| Research Article | ||
Open Vet. J.. 2025; 15(1): 428-436 Open Veterinary Journal, (2025), Vol. 15(1): 428-436 Research Article Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in ratsPutri Reno Intan1,2, Sukmayati Alegantina3, Hidayatul Fajri2,4, Fitrine Ekawasti5, Ani Isnawati3, Lisa Andriani Lienggonegoro2, Uly Alfi Nikmah2, Sunarno Sunarno2, Sela Septima Mariya2, Lina Noviyanti Sutardi6, Agus Setiyono7 and Ekowati Handharyani7*11Animal Biomedical Study Program, IPB Postgraduate School, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, West Java, Indonesia 2Center for Biomedical Research, Research Organization for Health, National Research and Innovation Agency (BRIN), Cibinong Science Center, Bogor, Indonesia 3Research Center for Pharmaceutical Ingredients and Traditional Medicine, Research Organization for Health, National Research and Innovation Agency (BRIN), Cibinong Science Center, Bogor, Indonesia 4Department of Biology, Faculty of Biology and Agriculture, Universitas Nasional, Jakarta, Indonesia 5Research Centre for Veterinary Science, Research Organization for Health, National Research and Innovation Agency, Bogor, Indonesia 6Division of Pharmacy, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, West Java, Indonesia 7is Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, West Java, Indonesia *Corresponding Author: Ekowati Handharyani. Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia. Email: ekowatieko [at] apps.ipb.ac.id Submitted: 21/10/2024 Accepted: 30/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
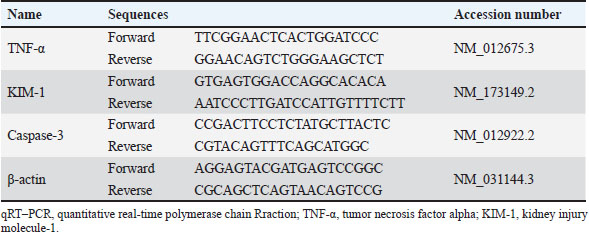
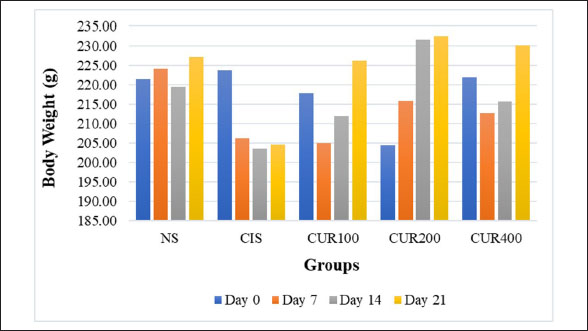
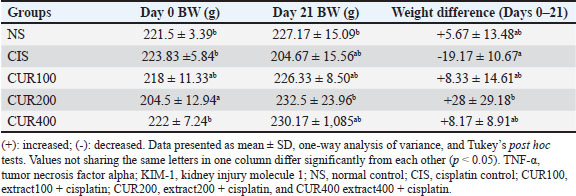
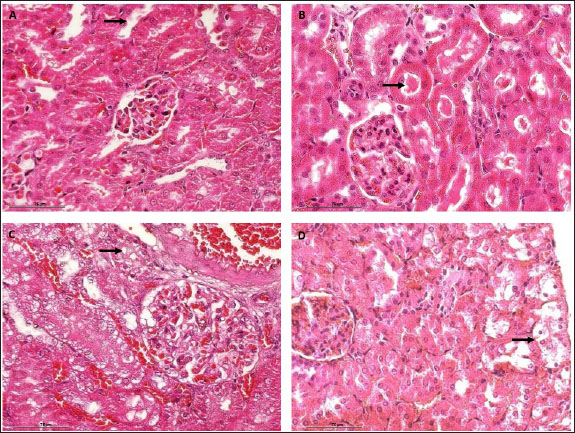
AbstractBackground: Cisplatin (CIS) is a highly effective chemotherapeutic drug. However, it is associated with various side effects, including kidney damage, due to its nephrotoxic properties. Aim: This study aimed to evaluate the renoprotective potential of the combined extract of Curcuma longa and Curcuma zedoaria in reducing nephrotoxicity by examining its effects on tumor necrosis factor-alpha (TNF-α), KIM-1, and caspase-3 levels. Methods: Twenty-five rats were divided into normal control groups (NS), CIS control groups, and three treatment groups that received doses of the combined extract at 100, 200, and 400 mg/kg (CUR100, CUR200, and CUR400), respectively, on day 1–20. All groups, except the NS group (receiving normal saline i.p.), received intraperitoneal CIS (1 mg/kg) on days 7 and 14 of the 20-day extract treatment. Results: Compared with the rats in the CIS group, rats given the combined extract had a considerable gain in body weight and decreased TNF-α, KIM-1, and caspase-3 expression levels. Histopathological examination revealed that the extract group experienced less kidney damage than the CIS group. The combined extract, administered at 200 mg/kg, exerted the most apparent protective effect, decreasing renal TNF-α, KIM-1, and caspase 3. Conclusion: The combined extract of C. longa and C. zedoaria has the potential to be a therapeutic agent for reducing nephrotoxicity by suppressing TNF-α, KIM-1, and caspase-3 levels. Further research is required to determine the potential of this combination therapy in humans. Keywords: Cisplatin, Curcuma longa, Curcuma zedoaria, Inflammation, Nephrotoxicity. IntroductionCurcuma longa, also known as turmeric, is indigenous to India, Indonesia, and South Asia. Numerous conditions involving endocrine, neurological, cardiovascular, digestive, pulmonary, renal, and other systems can be reversed by curcumin (Mantzorou et al., 2018). The Zingiberaceae family member Curcuma zedoaria tends to be referred to as ‟white turmeric” or ‟Zedoary” and contains various plants that are frequently used in traditional treatments (Sharifi-Rad et al., 2017). Curcumin has been isolated from C. longa and C. zedoaria. It also has a broad spectrum of pharmacological activities, including anti-inflammatory, antioxidant, antibacterial, and antineoplastic effects. Considerable scientific interest has been shown in the study of Zhang et al., 2023. Although C. longa and C. zedoaria have anti-inflammatory properties, much more must be learned about their combined actions and underlying molecular pathways to mitigate renal damage caused by cisplatin (CIS). We examined whether these plant anti-inflammatory qualities could lessen the well-known nephrotoxic adverse effects of CIS. The chemotherapy drug CIS (cis-diamminedichloroplatinum II) was administered. Nevertheless, substantial adverse effects, especially nephrotoxicity, accompanying kidney deposits, and CIS biotransformation limit its therapeutic efficacy. Nephrotoxicity is the main adverse effect affecting dosage and is a significant clinical issue, highlighting the significance of studies on the prevention of CIS-induced kidney damage (Volarevic et al., 2019). Apoptosis and inflammation are critical components of the pathophysiology of renal damage caused by CIS. Inflammation is the primary cause of kidney damage caused by CIS. Studies have demonstrated that CIS increases tumor necrosis factor-alpha (TNF-α) levels. Several studies have shown that anti-inflammatory drugs can inhibit CIS-induced inflammation (Ramesh and Brian Reeves, 2002). It has been hypothesized that DNA damage, decreased protein synthesis, and mitochondrial damage leading to apoptosis are the main causes of the adverse effects of CIS. Apoptosis, the primary cause of inflammation, has been linked to several kidney diseases caused by nephrotoxic medications (Santos et al., 2007). Current strategies for mitigating CIS-induced nephrotoxicity are frequently inadequate because of their limited effectiveness, unfavorable side effects, and inability to specifically target the pathways causing kidney damage. Thus, the development of innovative treatment approaches is urgently required. Numerous studies have examined the effects of these difficulties on CIS-induced nephrotoxicity. This study aimed to evaluate the renoprotective potential of the combined extract of C. longa and C. zedoaria in reducing nephrotoxicity by examining its effects on TNF-α, KIM-1, and caspase-3 levels. Materials and MethodsPlant material and extract preparationWe obtained and confirmed taxonomically authenticated rhizomes of C. longa and C. zedoaria (document number 306/IT3. L. P13/TA.00.03/M/B/2023, No. 305/IT3. L. P13/TA.00.03/M/B/2023) from the Tropical Biopharmaca Research Center of IPB University, Bogor, West Java, Indonesia. The extraction process for Simplicia used the maceration method with pharmaceutical-grade 96% ethanol for three consecutive periods of 24 hours each. A 1:10 sample-to-solvent ratio was established (Kemenkes, 2017), and extracts from C. longa and C. zedoaria were combined in equal amounts to create a 1:1 blend. CIS injection preparationCIS was purchased from PT. Dankos Farma, Indonesia. Each vial containing 50 mg/50 ml of water is included in each vial. The amount of CIS administered was determined based on the animal’s body weight (BW). CIS (1 mg/kg) was added without dilution. Animal and experimental designThe rats were procured from the PT. Biofarma, Indonesia. The rats were housed in an animal care facility with a 12-hour day-and-night cycle before the investigation, standard feed, unlimited access to water, temperature (20°C–25°C), and humidity levels (40%–70%) for 1 week to allow them to become acclimated to their environment. Twenty-five male Wistar albino rats were aged 12 weeks (weight range: 175–200 g). Five random groups (n=5 each) of rats were established: normal control (NS), CIS control, extract100 + CIS (CUR100), extract200 + CIS (CUR200), and extract400 + CIS (CUR400). The rats in the extract-treated groups were orally administered 100, 200, and 400 mg/kg of the combined extract of C. longa and C. zedoaria every day from day 1 to 20. Similarly, normal saline was administered to the NS and CIS groups. Two doses (1 mg/kg BW in saline solution) of CIS-induced kidney damage were administered at 7-day intervals (on days 7 and 14) and injected intraperitoneally (i.p.) in all groups, except the NS group, which received an intraperitoneal injection of saline. The dose of 1 mg/kg CIS and extracts was based on a previous study (John et al., 2009; El-Waseif et al., 2022) with modifications. The rats were administered a mixture of ketamine (80 mg/kg BW) and xylazine (10 mg/kg BW) on the 21st day of the experiment to induce their unconsciousness. The left kidney was stored at 80°C until molecular analysis, whereas the right kidney was maintained in 10% buffered neutral formalin for histological evaluation. Body and renal weightEach rat’s BW was measured at four time points: days 0, 7, 14, and 21 (final day) when the rat was sacrificed. The weight of each kidney was measured after kidney removal, divided by the weight of the body (g), and then multiplied by 100 (Nosrati et al., 2021). Histological examinationThe kidney tissue was cut into 5 µm slices and stained with hematoxylin and eosin (H&E). Hematoxylin and eosin (H&E) staining was performed by deparaffinizing the kidney tissue sections with xylol, followed by rehydration using graded ethanol. Preparations were stained by immersing them in Mayer’s H&E. The tissues were then dehydrated using absolute and 96% ethanol. The tissue was immersed in xylol for cleaning. Glass cover and gum attachment were the final steps in the observation of histopathology (Etriwati et al., 2023). An Olympus BX-51 light microscope (Olympus Corp., Tokyo, Japan) was used for inspection. Blinded, 200x magnification images of the renal cortical regions were acquired to assess the results. RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR) assayThe kidney tissue samples were processed for RNA extraction using a Tiangen Biotech kit (product number: 4992858). The RNA concentration was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific). Then, 10 μl of entire RNA was transferred to cDNA using the Sisco Research Laboratories cDNA Synthesis Kit (94837) according to the manufacturer’s instructions. qRT-PCR was performed using an Applied Biosystems 7500 Fast qRT-PCR system (Applied Biosystems, USA) to assess the expression levels of TNF-α, caspase 3, and KIM-1 RNA. The PCR mixture comprised 2 μl of cDNA, 100 ng of both forward and reverse primers, and 10 μl the KAPA SYBR Fast rox low qPCR kit (KK4619) from Kapa Biosystems (MA, USA) and nuclease-free water to a final volume of 20 μl. The cycling steps of PCR amplification were as follows: initial denaturation (95°C, 3 minute), with subsequent 40 cycles of amplification, denaturation (95°C, 5seconds), and annealing (57°C, 30 seconds). The procedure was performed in triplicate for each sample. The relative expression was calculated as follows: delta-Ct=Average Ct (ERCC1) − Average Ct (ACTB), Relative Expression=2−deltaCt (Takemoto et al., 2019). Table 1 lists the primer sequences for the target genes and the housekeeping gene, β-actin, in rats. Statistical data analysisThe body and relative kidney weights are presented as averages with standard deviations. A one-way ANOVA followed by Tukey’s post hoc test was used to assess the importance of data disparities and determine p-values. Statistical significance was set at p < 0.05. For quantitative real-time RNA expression analysis, data are presented as means accompanied by standard errors of the mean (SEM). Data analysis was performed using SPSS software (version 29.0) running on a Windows environment. Ethical approvalThe Maintenance and Ethics Commission for Animal Use of Indonesia’s National Research and Innovation Agency approved the animal experiments (Approval Number: 087/KE.02/SK/05/2023). Each step followed the recommendations of the Manual for Laboratory Animal Care and Use. ResultsThe combination of extract affected body and renal weight in CIS -treated ratsThe BW of each rat was measured at four time points: day 0, 7, 14, and 21 (Fig. 2). All groups experienced fluctuations in BW. The measured data show a decrease in BW over the observation period in the CIS group due to CIS injection (Fig. 2). In the measurement of the difference in BW at the beginning and end of the study, the CUR200 group experienced significant weight gain compared with the CIS group, wich experienced weight loss (Table 2). The kidney weight index did not differ significantly among the study groups (Fig. 3). The results of this study indicate that the combination of C. longa and C. zedoaria extracts can reduce the negative impact of CIS on BW, which is consistent with the anticipated protective effect. Histopathological resultRat kidney photomicrographs from this study are shown in Figure 4; the tubules in the NS group appeared normal and showed no histological alterations. The tubules in the cortex had a standard histological structure (H&E, × 400). H&E staining revealed tubular epithelial cell alterations in the CIS group, including areas exhibiting enlarged tubules with thinning of the epithelial cell layer (Fig. 4A), hyaline casts (Fig. 4B) within several proximal tubule lumens, cellular degradation (Fig. 4C) characterized by fluid-filled spaces, and localized tubular necrosis (Fig. 4D). Rats administered a combined extract of C. longa and C. zedoaria at 100 mg/kg BW (CUR100 group) for 20 days and 1 mg/kg of CIS intraperitoneally on days 7 and 14 experienced moderate proximal tubule alterations. However, only slight changes were observed in the CUR200 and CUR400 groups. Rats treated with the combined extract exhibited less structural damage on histological examination than rats treated with the CIS extract alone. Rats administered a combined extract at 100, 200, or 400 mg/kg BW (respectively) did not exhibit substantial pathological alterations in their kidneys. Table 1. qRT–PCR test primer sequences.
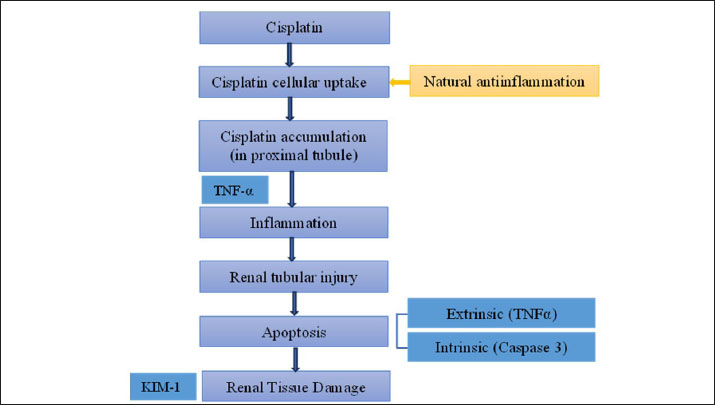
Fig. 1. Pathophysiological mechanisms underlying CIS-induced kidney damage. A pathophysiological diagram illustrates the primary molecular mechanisms involved in CIS-induced kidney damage. Although the interplay between these key pathways remains unclear, the accumulation of platinum in renal tissue is believed to be the main cause of CIS’s harmful nephrotoxic effects. CIS accumulation leads to enhanced production of TNF-α (Ramesh and Brian Reeves, 2002; Zhang et al., 2007), which in turn promotes inflammatory responses (Liu et al., 2020) and activates apoptotic cascades (Ni et al., 2019). These apoptotic processes subsequently damage kidney tissue, resulting in the characteristic clinical signs of nephrotoxicity. TNF-α, tumor necrosis factor alpha; KIM-1, kidney injury molecule 1.
Fig. 2. The effects of the combined extract of C. longa and C. zedoaria on BW were assessed weekly from day 0 to 21 of the trial. TNF-α, tumor necrosis factor alpha; KIM-1, kidney injury molecule 1; NS, normal control; CIS, cisplatin control; CUR100, extract100 + cisplatin; CUR200, extract200 + cisplatin, and CUR400 extract400 + cisplatin. Table 2. Effect of combined extract of C. longa and C. zedoaria administration on the CIS–induced rats’ BW in a treatment group.
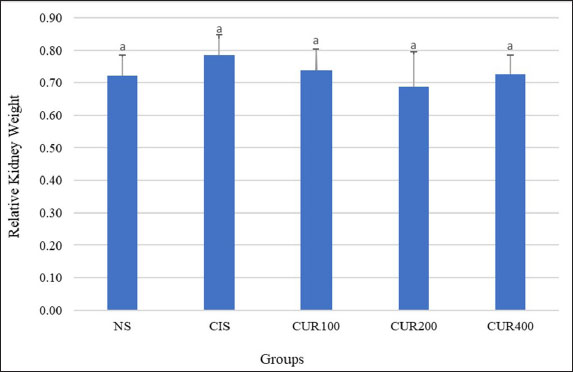
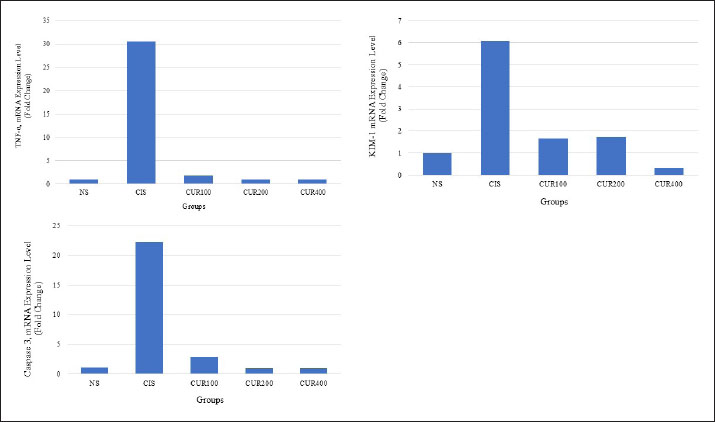
Fig. 3. The combined extracts of C. longa and zedoaria affect the relative kidney weight. Significant differences are indicated by bars with different superscript characters (p < 0.05). TNF-α, tumor necrosis factor alpha; KIM-1, kidney injury molecule 1; NS, normal control; CIS, cisplatin control; CUR100, extract100 + cisplatin; CUR200, extract200 + cisplatin, and CUR400 extract400 + cisplatin. qRT–PCR resultsTo evaluate and compare the results, qRT–PCR was performed by measuring changes in the gene expression cycle thresholds of TNF-α, KIM-1, and caspase 3. The results showed that the CIS group exhibited an increased mRNA expression of these markers compared with the NS group. However, as shown in Fig. 5A–C, the groups treated with the combined extract of C. longa and C. zedoaria showed significantly reduced expression levels, with the most pronounced decreases observed in the CUR200 and CUR400 groups. DiscussionThis study investigated the potential protective effects of combined C. longa and C. zedoaria extracts on rat kidneys against CIS-induced damage. This investigation focused on examining TNF-α, KIM-1, and caspase 3 markers to assess the efficacy of this herbal combination in mitigating the adverse renal effects of CIS. A pathophysiological diagram illustrates the primary molecular mechanisms involved in CIS-induced kidney damage (Fig. 1). The combined extract was administered in multiple doses for 20 days in a row, with the first dose of CIS administered on the seventh day and subsequent intraperitoneal injection of CIS (1 mg/kg) administered 1 week later.
Fig. 4. Photomicrographs of H&E-stained kidney sections from rats treated with C. longa and C. zedoaria extracts after CIS-induced nephrotoxicity. (A). Enlarged tubules with thinning of the epithelial cell layer (black arrow), (B) hyaline casts (black arrow), (C) cellular degradation (black arrow) characterized by fluid-filled spaces, and (D) localized tubular necrosis (black arrow). Bars=75 μ. The administration of C. longa and C. zedoaria resulted in BW fluctuations, with an overall upward trend observed at the end of the experiment. However, the NS group exhibited continuous weight gain throughout the study period. In contrast, the CIS group consistently decreased in weight every week until the end of the experiment (Fig. 2). These results are consistent with those of previous studies in which repeated i.p. administration of CIS 1 mg was associated with weight loss, reduced kidney weight, and increased kidney weight/BW ratio (El-Waseif et al., 2022). Changes in organ and BW are commonly used to indicate potential harmful effects in toxicological studies (Shafaei et al., 2015). Decreased renal tubular cell counts may contribute to decreased BW (Basile et al., 2012). The capacity of the manifold to absorb water may be diminished by renal tubular cell necrosis, which increases the amount of fluid lost in urine (Corman et al., 1981). By identifying inflammatory mediators such as TNF-α, CIS causes an inflammatory response in kidney tubular cells, as shown in both in vivo and in vitro studies (Domitrović et al., 2013; Teng et al., 2015). Studies have demonstrated the important roles of renal cell apoptosis and programmed cell death in CIS-induced acute kidney damage (Jin et al., 2020). Our findings revealed high TNF-α, KIM-1, and caspase 3 expression in the CIS group. At the same time, the administration of 200 mg/kg BW after two injections of CIS administered 7 days apart reduced the expression of TNF-α, caspase 3, and KIM-1, as the combined extract of C. longa and C. zedoaria effectively countered these changes. The results align with previous research demonstrating that TNF-α plays a crucial role in tubulointerstitial injury, CIS-induced nephrotoxicity, acute kidney injury (AKI), and chronic kidney disease in animal models, which is associated with elevated levels of TNF-α (Ramesh and Brian Reeves, 2002; Pabla and Dong, 2008; El-Waseif et al., 2022; Widowati et al., 2022). The well-known anti-inflammatory properties of the combined extract components may explain why the combined extract of C. longa and C. zedoaria fully or partially restored TNF-α levels to baseline (Pan et al., 2019). Renal epithelial cells, rather than immune cells, can generate TNF-α in urine and blood after CIS treatment. TNF-α also produces reactive oxygen species, triggering nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) (Zhang et al., 2007). Subsequently, this transcription factor produces pro-inflammatory cytokines, such as TNF-α. TNF-α is essential for nephrotoxicity caused by CIS (Ramesh and Brian Reeves, 2002).
Fig. 5. Kidney quantitative qRT-PCR histogram of TNF-α (A), KIM-1 (B), and caspase 3 (C) gene expression in the rats treated with C. longa and C. zedoaria extracts after CIS-induced nephrotoxicity. The CIS group had significantly higher TNF-α, caspase 3, and KIM-1 expression levels than the control group. Furthermore, compared with the CIS group, all groups treated with the combined extract showed reduced TNF-α, cCaspase 3, and KIM-1 expression. The data (n=5) are presented as the mean ± SEM. TNF-α, tumor necrosis factor alpha; KIM-1, kidney injury molecule 1; NS, normal control; CIS, cisplatin control; CUR100, extract100 + cisplatin; CUR200, extract200 + cisplatin, and CUR400 extract400 + cisplatin. The regulation of kidney cell populations during the development and progression of renal disease is significantly influenced by apoptosis (Havasi and Borkan, 2011). Numerous nephrotoxins can induce apoptosis in AKI. CIS, a commonly used drug, as well as other anticancer therapies, can result in severe kidney toxicity, which restricts their therapeutic effectiveness (Pabla and Dong, 2008; Miller et al., 2010). The findings of this study revealed that caspase-3 expression was highest in the CIS (CIS) group and lowest in the extract-treated group. KIM-1 is a biomarker of kidney injury in renal epithelial cells with proximal tubule damage (Li et al., 2019; Siddiqui et al., 2019). The current study demonstrated an increase in KIM-1 concentration in the CIS group. This increase could be mitigated by introducing a combined extract of C. longa and C. zedoaria, with particularly effective outcomes observed at 400 mg/kg twice daily. The proximal tubules exhibited a higher frequency of cytoplasmic and nuclear degenerative alterations than the distal tubules. This difference can be explained by the fact that the proximal tubules are the primary locations for reabsorption and active transport processes (Curthoys and Moe, 2014). Hyaline casts are formed from altered tubular epithelial cells that undergo molecular changes and contain cellular debris. The formation of these casts occurs when loose cells and fragments from the tubular basement membrane combine with proteins found in the lumen of the tubule. In addition, injury to the tubular epithelium leads to decreased sodium reabsorption, resulting in increased sodium levels in the tubule lumen. Elevated sodium concentrations facilitate protein polymerization and contribute to cast formation (Abuelo, 2007). The preventive effect of the combined extract of C. longa and C. zedoaria at 200 mg/kg BW, which considerably reduced tubular degeneration caused by CIS injection, was confirmed by histological testing. Clinically, CIS dosage can cause varying degrees of nephrotoxicity. Renal failure, which cannot be reversed, may occur in patients undergoing repeated therapy sessions (Cornelison and Reed, 1993). Furthermore, studies on drug behavior in the body have shown that the primary factors contributing to kidney damage are prolonged buildup of CIS in the renal tissue and its extensive distribution throughout the body (Ibrahim et al., 2019). This study revealed a correlation between the dosage of combined extracts and histological changes in CIS-induced nephrotoxicity. The combined extracts mitigated damage to epithelial cells within the proximal tubules of the kidneys. As CIS concentrates in renal tissue, it causes epithelial cell death, leading to their gradual detachment and formation of protein-rich casts (Yang et al., 2018). ConclusionIn conclusion, the above data indicated that the combined extract dose had beneficial renoprotective effects against CIS-induced nephrotoxicity. Its action may involve by suppressing TNF-α, KIM-1, and caspase-3 levels. AcknowledgmentsThe authors thank Indonesia’s National Research and Innovation Agency for funding this study. Conflict of interestThe authors declare no conflicts of interest. FundingThis study received funding from the National Research and Innovation Agency (BRIN) of Indonesia as part of its Research Projects Program for the 2023 Fiscal Year. Authors contributionsPRI, SA, HF, FE, AI, LAL, UAN, SS, SSM, LNS, AS, and EH developed the concepts and designs. PRI, SA, HF, SSM, LNS, AS, and EH contributed to the data analysis/interpretation. PRI, SA, HF, FE, AI, LAL, UAN, SS, SSM, LNS, AS, and EH drafted the manuscript. PRI, SA, HF, FE, AI, LAL, UAN, SS, SSM, LNS, AS, and EH contributed to the critical revision of the manuscript. PRI, SSM, LNS, AS, and EH contributed to the statistical analysis. PRI, HF, FE, SSM, LNS, AS, and EH contributed to admin, technical, or material support. PRI, SSM, LNS, AS, and EH supervised this study. PRI, SA, HF, FE, AI, LAL, UAN, SS, SSM, LNS, AS, and EH contributed to the final approval. Data availabilityAll data required to substantiate the findings of this research are included in the manuscript. ReferencesAbuelo, J.G. 2007. Normotensive ischemic acute renal failure. N. Engl. J. Med. 357(8), 797–805 Basile, D.P., Anderson, M.D. and Sutton, T.A. 2012. Pathophysiology of acute kidney injury. Compr. Physiol. 2, 1303–1353. Corman, B., Roinel, N. and De Rouffignac, C. 1981. Water reabsorption capacity of the proximal convoluted tubule: a microperfusion study on rat kidney. J. Physiol. 316, 379–392. Cornelison, T.L. and Reed, E. 1993. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol. Oncol. 50(2), 147–158. Curthoys, N.P. and Moe, O.W. 2014. Proximal tubule function and response to acidosis. Clin. J. Am. Soc. Nephrol. 9(9), 1627–1638. Domitrović, R., Cvijanović, O., Pugel, E.P., Zagorac, G.B., Mahmutefendić, H. and Škoda, M. 2013. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice tby inhibitinghrough inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 310, 115–123. El-Waseif, E.G., Sharawy, M.H. and Suddek, G.M. 2022. The modulatory effect of sodium molybdate against cisplatin-induced CKD: Role of TGF-β/Smad signaling pathway. Life Sci. 306, 120845. Etriwati, E., Agungpriyono, D.R., Setiyaningsih, S., Darniati, D., Ak, D.M. Erwin, E. and Handharyani, E. 2023. Comparative pathological and immunohistochemical analysespathology and immunohistochemistry of Newcastle disease in domestic chickenschicken (Gallus-gallus domesticus) and Alabio ducksduck (Anas platyrhynchos Borneo). Open Vet. J. 13, 433–442. Havasi, A. and Borkan, S.C. 2011. Apoptosis and acute kidney injury. Kidney Int. 80(1), 29–40. Ibrahim, M.E., Chang, C., Hu, Y., Hogan, S.L., Mercke, N., Gomez, M., O’Bryant, C.L., Bowles, D.W., George, B., Wen, X., Buckley, B., Aleksunes, L. and Joy, M.S. 2019. Pharmacokinetic determinants of cisplatin-induced subclinical kidney injury in oncology patients. Eur. J. Clin. Pharmacol. 75, 51–57. Jin, X., An, C., Jiao, B., Safirstein, R.L. and Wang, Y. 2020. AMP-activated protein kinase contributes to cisplatin-induced renal epithelial cell apoptosis and acute kidney injury. Am. J. Physiol. Ren. Physiol. 319, F1073–F1080. John, SouthS., Nikhil, S., Yaswanth, J., Bhaskar, A., Amit, A. and Sudha, S., 2009. Analgesic propertiesproperty of different extracts of Curcuma longa (Linn.): an experimental study in animals. J. Nat. Remedies 9, 116–120. Kemenkes, R.I. 2017. Farmakope herbal Indonesia, 2nd ed. Jakarta, Indonesia: Kementerian Kesehatan, R.I. Li, Y.F., Xu, B.Y., An, R., Du, X.F., Yu, K., Sun, J.H., Zhang, G.H., Wang, W., An, L.P. and Wu, G.L. 2019. Protective effect of anisodamine in rats with glycerol-induced acute kidney injury. BMC Nephrol. 20, 1–14. Liu, P., Li, X., Lv, W., Xu, Z. 2020. Inhibition of CXCL1-CXCR2 axis ameliorates cisplatin-induced acute kidney injury by mediating inflammatory response. Biomed. Pharmacother. 122, 109693. Mantzorou, M., Pavlidou, E., Vasios, G., Tsagalioti, E. and Giaginis, C. 2018. Effects of curcumin consumption on human chronic diseases: a narrative review of the most recent clinical data. Phyther. Res. 32, 957–975. Miller, R.P., Tadagavadi, R.K., Ramesh, G. and Reeves, W.B. 2010. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2, 2490–2518. Ni, J., Hou, X., Wang, X., Shi, Y., Xu, L., Zheng, X., Liu, N., Qiu, A., Zhuang, S. 2019. 3-deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression. Cell Death Dis. 10. Nosrati, H., Hamzepoor, M., Sohrabi, M., Saidijam, M., Assari, M.J., Shabab, N., Gholami Mahmoudian, Z. and Alizadeh, Z. 2021. The potential renal toxicity of silver nanoparticles after repeated oral exposure and its underlying mechanisms. BMC Nephrol. 22(1), 228. Pabla, N. and Dong, Z. 2008. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007. Pan, P., Huang, Y.W., Oshima, K., Yearsley, M., Zhang, J., Arnold, M., Yu, J. and Wang, L.S. 2019. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit. Rev. Food Sci. Nutr. 59(6), 992–1007. Ramesh, G. and Brian Reeves, W. 2002. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110, 835–842. Santos, N.A.G., Catão, C.S., Martins, N.M., Curti, C., Bianchi, M.L.P. and Santos, A.C. 2007. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state imbalance, energetic metabolism impairment, unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch. Toxicol. 81, 495–504. Shafaei, A., Esmailli, K., Farsi, E., Aisha, A.F.A., Abul Majid, A.M.S. and Ismail, Z. 2015. Genotoxicity, acute and subchronic toxicity studies of nano liposomes of Orthosiphon stamineus ethanolic extract in Sprague Dawley rats. BMC Complement. Altern. Med. 15, 1–14. Sharifi-Rad, M., Varoni, E.M., Salehi, B., Sharifi-Rad, J., Matthews, K.R., Ayatollahi, S.A., Kobarfard, F., Ibrahim, S.A., Mnayer, D., Zakaria, Z.A. and Sharifi-Rad, M. 2017. Plants of the genus Zingiber as a source of bioactive phytochemicals: from tradition to pharmacy. Molecules 22(12), 2145. Siddiqui, R.A., Simjee, S.U., Kabir, N., Ateeq, M., Shah, M.R. and Hussain, S.S. 2019. N-(2-hydroxyphenyl)acetamide and its gold nanoparticle conjugation prevent glycerol-induced acute kidney injury by attenuating inflammation and oxidative injury in mice. Mol. Cell. Biochem. 450, 43–52. Teng, Z.Y., Cheng, X.L., Cai, X.T., Yang, Y., Sun, X.Y., Xu, J. Di, Lu, W.G., Chen, J., Hu, C.P., Zhou, Q., Wang, X.N., Li, S.L. and Cao, P. 2015. Ancient Chinese formula Qiong-Yu-Gao protects against cisplatin-induced nephrotoxicity without reducing antitumoranti-tumor activity. Sci. Rep. 5, 1–13. Volarevic, V., Djokovic, B., Jankovic, M.G., Harrell, C.R., Fellabaum, C., Djonov, V. and Arsenijevic, N. 2019. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 26(1), 25. Widowati, W., Prahastuti, S., Hidayat, M., Hasiana, S.T., Wahyudianingsih, R., Afifah, E., Kusuma, H.S.W., Rizal, R. and Subangkit, M. 2022. Protective effect of ethanolic extract of Jati Belanda (Guazuma ulmifolia L.) by inhibiting oxidative stress and inflammatory processes in cisplatin-induced nephrotoxicity in rats. Pak. Vet. J. 42(3), 376–382. Yang, Y., Yu, X., Zhang, Y., Ding, G., Zhu, C., Huang, S., Jia, Z. and Zhang, A. 2018. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin. Sci. 132(7), 825–838. Zhang, B., Ramesh, G., Norbury, C.C. and Reeves, W.B. 2007. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int. 72, 37–44. Zhang, X., Zhu, L., Wang, X., Zhang, H., Wang, L. and Xia, L. 2023. Basic research on curcumin in cervical cancer: progress and perspectives. Biomed. Pharmacother. 162, 114590. | ||
| How to Cite this Article |
| Pubmed Style Intan PR, Alegantina S, Fajri H, Ekawasti F, Isnawati A, Lienggonegoro LA, Nikmah UA, Sunarno S, Mariya SS, Sutardi LN, Setiyono A, Handharyani E. Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. Open Vet. J.. 2025; 15(1): 428-436. doi:10.5455/OVJ.2025.v15.i1.38 Web Style Intan PR, Alegantina S, Fajri H, Ekawasti F, Isnawati A, Lienggonegoro LA, Nikmah UA, Sunarno S, Mariya SS, Sutardi LN, Setiyono A, Handharyani E. Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. https://www.openveterinaryjournal.com/?mno=225425 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.38 AMA (American Medical Association) Style Intan PR, Alegantina S, Fajri H, Ekawasti F, Isnawati A, Lienggonegoro LA, Nikmah UA, Sunarno S, Mariya SS, Sutardi LN, Setiyono A, Handharyani E. Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. Open Vet. J.. 2025; 15(1): 428-436. doi:10.5455/OVJ.2025.v15.i1.38 Vancouver/ICMJE Style Intan PR, Alegantina S, Fajri H, Ekawasti F, Isnawati A, Lienggonegoro LA, Nikmah UA, Sunarno S, Mariya SS, Sutardi LN, Setiyono A, Handharyani E. Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 428-436. doi:10.5455/OVJ.2025.v15.i1.38 Harvard Style Intan, P. R., Alegantina, . S., Fajri, . H., Ekawasti, . F., Isnawati, . A., Lienggonegoro, . L. A., Nikmah, . U. A., Sunarno, . S., Mariya, . S. S., Sutardi, . L. N., Setiyono, . A. & Handharyani, . E. (2025) Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. Open Vet. J., 15 (1), 428-436. doi:10.5455/OVJ.2025.v15.i1.38 Turabian Style Intan, Putri Reno, Sukmayati Alegantina, Hidayatul Fajri, Fitrine Ekawasti, Ani Isnawati, Lisa Andriani Lienggonegoro, Uly Alfi Nikmah, Sunarno Sunarno, Sela Septima Mariya, Lina Noviyanti Sutardi, Agus Setiyono, and Ekowati Handharyani. 2025. Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. Open Veterinary Journal, 15 (1), 428-436. doi:10.5455/OVJ.2025.v15.i1.38 Chicago Style Intan, Putri Reno, Sukmayati Alegantina, Hidayatul Fajri, Fitrine Ekawasti, Ani Isnawati, Lisa Andriani Lienggonegoro, Uly Alfi Nikmah, Sunarno Sunarno, Sela Septima Mariya, Lina Noviyanti Sutardi, Agus Setiyono, and Ekowati Handharyani. "Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats." Open Veterinary Journal 15 (2025), 428-436. doi:10.5455/OVJ.2025.v15.i1.38 MLA (The Modern Language Association) Style Intan, Putri Reno, Sukmayati Alegantina, Hidayatul Fajri, Fitrine Ekawasti, Ani Isnawati, Lisa Andriani Lienggonegoro, Uly Alfi Nikmah, Sunarno Sunarno, Sela Septima Mariya, Lina Noviyanti Sutardi, Agus Setiyono, and Ekowati Handharyani. "Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats." Open Veterinary Journal 15.1 (2025), 428-436. Print. doi:10.5455/OVJ.2025.v15.i1.38 APA (American Psychological Association) Style Intan, P. R., Alegantina, . S., Fajri, . H., Ekawasti, . F., Isnawati, . A., Lienggonegoro, . L. A., Nikmah, . U. A., Sunarno, . S., Mariya, . S. S., Sutardi, . L. N., Setiyono, . A. & Handharyani, . E. (2025) Combined extracts of Curcuma longa and Curcuma zedoaria ameliorates cisplatin-induced kidney damage in rats. Open Veterinary Journal, 15 (1), 428-436. doi:10.5455/OVJ.2025.v15.i1.38 |