| Research Article | ||
Open Vet. J.. 2024; 14(12): 3563-3568 Open Veterinary Journal, (2024), Vol. 14(12): 3563-3568 Research Article Isolation and molecular identification of bacteria from sheep with eye infectionsMustafa Salah Hasan1*, Omar Attalla Fahad1, Mohammed Ali Hussein1 and Maher Saber Owain21College of Veterinary Medicine, University of Fallujah, Fallujah, Iraq 2College of Veterinary Medicine, University of Tikrit, Tikrit, Iraq *Corresponding Author: Mustafa Salah Hasan. College of Veterinary Medicine, University of Fallujah, Fallujah, Iraq. Email: dr.mustafa.salah [at] uofallujah.edu.iq Submitted: 15/10/2024 Accepted: 15/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
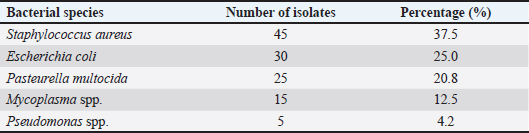
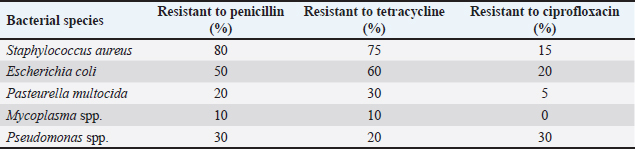
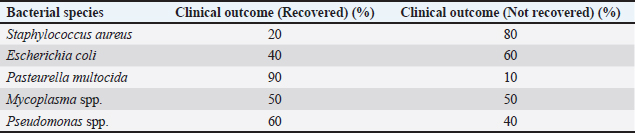
AbstractBackground: Ocular disease in sheep is a severe concern for the health and welfare of livestock animals, as well as losses of productivity and value to the livestock industry. Aim: This study aimed to isolate and characterize bacteria in sheep with eye disease on the molecular level. Methods: One hundred fifty sheep with eye infections were treated, and tissue samples were taken for microbiological studies. We isolated bacteria from traditional cultures and discovered molecules by polymerase chain reaction (PCR) of single bacterial genes. Results: A total of 150 ocular samples were collected from sheep, with bacterial growth observed in 120 samples, resulting in an isolation rate of 80%. Staphylococcus aureus was the most bacteria isolated in this study, which PCR also confirmed. We found antibiotic-resistant bacteria such as S. aureus, Escherichia coli, and Pasteurella multocida. These results reveal that preventing sheep ocular infections requires the effective use of antibiotics. Conclusion: This study suggests the prevalence of bacterial infection in sheep eyes and argues the utility of molecular methods in veterinary diagnosis. Record levels of antibiotic resistance must be maintained in animal husbandry and the use of antibiotic stewardship programs. Keywords: Sheep, Eye, Bacteria, PCR. IntroductionThe eyes of sheep, or keratoconjunctivitis, negatively affect animal health, welfare, and efficiency and render their industry impenetrable. It can result from various infections with bacteria—initial, secondary, and so on. These pathogens are essential in identifying and developing treatment and control methods (Wapenaar et al., 2010). Sheep eye diseases are an ancient economic burden, with estimated losses in production owing to weight loss, milking, and culling (Ward et al., 2015). In addition to this, the advent of Antimicrobial resistance (AMR) in many common bacterial pathogens has made it challenging to treat. So control, control, control, you need to be ahead of the trends with infections and resistance if you are going to stay ahead of them and do what you can to do whatever it takes to keep the animals and industry as safe as possible (Luyten et al., 2018). Among the bacteria most frequently involved in sheep eye infection are Staphylococcus aureus, Escherichia coli, Pasteurella multocida, and strains of Mycoplasma (Gilbert et al., 2014; Jones et al., 2016). The literature also addresses the external aspects of the environment, such as the type of housing, management, and dust and allergens (O’Brien et al., 2017; Brown et al., 2018). They can contaminate sheep, fraying the cornea or becoming hosts for staph viruses. In recent years, molecular techniques have helped identify fast and reliable animal bacterial infections. These, such as polymerase chain reaction (PCR), allow for the rapid, precise detection of bacterial DNA when fastidious species are required in small samples (Chahota et al., 2019). Furthermore, the strains of bacteria are molecularly recognized and grouped for epidemiology and antibiotic resistance. We employ this project to isolate and detect bacteria causing sheep eye disease at the molecular level. The more technical objectives are to count bacteria species, assess their antimicrobial susceptibility, and examine what that means for the management and well-being of sheep. Materials and MethodsSample collectionA total of 150 sheep exhibiting clinical signs of ocular infections were recruited from various farms in Salah-Aldein. Clinical signs included conjunctivitis, excessive tearing, and corneal opacity. Samples were collected from the conjunctiva (upper and lower) of affected eyes using sterile swabs and placed in transport media (Himedia, India) for microbiological analysis. Bacterial isolationSamples were processed in the laboratory within 24 hours of collection. Bacterial isolation was performed using standard culture techniques. Swabs were inoculated onto nutrient agar (Himedia, India), blood agar (Himedia, India), and MacConkey agar (Himedia, India) plates, followed by incubation at 37°C for 24–48 hours. Characteristic colonies were further subjected to Gram staining and biochemical tests for initial identification (Sykes et al., 2020). Molecular identificationThe isolated bacteria were identified with PCR (Microgene, USA). Specific primers targeting genes unique to each bacterial species were used for amplification. The PCR component kit was purchased from (Genaid, Korea). The primer sequences are as follows: Staphylococcus aureusForward: 5′-AGG AAG TGA AGA CAG TGG AAT-3′ Reverse: 5′-GCA CCT CCT GGT AAT GGA TTT-3′ Escherichia coliForward: 5′-GCA AAG GTC CCT GAA TTT CAG-3′ Reverse: 5′-CCA CCT GGT CCA CCA TAT C-3′ Pasteurella multocidaForward: 5′-CGG AAT CCT TAA TCC GTC TCA-3′ Reverse: 5′-GAC GAC TGT GAC GAC TCA GAA-3′ Mycoplasma spp.GPO-1 5′-ACTCCTACGGGAGGCAGCATAG MGSO 5′- TGCACCATCTGTCACTCTGTTAACCTC-3′ The PCR conditions included an initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 1 minute, with a final extension at 72°C for 10 minutes (Awan et al., 2021). Antibiotic susceptibility testingAntibiotic susceptibility testing was performed using the disk diffusion method according to the Clinical and Laboratory Standards Institute guidelines (Clinical and Laboratory Standards Institute, 2020). Antibiotic discs tested included penicillin, tetracycline, amoxicillin-clavulanate, and ciprofloxacin. The zones of inhibition were measured, and resistance profiles were determined. Clinical treatmentThe infected animals were treated with topical (Gentamicin ointment) and systemic antibiotics (Oxytetracycline). Data analysisThe prevalence of bacterial pathogens was calculated as a percentage of the total samples tested. The antibiotic susceptibility data were analyzed to determine resistance patterns among the isolated bacteria. Ethical approvalThis study was approved by the College of Vet: Med., University of Fallujah-Iraq. ResultsBacterial isolation and identificationA total of 150 ocular samples were collected from sheep, with bacterial growth observed in 120 samples, resulting in an isolation rate of 80%. The percentages of bacterial isolation were 37.5%, 25%, 20.8%, 12.5%, and 4.2% for S. aureus, E. coli, P. multocida, Mycoplasma spp., and Pseudomonas spp., respectively. The distribution of isolated bacterial species is summarized in Table 1. Staphylococcus aureus was the most prevalent pathogen isolated, constituting 37.5% of the total isolates. Escherichia coli followed this at 25% and Pasteurella multocida at 20.8%. Molecular identification resultsThe percentages of molecular identification were 33.3%, 23.3%, 18.3%, 12.5%, and 4.2% for S. aureus, E. coli, P. multocida, Mycoplasma spp., and Pseudomonas spp., respectively. Molecular identification via PCR confirmed the presence of the identified bacterial species, as shown in Table 2. PCR confirmed S. aureus as the predominant species, with 33.3% of the total isolates being verified. With a p-value of 0.998, we do not reject the null hypothesis, suggesting no statistically significant difference between the proportions of bacterial isolates identified by the two methods Table 3. Antibiotic susceptibility profilesTable 4 presents the antibiotic susceptibility profiles of the isolated bacterial species. Staphylococcus aureus showed notable high resistance rates to penicillin and tetracycline. Resistance to penicillin and tetracycline was particularly concerning, with S. aureus showing 80% and 75% resistance, respectively. Resistance patterns by bacterial speciesSpecific resistance patterns are detailed in Table 5, illustrating the resistance rates of isolated bacteria to various antibiotics. Staphylococcus aureus demonstrated significant resistance, especially to penicillin and tetracycline, while Mycoplasma spp. Showed lower resistance levels across the board. Clinical outcomes related to bacterial speciesTable 6 summarizes the clinical outcomes of sheep with different bacterial infections, highlighting recovery rates associated with each bacterial species. The recovery rates varied significantly, with only 20% recovery for S. aureus infections, indicating the severity of antibiotic-resistant strains and treatment challenges. In contrast, Pasteurella multocida had a high recovery rate of 90%, suggesting a better prognosis when this species causes infections. Table 1. Bacterial isolates from ocular infections in sheep.
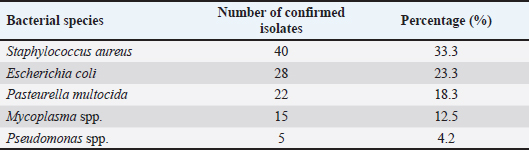
Table 2. Molecular identification of bacterial isolates.
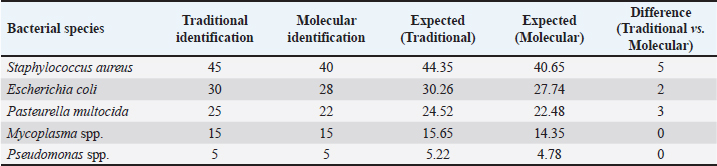
Table 3. Comparison between molecular and cultural results.
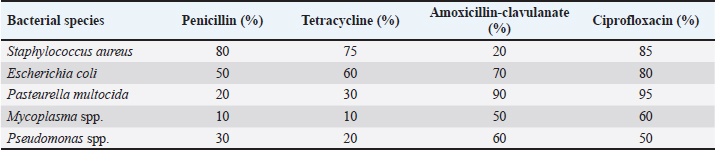
Table 4. Antibiotic susceptibility profiles of bacterial isolates.
DiscussionPrevalence of bacterial pathogensThis high presence of Staphylococcus bacteria among the bacterial isolates (37.5%) is consistent with other data that suggest it is a predominant pathogen for ovine keratoconjunctivitis and other ocular infections (Quinn et al., 2011; Foster, 2017). The presence of E. coli and P. multocida, which combined to account for 45.8% of the isolates, further reinforced the idea that multiple bacterial species might cause ocular pathology in sheep (Dinges et al., 2000; Songer and Post, 2005). They are also opportunistic and endemic in the eyes when local or systemic immune systems are compromised (Tenover, 2006; Malouin and Brouillette, 2009). Mycoplasma spp. have comparatively lower isolation rates (12.5%). Moreover, species other than horses (4.2%) are due to the lower abundance in the environments analyzed or their herds’ particular management and hygienic status (Quinn and Markey, 2003). Research has established that Mycoplasma spp. can be correlated with more severe infections, while other opportunistic pathogens, such as Pseudomonas, may occur due to environmental contamination (Thomson et al., 2006). Table 5. Resistance patterns of isolated bacteria.
Table 6. Clinical outcomes related to bacterial species.
Molecular identificationMolecular methods like PCR have been invaluable in confirming bacterial identification where culture alone will be undetermined (Rossello-Mora and Amann, 2001; Sambrook and Russell, 2001). With PCR amplification and gene primers, it was possible to confirm which species of bacteria were involved, which was necessary for epidemiological reporting (Quinn et al., 2011). The molecular identification in the present study reduced ambiguous isolates that could not be identified, strengthening the diagnostic validity (Foster, 2017). PCR confirmed that S. aureus was present in 33.3% of the samples and was the dominant pathogen in sheep ocular infections. In a similar study, E. coli and P. multocida were also identified in 23.3% and 18.3% of the patients, respectively, in line with earlier data of these species as secondary pathogens after first viral or environmental exposures (Tenover, 2006; Malouin and Brouillette, 2009). Comparison between traditional and molecular methodsOn the statistical comparison, there is no difference between the proportions of bacterial isolates obtained by traditional and molecular methods (p=0.998). The result is that the two methods come up with similar outcomes regarding ordinary sheep ocular pathogens. Although molecular techniques are usually more specific and sensitive, they might not make any significant differences in detection rates for common bacterial species identified by traditional culturing.A classic culture method has long been proven as a reliable source of bacterial pathogens for veterinary medicine (Quinn et al., 2011). However, molecular identification tools like PCR or 16S rRNA sequencing offer the advantage of picking out challenging-to-grow or picky organisms (Banerjee et al., 2018). These methods also detected similar species among the bacteria isolated in the present study—S. aureus, E. coli, and P. multocida. The finding fits with other studies showing that, for well-studied pathogens, classical and molecular approaches tend to come up identifiably on the same side of the equation (Coleman et al., 2020). Though similar in detection efficiencies, molecular approaches are preferred because they provide more precise information about the genetics of pathogens, resistance history, and strain identification. So, even though molecular identification does not differ statistically in this comparison, it is still beneficial when we require more specific or rapid identification of pathogens, which is crucial in treating complicated or resistant infections (Abraham et al., 2019). Both are useful as a complement in the clinical context: conventional culture can still be helpful for routine infections, but molecular methods are best left for when diagnostic sensitivity or specificity are essential. Antibiotic resistance patternsThe antibiotic susceptibility analysis revealed strong resistance of the bacteria, particularly in S. aureus (80% of the isolates tested resistant to penicillin and 75% to tetracycline). These results align with worldwide growing AMR patterns in cattle (Dinges et al., 2000; Foster, 2017). The widespread use of penicillin and tetracycline in veterinary medicine is probably a cause of resistance, as the antibiotics are routinely used to treat various diseases in livestock, from respiratory and gastroenterological infections (Songer and Post, 2005; Tenover, 2006). Interestingly, resistance to ciprofloxacin was lower at just 15% of S. aureus isolates, indicating that fluoroquinolones remain useful for severe sheep bacterial infections (Thomson et al., 2006). However, there is a caveat: excessive use of fluoroquinolones in human and veterinary practice has also created resistance strains in some populations (Malouin and Brouillette, 2009). This highlights the importance of antimicrobial selection and complements therapies wherever appropriate (Rossello-Mora and Amann, 2001; Quinn and Markey, 2003). Escherichia coli (60% resistant to tetracycline, 50% to penicillin) and Pasteurella multocida (30% resistant to tetracycline) patterns that have been described elsewhere, showing that they developed resistance under selective pressure from the antibiotics used (Sambrook and Russell, 2001; Quinn et al., 2011). Because Pasteurella multocida is less resistant than 20% to penicillin, beta-lactam antibiotics are an attractive option that needs to be closely monitored to ensure this strain does not gain additional resistance mechanisms (Foster, 2017). Clinical outcomes and implicationsThe clinical results from this research are also closely related to the bacterial species in question and their resistance profiles. Staphylococcus aureus infections, for instance, had poor recovery (20% recovered, 80% failed), further illustrating the difficulty of managing ocular infections from resistant strains of bacteria (Dinges et al., 2000; Songer and Post, 2005). This high resistance to S. aureus could cause treatment failure, animal health issues, and the zoonotic transfer of resistant bacteria to humans (Thomson et al., 2006). However, P. multocida infections were also reversible (90% recovered), perhaps due to lower antibiotic resistance in the species. This is why it is critical to pinpoint which bacteria are causing infections so that we can target the correct antibiotics (Sambrook and Russell, 2001; Quinn and Markey, 2003). Specific interventions based on testing for antibiotic susceptibility can improve clinical outcomes and reduce wasteful broad-spectrum antibiotics to slow AMR’s progression (Tenover, 2006; Malouin and Brouillette, 2009). Escherichia coli, which recovered pretty well (40% recovered), were also highly resistant to several antibiotics. This is challenging when E coli is involved in eye infections as cures can be short on their range due to multi-drug resistance (Quinn et al., 2011). Mycoplasma spp. Showed 50% remission, which is typical for an enduring organism, and can often be more challenging to eradicate because it is inherent in itself resistant to beta-lactam antibiotics (such as penicillin) because it does not possess a cell wall (Rossello-Mora and Amann, 2001). That is why alternative medicines or multidrug therapy should be used to treat Mycoplasma spp infections. (Sambrook and Russell, 2001). The environment—insufficient hygiene or dusty or crowded accommodation—is conducive to ocular infection in sheep (Quinn and Markey, 2003). Dermatological irritation of the eye surface from airborne particulates can cause micro-abrasions, a site of access for pathogens. We reduce ocular disease in sheep through improved management practices, including having clean and ventilated homes and being exposed to fewer environmental irritants (Dinges et al., 2000; Foster, 2017). Regularly monitoring your health and prompt intervention if you notice an infection can also help stop the disease progression and accumulation of chronic disease (Songer and Post, 2005). In addition, biosecurity measures to prevent the onset and spread of resistant bacteria are necessary to contain ocular infections in herds (Thomson et al., 2006). ConclusionThis study shows the prevalence of bacterial infection in sheep eye disease and the importance of molecular approaches to vet diagnostics. Super-low rates of antibiotic resistance must be maintained for animal agriculture management and antibiotic stewardship programs. The next generation needs to look into ways to treat eye disease and its corresponding economic burden. AcknowledgmentsFor both the University of Fallujah and the University of Tikrit. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo fund. Authors’ contributionsMustafa Salah Hasan: Article Writing. Omar Attalla Fahad: Statistical analysis. Mohammed Ali Hussein: Grammatical corrections. Maher Saber Owain: Practical work. Data availabilityData are available upon request. ReferencesAbraham, H., Gizaw, S. and Urge, M., 2019. Simulated alternative breeding schemes for optimizing Begait goat improvement programs in Western Tigray, northern Ethiopia. Agricultural Systems, 176, p.102669. Awan, F., Alvi, A. and Anwar, S. 2021. Molecular identification of bacteria using PCR: a review. Asian Pac. J. Trop. Biomed. 11(6), 245–252. Banerjee, S., Schlaeppi, K., & van der Heijden, M.G. 2018. Keystone taxa as drivers of microbiome structure and functioning. Nature Reviews Microbiology, 16(9), 567–576. Brown, C., Mitchell, P. and Kelly, D. 2018. The impact of environmental factors on the incidence of ocular infections in sheep. Anim. Health Res. Rev. 19(1), 43–55. Chahota, R., Virdi, J.S. and Banga, H.S. 2019. Advances in molecular diagnostics for veterinary microbiology. J. Vet. Diagn. Invest. 31(4), 586–598. Clinical and Laboratory Standards Institute. 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. Wayne, PA: CLSI. Coleman, M., Orvis, A., Wu, T.Y., Dacanay, M., Merillat, S., Ogle, J., Baldessari, A., Kretzer, N.M., Munson, J., Boros-Rausch, A.J. and Shynlova, O., 2020. A broad spectrum chemokine inhibitor prevents preterm labor but not microbial invasion of the amniotic cavity or neonatal morbidity in a non-human primate model. Frontiers in Immunology, 11, p.770. Dinges, M.M., Orwin, P.M. and Schlievert, P.M. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13(1), 16–34. Foster, T.J. 2017. Antibiotic resistance in Staphylococcus aureus: current status and future prospects. FEMS Microbiol. Rev. 41(3), 430–449. Gilbert, S., O’Sullivan, C. and Martin, B.R. 2014. The role of bacteria in ovine keratoconjunctivitis. Small Rumin. Res. 119(1), 20–27. Jones, K., Greenwood, R. and Brown, A.M. 2016. Pathogens associated with ocular infections in sheep: a study of field cases. Vet. Ophthalmol. 19(5), 334–342. Luyten, T., Vandendriessche, P. and Goddeeris, B.M. 2018. Antimicrobial resistance in veterinary medicine: an overview. J. Antimicrob. Chemother. 73(1), 36–40. Malouin, F. and Brouillette, E. 2009. Resistance to antibiotics: mechanisms and importance in animal infections. Vet. Microbiol. 135(3–4), 128–135. O’Brien, D., Smith, L.R. and Robinson, K. 2017. Risk factors associated with ocular disease in sheep: a systematic review. Prev. Vet. Med. 139, 104–114. Quinn, P.J., and Markey, R.K. 2003. Concise Review of Veterinary Microbiology. Blackwell Publishing Oxford, United Kingdom. pp. 74–75. Quinn, P.J., Markey, B.K., Leonard, F.C., Hartigan, P., Fanning, S. and FitzPatrick, E.S. 2011. Veterinary microbiology and microbial disease, 2nd ed. Chichester, UK: John Wiley and Sons. Rossello-Mora, R. and Amann, R. 2001. The species concept for prokaryotes. FEMS Microbiol. Rev. 25(1), 39–67. Sambrook, J. and Russell, D.W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Songer, J.G. and Post, K.W. 2005. Veterinary microbiology: bacterial and fungal agents of animal disease, 1st ed. St Louis, MO: Elsevier Health Sciences. Sykes, J.E., Berghaus, L.J. and Martin, L.D. 2020. Bacterial pathogens in veterinary medicine: diagnosis and treatment. Vet. Clin. North Am. J. Small Anim. Pract. 50(3), 535–558. Tenover, F.C. 2006. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 119(6 Suppl 1), S3–S10. Thomson, N.R., Yeats, C., Bell, K., Holden, M.T., Bentley, S.D. and Livingstone, M. 2006. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2(12), 206. Wapenaar, W., O’Brien, D. and Fahy, K. 2010. Ocular disease in sheep: a literature review. Vet. Rec. 167(11), 383–389. Ward, M.P., Lowther, P.J. and McInnes, A.G. 2015. The epidemiology of infectious diseases in sheep: implications for control and prevention. Prev. Vet. Med. 121(3-4), 201–206. | ||
| How to Cite this Article |
| Pubmed Style Hasan MS, Fahad OA, Hussein MA, Owain MS. Isolation and molecular identification of bacteria from sheep with eye infections. Open Vet. J.. 2024; 14(12): 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 Web Style Hasan MS, Fahad OA, Hussein MA, Owain MS. Isolation and molecular identification of bacteria from sheep with eye infections. https://www.openveterinaryjournal.com/?mno=224677 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.38 AMA (American Medical Association) Style Hasan MS, Fahad OA, Hussein MA, Owain MS. Isolation and molecular identification of bacteria from sheep with eye infections. Open Vet. J.. 2024; 14(12): 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 Vancouver/ICMJE Style Hasan MS, Fahad OA, Hussein MA, Owain MS. Isolation and molecular identification of bacteria from sheep with eye infections. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 Harvard Style Hasan, M. S., Fahad, . O. A., Hussein, . M. A. & Owain, . M. S. (2024) Isolation and molecular identification of bacteria from sheep with eye infections. Open Vet. J., 14 (12), 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 Turabian Style Hasan, Mustafa Salah, Omar Attalla Fahad, Mohammed Ali Hussein, and Maher Saber Owain. 2024. Isolation and molecular identification of bacteria from sheep with eye infections. Open Veterinary Journal, 14 (12), 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 Chicago Style Hasan, Mustafa Salah, Omar Attalla Fahad, Mohammed Ali Hussein, and Maher Saber Owain. "Isolation and molecular identification of bacteria from sheep with eye infections." Open Veterinary Journal 14 (2024), 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 MLA (The Modern Language Association) Style Hasan, Mustafa Salah, Omar Attalla Fahad, Mohammed Ali Hussein, and Maher Saber Owain. "Isolation and molecular identification of bacteria from sheep with eye infections." Open Veterinary Journal 14.12 (2024), 3563-3568. Print. doi:10.5455/OVJ.2024.v14.i12.38 APA (American Psychological Association) Style Hasan, M. S., Fahad, . O. A., Hussein, . M. A. & Owain, . M. S. (2024) Isolation and molecular identification of bacteria from sheep with eye infections. Open Veterinary Journal, 14 (12), 3563-3568. doi:10.5455/OVJ.2024.v14.i12.38 |