast media uptake in the brain or surrounding structures were detected (Fig. 1). Regrettably, cerebrospinal fluid collection for further diagnostic evaluation was not performed. Recovery from anesthesia was uneventful. Without any evidence of structural brain disease, an anti-epileptic drug (AED) therapy was initiated with phenobarbital (Phentotyl 60 mg, Chanelle Pharmaceuticals Manufacturing Ltd., Galway) at 10 mg/animal dose twice a day (BID) (Portier et al., 2016), instead of gabapentin. Two weeks into treatment, a blood sample was collected to measure serum phenobarbital concentration. A phenobarbital level of 41 μg/ml was measured. No seizure activity or adverse drug effects were observed following the initiation of phenobarbital therapy. The patient has remained seizure-free for 13 months under treatment. Furthermore, no evidence of phenobarbital-associated hepatotoxicity was detected in subsequent biochemical blood analyses. DiscussionTo our knowledge, this is the first report describing the diagnostic approach, management of epileptic seizures, and monitoring of AED therapy in captive ring-tailed lemur. Although a few cases of epileptic seizures in captive ring-tailed lemurs are found in the literature (Croll et al., 2019), none has described a full diagnostic approach and successful management of seizures together with AED monitoring. Epilepsy observed in nonhuman primates typically manifests in one of two forms: spontaneous epilepsy, often with a genetic component, and epilepsy resulting from structural causes such as infections or trauma (Croll et al., 2019).
Fig. 1. (A) Mid-sagital T2W; (B) T2W, (C) FLAIR and (D) T1 post-contrast images at the level of thalamus. No structural abnormalities, nor pathological contrast media uptake in the brain or surrounding structures were detected. Several infectious agents were described in the literature, causing seizures in primates, including cerebral tuberculosis in a Rhesus monkey (Machotka et al., 1975), Herpes virus encephalitis in ruffed lemur (Kornegay et al., 1993), Herpesvirus type 1 infection in White-handed gibbon (Landolfi et al., 2005), Cryptococcosis in Red-tailed guenon (Helke et al., 2006), Staphylococcal meningoencephalitis in Guyanese squirrel monkey (García et al., 2009), and Balamuthia mandrillaris meningoencephalitis in Western low-land gorilla (Gjeltema et al., 2016). All of these infections are known to cause structural changes in the brain, which in our case would have been identifiable through MRI imaging. Moreover, the patient’s long history of seizures, in the absence of additional symptoms or abnormalities in multiple blood examinations, makes an infectious etiology less likely. In the case of the ring-tailed lemur described in this report, a genetic basis for seizures is unlikely, as neither the parents nor a sibling of the lemur have shown any documented signs of epilepsy. Toxoplasma gondii has been reported in ring-tailed lemurs (Rocchigiani et al., 2022), where it typically leads to acute death but can also present with neurological clinical signs such as epilepsy and tremor. In this case, the rapid Toxoplasma IgG/IgM antibody test was negative, suggesting that toxoplasmosis was not the cause of the seizures in this lemur. In addition, extended cerebrospinal fluid analysis incorporating additional infectious agents could have provided valuable diagnostic insights in this case. However, it is a subject for future research. The timing of epileptic episodes in this patient has not supported the hypothesis that external stressors, such as increased zoo visitor numbers, may contribute to the onset of seizures. To assess the potential impact of external stressors on seizure occurrence, a series of cortisol measurements could provide valuable insights. This represents a promising area for future research to better understand the relationship between stress, cortisol levels, and seizure activity in captive primates. The limited information available in the literature regarding phenobarbital dosage and its therapeutic serum concentration in ring-tailed lemurs posed a significant challenge for decision-making. However, extensive data available for dogs and cats with epilepsy—whose phenobarbital dosages and therapeutic serum concentrations are comparable to those used in adult humans (Trinka, 2023)—provided a valuable reference and aligned with our approach in this case. In summary, this case presents suspected idiopathic epilepsy in ring-tailed lemur and highlights the importance of a thorough diagnostic work-up, including imaging and laboratory testing, to rule out potential structural causes of seizures. The successful management of seizures with phenobarbital further emphasizes the need for ongoing monitoring and individualized treatment plans in similar cases. This case report contributes to the limited body of knowledge on epilepsy in ring-tailed lemurs and underscores the need for further research to better understand and manage this condition in non-human primates. AcknowledgmentsNone reported. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe authors have nothing to disclose. Author contributionsDVM Ieva Plungytė—anesthesia management and preparation of the manuscript; DVM Aistė Gradeckienė—MRI diagnostics and preparation of the manuscript; DVM Martinas Jankauskas, PhD Dmitrij Kvitka and PhD Dalia Juodžentė revision and correction of the manuscript. All authors contributed to the article and approved the submitted version. DisclosuresChat GPT language correction tool was used in the preparation of this manuscript. ReferencesCroll, L., Szabo, C.A., Abou-Madi, N. and Devinsky, O. 2019. Epilepsy in nonhuman primates. Epilepsia 60, 1526–1538; doi:10.1111/epi.16089. García, A., Nambiar, P.R., Marini, R.P. and Fox, J.G. 2009. Staphylococcal meningoencephalitis, nematodiasis, and typhlocolitis in a squirrel monkey (Saimiri sciureus). J. Med. Primatol. 38, 377–381; doi:10.1111/j.1600-0684.2009.00363.x. Gjeltema, J.L., Troan, B., Muehlenbachs, A., Liu, L., Da Silva, A.J., Qvarnstrom, Y., Tobias, J.R., Loomis, M.R. and De Voe, R.S. 2016. Amoebic meningoencephalitis and disseminated infection caused by Balamuthia mandrillaris in a Western lowland gorilla (Gorilla gorilla gorilla). J. Am. Vet. Med. Assoc. 248, 315–321; doi:10.2460/javma.248.3.315. Helke, K.L., Denver, M.C., Bronson, E. and Mankowski, J.L. 2006. Disseminate Cryptococcosis in a guenon (Cercopithecus ascanius). Vet. Pathol. 43, 75–78; doi:10.1354/vp.43-1-75. Kornegay, R.W., Baldwin, T.J. and Pirie, G. 1993. Herpesvirus encephalitis in a ruffed lemur (Varecia variegatus). J. Zoo. Wildl. Med. 24, 196–203. Landolfi, J.A., Wellehan, J.F.X., Johnson, A.J. and Kinsel, M.J., 2005. Fatal human herpesvirus type 1 infection in a white-handed gibbon (Hylobates Lar). J. Vet. Diagn. Invest. 17, 369–371; doi:10.1177/104063870501700412. Machotka, S.V., Chapple, F.E. and Stookey, J.L. 1975. Cerebral tuberculosis in a rhesus monkey. J. Am. Vet. Med. Assoc. 167, 648–650. Portier, K., Viguier, E. and Quintard, B. 2016. The anaesthetic management of a lemur (Prolemur simus) undergoing craniotomy for brain tumour resection. Vet. Rec. Case Rep. 4, e000332; doi:10.1136/vetreccr-2016-000332. Rocchigiani, G., Fonti, N., Nardoni, S., Cavicchio, P., Mancianti, F. and Poli, A., 2022. Toxoplasmosis in captive ring-tailed lemurs (Lemur catta). Pathogens 11, 1142; doi:10.3390/pathogens11101142. Trinka, E. 2023. Phenobarbital in status epilepticus – rediscovery of an effective drug. Epilepsy Behav. 141, 109104; doi:10.1016/j.yebeh.2023.109104. | ||
| How to Cite this Article |
| Pubmed Style Plungytė I, Jankauskas M, Kvitka D, Juodžentė D, Gradeckienė A. MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). Open Vet. J.. 2025; 15(1): 478-481. doi:10.5455/OVJ.2025.v15.i1.44 Web Style Plungytė I, Jankauskas M, Kvitka D, Juodžentė D, Gradeckienė A. MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). https://www.openveterinaryjournal.com/?mno=224153 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.44 AMA (American Medical Association) Style Plungytė I, Jankauskas M, Kvitka D, Juodžentė D, Gradeckienė A. MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). Open Vet. J.. 2025; 15(1): 478-481. doi:10.5455/OVJ.2025.v15.i1.44 Vancouver/ICMJE Style Plungytė I, Jankauskas M, Kvitka D, Juodžentė D, Gradeckienė A. MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 478-481. doi:10.5455/OVJ.2025.v15.i1.44 Harvard Style Plungytė, I., Jankauskas, . M., Kvitka, . D., Juodžentė, . D. & Gradeckienė, . A. (2025) MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). Open Vet. J., 15 (1), 478-481. doi:10.5455/OVJ.2025.v15.i1.44 Turabian Style Plungytė, Ieva, Martinas Jankauskas, Dmitrij Kvitka, Dalia Juodžentė, and Aistė Gradeckienė. 2025. MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). Open Veterinary Journal, 15 (1), 478-481. doi:10.5455/OVJ.2025.v15.i1.44 Chicago Style Plungytė, Ieva, Martinas Jankauskas, Dmitrij Kvitka, Dalia Juodžentė, and Aistė Gradeckienė. "MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta)." Open Veterinary Journal 15 (2025), 478-481. doi:10.5455/OVJ.2025.v15.i1.44 MLA (The Modern Language Association) Style Plungytė, Ieva, Martinas Jankauskas, Dmitrij Kvitka, Dalia Juodžentė, and Aistė Gradeckienė. "MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta)." Open Veterinary Journal 15.1 (2025), 478-481. Print. doi:10.5455/OVJ.2025.v15.i1.44 APA (American Psychological Association) Style Plungytė, I., Jankauskas, . M., Kvitka, . D., Juodžentė, . D. & Gradeckienė, . A. (2025) MRI diagnostics and management of idiopathic epilepsy in ring-tailed lemur (Lemur catta). Open Veterinary Journal, 15 (1), 478-481. doi:10.5455/OVJ.2025.v15.i1.44 |