| Research Article | ||
Open Vet. J.. 2025; 15(1): 416-427 Open Veterinary Journal, (2025), Vol. 15(1): 416-427 Research Article Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extractWurlina Wurlina1, Imam Mustofa1*, Dewa Ketut Meles2, Aswin Rafif Khairullah3, Adeyinka Oye Akintunde4, Kadek Rachmawati2, Niluh Suwasanti5, Dewa Made Sucipta Putra6, Sri Mulyati1, Suzanita Utama1, Ulul Khoiriyah7, Baich R. Tyarraushananda Defvyanto7, Sila Faredy Heriana7, Katty Hendriana Priscilia Riwu8, Riza Zainuddin Ahmad3 and Audrey Gracelia Riwu91Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 5Department of Clinical Pathology, Faculty of Medicine, Universitas Katolik Widya Mandala Surabaya, Surabaya, Indonesia 6Dr. R. Soedjono Selong Hospital, East Lombok, Indonesia 7Profession Program of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 8Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia 9Faculty of Medicine and Veterinary Medicine, Universitas Nusa Cendana, Kupang, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 26/10/2024 Accepted: 31/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
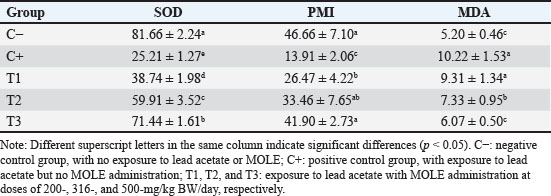
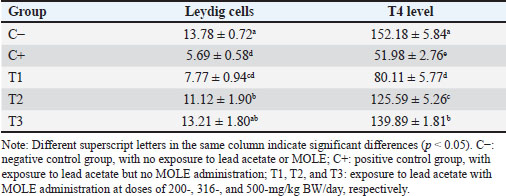
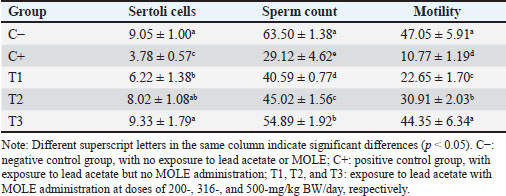
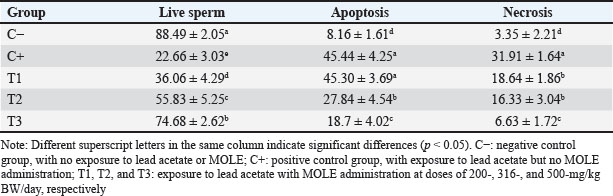
AbstractBackground: Lead intoxication triggers testicular toxicity via oxidative stress. Aim: This study aimed to explore the antioxidant potential of Moringa oleifera leaf extract (MOLE) in enhancing the semen quality of rats exposed to lead acetate. Methods: Twenty-five healthy rats were randomly and equally divided into five groups. Group C served as the negative control, whereas group C+ was exposed to lead acetate at 50-mg/kg body weight (BW)/day without MOLE. The T1, T2, and T3 groups were exposed to lead acetate at 50-mg/kg BW and concurrently received MOLE at doses of 200-, 316-, and 500-mg/kg BW/day, respectively, for 20 days. On the 21st day, all rats were euthanized for blood collection and testicle harvesting. Results: The result showed that exposure to lead acetate at 50-mg/kg BW/day in group C+ led to significant decreases (p < 0.05) in superoxide dismutase (SOD) levels, plasma membrane integrity, Leydig and Sertoli cell counts, spermatozoa numbers, sperm motility, and live spermatozoa, as well as significant increases (p < 0.05) in malondialdehyde levels and apoptotic and necrotic sperm, compared with control group C–. The administration of MOLE to rats exposed to lead acetate resulted in improvement in all of these variables. However, SOD and testosterone levels, as well as spermatozoa numbers, viability, apoptosis, and necrosis, did not recover in group T3 (p < 0.05) compared with control group C–. Conclusion: MOLE effectively restores sperm quality in lead acetate-induced rats. Keywords: Leydig cells, Malondialdehyde, Membrane integrity, Reproductive health, Sertoli cells. IntroductionLead pollution affects soil, water, and air on a global scale (Raj and Das, 2023). Elevated lead concentrations in aquatic environments, which exceed standard limits, pose threats to both the environment and human populations in the vicinity (Olufemi et al., 2022). Reports from Indonesia have documented lead concentrations exceeding established thresholds in urban river water samples (Fadlillah et al., 2023). Furthermore, several major cities have experienced lead air pollution exceeding both Indonesian regulations and WHO standards on an annual basis (Istiqomah and Marleni, 2020). Global lead production has increased due to the increased production of conventional cars, electric vehicles, and cellphone batteries (Raj and Das, 2023). Lead accumulates in the bodies of humans and animals through various processes, including absorption, bioavailability, bioconcentration, and biomagnification (Collin et al., 2022). At varying exposure levels, lead is a pervasive pollutant that jeopardizes health. A literature review on lead exposure revealed that exposure results in nervous system dysfunction, anemia, and cognitive impairment in children, and cardiovascular dysfunction, neurological decline, and male infertility in adults (Obeng-Gyasi, 2019). Oxidative stress and the ensuing imbalance between the antioxidant capacity of the testes and free-radical generation are the main causes of lead’s harmful effects on the testicles (Patra et al., 2011). Antioxidants can stabilize free radicals by donating hydrogen atoms or single electrons, which can counteract or lessen their harmful effects on the body (Rabeta and Faraniza, 2013). Antioxidant activity plays a critical role in mitigating heavy metal toxicity in testicles (Asadi et al., 2017; Albasher et al., 2021). Natural antioxidants can be found in plants, such as Moringa oleifera Lam (Fitriana et al., 2016). Moringa oleifera contains flavonoids, polyphenols, lycopene, and ß-carotene, and its primary flavonoid is quercetin (Makita et al., 2016). Moringa oleifera leaves contain a quercetin concentration of 384.61 mg/100 g (Bhagawan et al., 2017). Notably, the antioxidant potency of quercetin exceeds that of vitamin C and vitamin E (Rarinca et al., 2023). Studies on the harmful effects of lead exposure on male infertility are frequently included in review articles (Giulioni et al., 2023). Experimental research has been conducted to explore fertility disorders resulting from lead exposure in various animal models (Dolati et al., 2021; Osowski et al., 2023). Several studies have documented the use of M. oleifera leaf extract (MOLE) as an antioxidant to counteract oxidative damage to male reproduction in various animals (Abd et al., 2020; Laoung-On et al., 2021). MOLE is expected to alleviate oxidative stress in relation to male infertility (Mohlala et al., 2023). However, the use of MOLE as an antioxidant against lead exposure-induced male infertility remains unclear. Therefore, the present study aimed to ascertain the impact of MOLE on malondialdehyde (MDA) levels in the testicles as an indicator of plasma membrane damage, superoxide dismutase (SOD) as a measure of antioxidant capacity, as well as the number of Sertoli and Leydig cells, serum testosterone levels, sperm count, viability, motility, intact plasma membrane, and apoptosis and necrosis of spermatozoa in rats exposed to lead acetate. Materials and MethodsEthical approvalThe research was approved by the Animal Care and Use Committee, Universitas Airlangga (No: 158/HRECCFODM/XI/2022). PreparationLead acetate (Pb(CH3COO)2) was obtained from Sigma-Aldrich (USA; Cat. No. 6080-56-4). Treatments were prepared by diluting lead acetate in distilled water, which was administered as a single dose of 50-mg/kg body weight (BW)/day (Septiani et al., 2022). MOLE was obtained via extraction through maceration using 96% ethanol, followed by freeze drying, and subsequently dilution in 0.5% carboxymethyl cellulose sodium (CMC Na) (Mardatillah et al., 2022). Experimental animalsMale rats weighing approximately 200–250 g and aged 2.5 to 3.0 months were individually housed in plastic cages in a climate-controlled environment. The temperature was maintained at 26°C ± 2°C under a 12/12 hours alternating light/dark cycle. Rats were provided ad libitum access to drinking water and standard commercial feed. Twenty-five rats were randomly and equally divided into five groups. The negative control group (C–) consisted of rats exposed to distilled water (used as a solvent for lead acetate) and administered 0.5% CMC Na. The positive control group (C+) was exposed to lead acetate at a concentration of 50-mg/kg BW/day and 0.5% CMC Na solution. Groups T1, T2, and T3 were exposed to lead acetate at a dose of 50-mg/kg BW and MOLE at doses of 200-, 316-, and 500-mg/kg BW/day, respectively. Rats were orally exposed to a 0.5-ml lead acetate solution at 07.00 before feeding, and 4 hours later, they were orally administered 0.5-ml MOLE solution. This regimen was continued for 20 days. All rats were anesthetized with intraperitoneal ketamine at a dose of 40-mg/kg BW and euthanized on day 21. Blood was collected from the heart via subcostal arch laparotomy. Serum samples were also collected to measure testosterone levels. Testicles were harvested to determine MDA levels and SOD activity, and Leydig and Sertoli cell counts were assessed using hematoxylin–eosin-stained slides examined microscopically. Semen was collected from the cauda epididymis for quality evaluation, including spermatozoa number, motility, viability, and plasma membrane integrity (PMI) (Octaviani et al., 2021). Testosterone levelsSerum testosterone levels were determined using the solid-phase competitive chemiluminescence enzyme immunoassay technique with a Seimens testosterone test kit (Immulite® 1000 total testosterone) (Cinquanta et al., 2017). MDA levelsThe thiobarbituric acid-reactive substance assay was used to measure the amount of MDA in testicular tissue using a Thiobarbituric Acid Kit (NWLSS, USA, Cat. No. NWK-MDA01). A UV-1601 spectrophotometer was used to measure absorbance at a maximum wavelength of 535 nm (Fogarasi et al., 2016; Steffensen et al., 2020). SOD levelsSOD activity in testicular tissue was assessed using a test kit (Cayman Chemicals (USA, Cat. No. 706,002). The wavelength at which absorbance was measured was 550 nm (Weydert and Cullen, 2010). Leydig cell countTo determine the number of Leydig cells, five interstitial seminiferous tubules were randomly selected, and the average count was recorded (Ngizzah et al., 2023). An Optilab viewer software version 2.2-equipped light microscope was used for observations at 400× magnification. Sertoli cell countSertoli cells were counted using a Nikon E200 light microscope equipped with Optilab viewer software version 2.2 at 400× magnification. Five average observation replications of seminiferous tubules were performed randomly (Panggalih et al., 2021). Sperm countSemen was collected from the cauda epididymis in a 3.5 cm Petri dish (Nunc™, Thermo Scientific) containing 1 ml of 0.9% NaCl and gently stirred to ensure homogeneity (Octaviani et al., 2021). A microscope with a 100× magnification was used to count the spermatozoa (Lemoine et al., 2018). Sperm motilityA glass slide containing the spermatozoa suspension was covered with a cover glass. A Nikon EclipseE100 light microscope with 400× magnification was used to make the observations. Sperm motility was determined based on the percentage of sperm that exhibited progressive movement out of 100 observed sperm (Sari et al., 2023). Sperm viabilityA drop of semen and two drops of eosin nigrosine solution were smeared on a glass slide and rapidly dried using a Bunsen flame. Sperm viability was evaluated on an eosin nigrosine-stained slide under a light microscope (Nikon EclipseE100) at 400× magnification. Live sperm were unstained, whereas dead sperm had a reddish appearance in the head of the cytoplasm. Sperm viability (percentage of live spermatozoa) was determined using 100 spermatozoa (Sari et al., 2023). Plasma membrane integrityA 0.1-ml semen suspension was added to 0.9-ml of hypoosmotic solution in a microtube, gently stirred, and incubated at 37°C for 30 minutes. For 100 spermatozoa, a drop of the mixture was observed under a light microscope at 400× magnification. Intact sperm plasma membranes appeared swollen or coiled, whereas damaged sperm plasma membranes displayed straight tails. The PMI was calculated as the percentage of spermatozoa with coiled tails (Silviani et al., 2022). Apoptotic and necrotic sperm countsSamples of dry-smeared semen were preserved for 15 minutes with absolute methanol and glacial acid, stained for 10 minutes with acridine orange, and then seen at 100× magnification using a Nikon Eclipse E800 fluorescent microscope (Tokyo, Japan). Necrotic sperm appeared brownish-orange, whereas apoptotic sperm exhibited yellow to reddish coloration. The sperm cells were dyed green. A sample of 300 spermatozoa was used to determine the percentage of necrotic and apoptotic sperm (Meles et al., 2022). Data analysisThe one-way ANOVA test and Duncan’s multiple range test were used. If there was a significant difference (p < 0.05), then continue with the Mann–Whitney test at a 95% confidence level. The statistical software (Statistical Program and Service Solution, IBM Corporation) version 26 for Windows was used in all statistical studies. ResultExposure to lead acetate at 50-mg/kg BW/day in rats led to a significant decrease (p < 0.05) in both SOD and PMI and a corresponding increase (p < 0.05) in MDA levels compared with the control rats. In general, MOLE administration in rats exposed to lead acetate resulted in a significant increase (p < 0.05) in SOD and PMI levels and reduced MDA levels. Notably, a dose of 500-mg/kg BW/day MOLE restored MDA levels and PMI levels did not differ significantly (p > 0.05) from those of control rats (Table 1). The mean number of Leydig cells (Fig. 1) and testosterone levels in rats exposed to lead acetate were significantly lower (p < 0.05) than those in control rats. The administration of MOLE to rats exposed to lead acetate significantly increased (p < 0.05) both the number of Leydig cells and testosterone levels compared with the C+ group. Notably, in the T3 group, the number of Leydig cells did not differ (p > 0.05) from that in the control group. However, serum testosterone levels remained significantly lower (p < 0.05), not reaching levels observed in control rats (Table 2). Exposure to lead acetate in rats in the positive control group resulted in a significant reduction (p < 0.05) in both the number of Sertoli (Fig. 1) cells, spermatozoa count, and motility, compared with the negative control rats. MOLE administration, starting at a dose of 200-mg/kg BW (T1), significantly increased (p < 0.05) the number of Sertoli cells, the spermatozoa count, and their motility compared with the positive control group. At a dose of 500-mg/kg BW, the number of Sertoli cells and sperm motility were not significantly different (p > 0.05) from those observed in the control group rats. However, the spermatozoa counts observed under the highest MOLE dose remained significantly lower (p < 0.05) remained significantly lower than those in the control group (Table 3). Exposure to lead acetate in rats resulted in a decrease in the percentage of live sperm cells and an increase in the percentage of apoptotic and necrotic sperm (p < 0.05) compared with the negative control group (Fig. 2). The administration of MOLE to rats exposed to lead acetate resulted in an increase in the percentage of live sperm cells and a reduction in the percentage of apoptotic and necrotic sperm (p < 0.05) compared with rats exposed to lead acetate. However, at a dose of 500-mg/kg BW, MOLE did not fully restore the percentage of live sperm cells or reduce the percentage of apoptotic and necrotic sperm; these values remained significantly lower (p < 0.05) than those observed in the control group rats (Table 4). Table 1. SOD, % activity, intactness of the sperm (PMI, %), and MDA, nmol/ml levels in lead acetate-induced rats (Rattus norvegicus) orally administered MOLE.
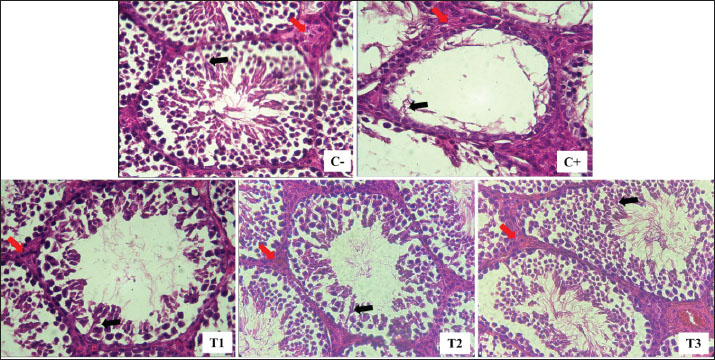
Fig. 1. Leydig (red arrow) and Sertoli (black arrow) cells were isolated from lead acetate-induced rats (Rattus norvegicus) orally administered MOLE. HE staining under light microscope in 400× magnification. C−: negative control group, with no exposure to lead acetate or MOLE; C+: positive control group, with exposure to lead acetate but no MOLE administration; T1, T2, and T3: exposure to lead acetate with MOLE administration at doses of 200-, 316-, and 500-mg/kg BW/day, respectively. DiscussionIn this study, exposure to lead acetate resulted in a significant decrease in SOD activity (31% remaining), a reduction in the percentage of spermatozoa with intact plasma membranes (30% remaining), and an increase in MDA levels (197%) compared with control rats. Lead exposure inhibits ð-aminolevulinic acid dehydratase, resulting in increased ð-aminolevulinic levels and 4,5-dioxovaleic acid content, leading to the generation of reactive oxygen species (ROS) in the form of hydroxyl radicals, superoxide radicals, hydroxyl radicals, and nitric oxide radicals, thereby inducing oxidative stress (Ibrahem et al., 2020; Irawati et al., 2022). ROS, being unstable and reactive oxygen compounds with unpaired electrons in the outer orbitals, interact with crucial molecules, such as proteins, lipids, and DNA, attracting electrons from their vicinity (Darbandi et al., 2018). Although the body’s endogenous antioxidant enzymes can neutralize ROS to some extent, an excess of ROS overwhelms these defenses, leading to oxidative stress and subsequent cell membrane damage (Murphy et al., 2022). Lipid peroxidation is the result of excessive ROS reaction with polyunsaturated fatty acids in cell membranes (Steffensen et al., 2020). Molecules that have their electrons taken up by ROS can undergo lipid peroxidation, leading to subsequent cell damage (El-Magd et al., 2016). ROS also reduces antioxidant capacity by decreasing the activity of various enzymes, such as glutathione peroxidase (GPx), SOD, and catalase (Ighodaro and Akinloye, 2018). The process of lipid peroxidation results in the production of MDA as a secondary product, and elevated MDA levels indicate cell membrane damage. Decreased SOD activity and increased MDA concentrations provide evidence of tissue damage caused by lead toxicity (Pizzino et al., 2017). Table 2. Leydig cell count per interstitial seminiferous tubule and serum testosterone (T4, ng/dL) levels in lead acetate-induced rats (Rattus norvegicus) orally administered MOLE.
Table 3. Numbers of Sertoli cells per seminiferous tubule and sperm cells (million/ml), as well as the percentage of motile sperm (%), in lead acetate-induced rats (Rattus norvegicus) orally administered MOLE.
Lead exposure also damaged the plasma membranes of Leydig and Sertoli cells, resulting in a decrease in their numbers (41% and 42% remaining, respectively). Leydig cells are responsible for synthesizing testosterone, and reduced Leydig cell counts led to decreased serum testosterone levels (34% remaining). An androgen-binding protein (ABP) that binds testosterone is produced by Sertoli cells during spermatogenesis (Gill-Sharma, 2018). The ABP–testosterone complex supports the proliferation of spermatogonia into spermatocytes, spermatids, and spermatozoa (Griswold, 2018). The reduction in Leydig and Sertoli cell counts, along with lower testosterone levels, resulted in fewer spermatozoa (46% remaining), diminished motility (23% remaining), and decreased viability (26% remaining). Furthermore, these changes were accompanied by an increase in the percentage of spermatozoa undergoing apoptosis (557%) and necrosis (952%). The results of this investigation are in line with those of Septiani et al. (2022), who found that rats exposed to lead acetate had fewer Leydig cells. Lead directly reduces the numbers of Sertoli and Leydig cells and alters the arrangement and shape of spermatogonia, spermatocytes, and spermatozoa due to lipid peroxidation of cell membranes (Abdel-Emam et al., 2021). Furthermore, damage to the sperm plasma membrane negatively affects spermatozoa viability and motility (Oluwakemi and Olufeyisipe, 2016; Pereira et al., 2017). The present results align with the findings of El-Magd et al. (2017) and Offor et al. (2019), who highlighted lead’s adverse effects on sperm count and motility.
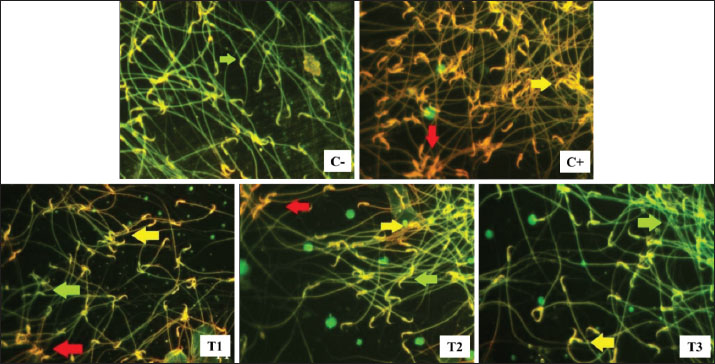
Fig. 2. Live (green arrow), apoptosis (yellow arrow), and necrosis (red arrow) spermatozoa in lead acetate-induced rats (Rattus norvegicus) orally administered MOLE. Acridine orange-ethidium bromide staining and fluorescent microscope in 400× magnification. C−: negative control group, no exposure to lead acetate or MOLE; C+: positive control group, exposure to lead acetate but no MOLE administration; T1, T2, and T3: exposure to lead acetate with MOLE administration at doses of 200-, 316-, and 500-mg/kg BW/day, respectively. Table 4. Percentage of live, apoptotic, and necrotic sperm cells (%) in lead acetate-induced rats (Rattus norvegicus) orally administered MOLE.
Leydig cells express androgen receptors (ARs) on their membranes, which mediate the steroidogenesis pathway (Zirkin and Papadopoulos, 2018). Elevated ROS levels lead to increased CYP450 expression in Leydig cells, which can bind to ARs, subsequently reducing testosterone synthesis (Huang et al., 2021) and lowering testosterone levels (Wurlina et al., 2021). Another method is that exposure to lead damages the mitochondria and endoplasmic reticulum of Leydig cells, disrupting the enzymes that produce androgen (Huang et al., 2021). Oxidative stress resulting from lead exposure can damage the plasma membrane of endocrine cells in the hypothalamo–pituitary–testicular axis (Gandhi et al., 2017; Darbandi et al., 2018). The hypothalamus produces gonadotropin-releasing hormone (GnRH), which is a crucial hormone for reproductive endocrine regulation. The arcuate nucleus of the hypothalamus produces and secretes GnRH, which promotes the synthesis of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (Wang et al., 2021). FSH regulates Sertoli cells to produce ABP, whereas LH regulates Leydig cells to synthesize testosterone. Direct testosterone biosynthesis from cholesterol occurs only in Leydig cells via the ∆5 pathway (Eacker et al., 2008). A decline in Leydig cell numbers results in reduced testosterone production, leading to a decrease in spermatogenesis (Huang et al., 2021). In the present study, administration of 50 mg/kg/day of lead acetate for 20 days led to Leydig cell damage and reduced testosterone levels. Suppression of testosterone biosynthesis can inhibit spermatogenesis, triggering apoptosis in spermatogenic cells in the seminiferous tubules and spermatozoa in the epididymis (Oduwole et al., 2018). The process of programmed cell death, known as apoptosis, is typified by chromosomal and cell nucleus ruptures that result in apoptotic bodies devoid of inflammation (Elmore, 2007). This process can be initiated by ROS (Redza-Dutordoir and Averill-Bates, 2016). Excessive ROS levels damage both DNA and the mitochondrial plasma membrane. DNA damage is followed by increased levels of p53, promoting an increase in Bax protein content and a decrease in Bcl2 protein levels, ultimately raising the Bax/Bcl ratio and potentially triggering the apoptosis cascade (George and Abrahamse, 2019). Moreover, ROS increases cell membrane permeability and decreases mitochondrial membrane potential, both of which result in the release of cytochrome c. The combination of an increased Bax/Bcl ratio and elevated cytochrome c levels induces the expression of caspase-3, which acts as an apoptotic executor (Qu et al., 2019). Excessive ROS also leads to changes in the expression of genes related to apoptosis (e.g., caspase-3, caspase-9, Bax, and Bcl2) in testicular histology (Famurewa et al., 2023). Lead intoxication results in elevated ROS levels, reducing antioxidant enzyme capacity and affecting several macromolecules, ultimately leading to cell necrosis (Pal et al., 2015). As cell membranes become damaged and unable to maintain homeostasis, they permit the entry of extracellular ions and water, leading to necrosis, an abrupt and irreversible form of cell death. The breakdown of organelles within the cell membrane, including intracytoplasmic fluid in ribosomes and mitochondrial membranes, results in cell necrosis and eventual lysis (Gudipaty et al., 2018). Lead poisoning upregulates the expression of necrosis-related proteins, particularly interleukin-6 and tumor necrosis factor-alpha (Famurewa et al., 2023). The apoptosis and necrosis observed in spermatozoa due to excess ROS resulting from exposure to lead acetate in the present study are similar to the effects of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in a previous study (Meles et al., 2022). Rats exposed to lead acetate without MOLE in the current study had a greater proportion of spermatozoa experiencing necrosis than those experiencing apoptosis, indicating a transition from apoptosis to necrosis (necroptosis). Necroptosis is a controlled type of cell death that resembles necrosis in appearance and requires receptor-interacting serine-threonine kinase 3 (RIPK3) and mixed lineage kinase domain-like (MLKL) (Galluzzi et al., 2017). Cell surface death receptors, such as Fas, TNF receptor 1, IFN receptors, TLRs, and intracellular RNA- or DNA-sensing molecules, can initiate the necroptosis pathway, especially in response to apoptotic insults (Choi et al., 2019). Furthermore, lead exposure induces oxidative stress in the endoplasmic reticulum membrane, followed by the expression of caspase-9 and caspase-8. In the apoptosis cascade, inhibition of caspase-8 activates RIPK3 and MLKL (Dhuriya and Sharma, 2018; Chen et al., 2019), ultimately leading to regulation of necrosis through the formation of a necrosome complex (Chaouhan et al., 2022). Cellular damage caused by oxidants can be mitigated by administering antioxidants that donate electrons. Antioxidants are categorized into enzyme and nonenzyme antioxidants (Martemucci et al., 2022). Antioxidant enzymes, such as SOD, catalase, and GPx, are found in many fruits and vegetables, along with nonenzyme antioxidants such as lipoic acid, reduced glutathione (GSH), vitamins C and E, β-carotene, flavones, flavonoids, anthocyanins, catechins, isoflavone, and isocatechins (Flieger et al., 2021). The lowest dose of MOLE (200 mg/kg BW) administered to rats exposed to lead acetate resulted in increased SOD and PMI levels, although MDA levels remained unchanged compared with rats exposed to lead acetate. The percentage of Leydig cells increased, accompanied by elevated testosterone levels. Higher testosterone levels and an increased number of Sertoli cells naturally contribute to greater numbers of spermatozoa, higher percentages of live and motile spermatozoa, and decreased percentages of necrotic spermatozoa. However, the percentage of apoptotic spermatozoa remained unchanged in rats exposed to lead acetate without MOLE treatment. This is explained by the fact that rats exposed to lead acetate alone and those exposed to lead acetate plus 200-mg/kg BW MOLE had comparable MDA levels. The administration of MOLE at a dose of 316-mg/kg BW improved all observed variables. Moringa oleifera leaves alleviate male infertility by reducing oxidative stress and restoring reproductive hormone levels, steroidogenesis, and sperm parameters (Mohlala et al., 2023). Reduced antioxidant enzyme content can serve as a marker of elevated free radical levels (Flieger et al., 2021). Moringa oleifera leaves are rich in various compounds, such as ascorbic acid (vitamin C) (Peñalver et al., 2022), flavonoids, phenolics, carotenoids, phytoestrogens, β-sitosterol, iron, calcium, phosphorus, copper, vitamin A, vitamin B, riboflavin, α-tocopherol (vitamin E), pyridoxine, β-carotene, folic acid, and essential amino acids, including methionine, cystine, tryptophan, and lysine (Fitriana et al., 2016). The high antioxidant capacity of M. oleifera leaves is primarily attributed to their high phenolic content (Peñalver et al., 2022), vitamin C (Paramita, 2023), and vitamin E, for which the activity against superoxide radicals and hydroxyl radicals has previously been observed at 92.4% and 73.1%, respectively (Fejér et al., 2019). Vitamin C is an antioxidant that breaks radical chain reactions, thereby inhibiting ROS and oxidative stress. Vitamin C also affects endocrine function and improves the testicular structure and the number of spermatozoa in rats with testicular oxidative damage (Behairy et al., 2020). Vitamin E protects unsaturated fatty acids in biological membranes from free radicals, captures free radicals and oxygen compounds, and prevents lipid peroxidation (Górnicka et al., 2019). It also neutralizes superoxide, hydrogen peroxide, and hydroxyl radicals. By donating a hydrogen atom to convert peroxyl radicals to less reactive and non-damaging radicals, vitamin E inhibits lipid peroxide and protects cells from harm (Ochi and Takeda, 2015; Górnicka et al., 2019). Vitamins C and E have antioxidant properties that help mitigate free radicals (Traber and Stevens, 2011), thereby improving spermatogenesis in rats exposed to lead. It is well-established that cholesterol is transported to the mitochondrial membrane of Leydig cells via peripheral-type benzodiazepine receptors and acute regulatory proteins during steroidogenesis. The inner mitochondrial membrane’s P450scc/CYP11A1 then catalyzes the conversion of cholesterol to pregnenolone (Manna et al., 2016). Through a sequence of enzymatic events, pregnenolone is transported to the endoplasmic reticulum for steroidogenesis. There, 3β-HSD, which is catalyzed by P450 17α, converts it to progesterone to form 17-hydroxyprogesterone and androstenedione, which are then transformed into testosterone (Agdam et al., 2017). Excessive free-radical generation by lead exposure disrupts testosterone synthesis and reduces the stages of spermatogenesis and spermiogenesis. The present study revealed that MOLE administration revitalizes the formation of spermatozoa. Vitamin E can effectively halt free-radical chain reactions, ensuring the normal process of spermatogenesis in seminiferous tubules (Malmir et al., 2021), even without lead intoxication. Vitamin E regulates lipid peroxidation by donating hydrogen to convert peroxyl radicals into less reactive tocopherol radicals, thereby preventing damage to fatty acid chains and preserving cell integrity (Shastak et al., 2023). Being fat-soluble, vitamin E can easily penetrate cells through the phospholipid layers of cell membranes (Vergara-Jimenez et al., 2017; Ahmed et al., 2020). In the current study, the administration of MOLE at a dose of 500-mg/kg BW led to an even higher SOD activity compared with MOLE at a dose of 200-mg/kg BW, with MDA and PMI levels similar to those in normal rats. This suggests that oxidative stress is effectively reduced in spermatogenesis-related cells. Spermatogenesis is regulated by the hypothalamus, pituitary gland, and testes. Specific neurons in the hypothalamus synthesize GnRH, which, in turn, stimulates the production of LH and FSH. LH induces testosterone synthesis in Leydig cells, and FSH influences Sertoli cells, promoting the production of ABP, which is essential for binding testosterone during spermatogenesis (Ramaswamy and Weinbauer, 2015). Several studies have demonstrated the detrimental effects of lead-induced toxicity on male reproductive function. However, GSH, GPX, and testicular catalase levels, sperm count and motility, and FSH, LH, and testosterone content were all restored in male rats exposed to MOLE, thereby reducing reproductive toxicity (El-Sheikh et al., 2016; Ngizzah et al., 2023). Furthermore, studies have found that MOLE administered to rats exposed to gentamicin leaf extract increases the number of Leydig and spermatogenic cells (Ngizzah et al., 2023). In addition, MOLE at a dose of 400-mg/kg BW/day has been shown to maintain spermatozoa viability and motility in rats subjected to 1 hour of daily exposure to 40°C for 14 days (Octaviani et al., 2021). MOLE administered at a dose of 300-mg/kg BW per day for 28 days was found to restore semen quality (total sperm count, viability, and motility), as well as antioxidant markers (catalase, SOD, and GSH), oxidative stress markers (MDA), and hormonal profiles (FSH, LH, and testosterone) in drug toxicity-induced rats (Ogunlade et al., 2022). The improvements observed in cellular parameters based on SOD markers, MDA, and PMI levels led to increased numbers of Leydig and Sertoli cells, effectively restoring counts to normal levels. However, despite the restoration of Leydig cell numbers to normal levels, serum testosterone content remained somewhat lower (92%) than that in normal rats. Furthermore, the highest dose administered in the present study did not completely restore the numbers of spermatozoa (86%), live spermatozoa (84%), apoptotic sperm (229%), and necrotic sperm (192%) compared with control rats. This outcome may be attributed to the marginal improvement in SOD activity, which was only 0.87% compared with that of the control rats. Nevertheless, sperm motility, a vital fertility indicator, was restored to levels observed in normal rats. ConclusionIt can be inferred that MOLE enhances antioxidant capacity, restores the integrity of the spermatogenic support system, and effectively improves semen quality in rats with lead acetate–induced reproductive toxicity. AcknowledgmentsThe researchers would like to thank the Faculty of Veterinary Medicine, Airlangga University, for all the facilities provided during the research. Conflict of interestThe authors declare no conflict of interest. FundingThe authors express their sincere gratitude to the Directorate of Research and Community Service, Deputy for Strengthening Research and Technology, Ministry of Research and Technology/National Research and Innovation Agency for the 2022 fiscal year, Chancellor’s Decree number: 770/UN3.14/PT/2022. Authors’ contributionsWW, ARK, AGR, RZA, and IM: conceived the idea and manuscript drafting. DKM, SM, KR, UK, BRTD, SFH, and SU: acquisition, analysis, and interpretation of data. AOA, KHPR, NS, and DMSP: the manuscript was critically read and revised for intellectual content. All authors have read and approved the final manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data are available in the manuscript. ReferencesAbd, H.H., Ahmed, H.A. and Mutar, T.F. 2020. Moringa oleifera leaf extract modulates toxicity, sperm alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol. Res. 9(2), 101–106. Abdel-Emam, R.A. and Ahmed, E.A. 2021. Ameliorative effect of L-carnitine on chronic lead-induced reproductive toxicity in male rats. Vet. Med. Sci. 7(4), 1426–1435. Agdam, H.R., Razi, M., Amniattalab, A., Malekinejad, H. and Molavi, M. 2017. Co-administration of vitamin E and testosterone attenuates atrazine-induced toxic effects on sperm quality and testes in rats. Cell J. 19(2), 292–305. Ahmed, N.F., Sadek, K.M., Soliman, M.K., Khalil, R.H., Khafaga, A.F., Ajarem, J.S., Maodaa, S.N. and Allam, A.A. 2020. Moringa oleifera leaf extract repairs oxidative misbalance following sub-chronic exposure to sodium fluoride in Nile tilapia Oreochromis niloticus. Animals 10(4), 626. Albasher, G., Alrajhi, R., Alshammry, E. and Almeer, R. 2021. Moringa oleifera leaf extract attenuates Pb acetate-induced testicular damage in rats. Comb. Chem. High Throughput Screen. 24(10), 1593–1602. Asadi, N., Bahmani, M., Kheradmand, A. and Rafieian-Kopaei, M. 2017. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J. Clin. Diagn. Res. 11(5), IE01–IE05. Behairy, A., El-Sharkawy, N.I., Saber, T.M., Soliman, M.M., Metwally, M.M., Abd El-Rahman, G.I., Abd-Elhakim, Y.M. and El Deib, M.M. 2020. The modulatory role of vitamin C in boldenone undecylenate-induced testicular oxidative damage and androgen receptor dysregulation in adult male rats. Antioxidants 9(11), 1053. Bhagawan, W.S., Atmaja, R.R.D. and Atiqah, S.N. 2017. Optimization and quercetin release of Moringa leaf extract (Moringa oleifera) in gel-microemulsion preparation. J. Islam. Pharm. 2(2), 34–42. Chaouhan, H.S., Vinod, C., Mahapatra, N., Yu, S.H., Wang, I.K., Chen, K.B., Yu, T.M. and Li, C.Y. 2022. Necroptosis: a pathogenic negotiator in human diseases. Int. J. Mol. Sci. 23(21), 12714. Chen, J., Kos, R., Garssen, J. and Redegeld, F. 2019. Molecular insights into the mechanism of necroptosis: the necrosome is a potential therapeutic target. Cells 8(12), 1486. Choi, M.E., Price, D.R., Ryter, S.W. and Choi, A.M.K. 2019. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 4(15), e128834. Cinquanta, L., Fontana, D.E. and Bizzaro, N. 2017. Chemiluminescent immunoassay technology: what does it mean for autoantibody detection?. Auto Immun. Highlights 8(1), 9. Collin, M.S., Venkatraman, S.K., Vijayakumar, N., Kanimozhi, V., Arbaaz, S.M., Stacey, R.G.S., Anusha, J., Choudhary, R., Lvov, V., Tovar, G.I., Senatov, F., Koppala, S. and Swamiappan, S. 2022. Bioaccumulation of lead (Pb) and its effects on humans: a review. J. Hazard. Mater. Adv. 7(1), 100094. Darbandi, M., Darbandi, S., Agarwal, A., Sengupta, P., Durairajanayagam, D., Henkel, R. and Sadeghi, M.R. 2018. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 16(1), 87. Dhuriya, Y.K. and Sharma, D. 2018. Necroptosis: a regulated inflammatory mode of cell death. J. Neuroinflammation 15(1), 199. Dolati, P., Zamiri, M.J., Akhlaghi, A., Khodabandeh, Z., Mehrabani, D., Atashi, H. and Jamhiri, I. 2021. Reproductive and embryological toxicity of lead acetate in male mice and their offspring and the mitigation effects of quercetin. J. Trace Elem. Med. Biol. 67(1), 126793. Eacker, S.M., Agrawal, N., Qian, K., Dichek, H.L., Gong, E.Y., Lee, K. and Braun, R.E. 2008. Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol. Endocrinol. 22(3), 623–635. El-Magd, M.A., Kahilo, K.A., Nasr, N.E., Kamal, T., Shukry, M. and Saleh, A.A. 2017. A potential mechanism associated with lead-induced testicular toxicity in rats. Andrologia 49(9), e12750. El-Sheikh, S., Khairy, M., Fadil, H.A. and Abo-Elmaaty, A. 2016. Ameliorative effect of Moringa oleifera extract on male fertility in paroxetine treated rats. Zag. Vet. J. 44(3), 244–250. Elmore, S. 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35(4), 495–516. Fadlillah, L.N., Utami, S., Rachmawati, A.A., Jayanto, G.D. and Widyastuti, M. 2023. Environmental risk and source identification of heavy metal contamination in water and surface sediments from the anthropogenic impacts of urban river, Indonesia. Heliyon 9(4), e15485. Famurewa, A.C., Hamdi, H. and Sedky, A. 2023. Lipoic acid abates testis lead accumulation, sperm-endocrine deficits, testicular oxidative inflammation, and apoptosis and modulates gene expression of Bax and Bcl-2 in rats. Sci. Afr. 21(1), e01842. Fejér, J., Kron, I., Pellizzeri, V., Pľuchtová, M., Eliašová, A., Campone, L., Gervasi, T., Bartolomeo, G., Cicero, N., Babejová, A., Konečná, M., Sedlák, V., Poráčová, J. and Gruľová, D. 2019. First report on the evaluation of the basic nutritional and antioxidant properties of Moringa oleifera Lam. from the Caribbean Islands of Saint Lucia. Plants 8(12), 537. Fitriana, W.D., Ersam, T., Shimizu, K. and Fatmawati, S.. 2016. Antioxidant activity of Moringa oleifera extracts. Indones. J. Chem. 16(3), 297–301. Flieger, J., Flieger, W., Baj, J. and Maciejewski, R. 2021. Antioxidants: classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 14(15), 4135. Fogarasi, E., Croitoru, M.D., Fülöp, I., Nemes-Nagy, E., Tripon, R.G., Simon-Szabo, Z. and Muntean, D.L. 2016. Malondialdehyde levels can be measured in serum and saliva using a fast HPLC method with visible detection/Determinarea printr-o metodă HPLC-VIS rapidă a concentraţiilor serice şi salivare ale malondialdehidei. Rev. Rom. Med. Lab. 24(3), 319–326. Galluzzi, L., Kepp, O., Chan, F.K. and Kroemer, G. 2017. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. 12(1), 103–130. Gandhi, J., Hernandez, R.J., Chen, A., Smith, N.L., Sheynkin, Y.R., Joshi, G. and Khan, S.A. 2017. Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote 25(2), 103–110. George, B.P. and Abrahamse, H. 2019. Increased oxidative stress induced by Rubus bioactive compounds induce apoptotic cell death in human breast cancer cells. Oxid. Med. Cell. Longev. 2019(1), 6797921. Gill-Sharma, M.K. 2018. Testosterone retention mechanism in Sertoli cells: a biochemical perspective. Open Biochem. J. 12(1), 103–112. Giulioni, C., Maurizi, V., De Stefano, V., Polisini, G., Teoh, J.Y., Milanese, G., Galosi, A.B. and Castellani, D. 2023. The influence of lead exposure on male semen parameters: a systematic review and meta-analysis. Reprod. Toxicol. 118(1), 108387. Górnicka, M., Ciecierska, A., Hamulka, J., Drywień, M.E., Frackiewicz, J., Górnicki, K. and Wawrzyniak, A. 2019. α-Tocopherol protects the heart, muscles, and testes from lipid peroxidation in growing male rats subjected to physical efforts. Oxid. Med. Cell. Longev. 2019(1), 8431057. Griswold, M.D. 2018. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol. Reprod. 99(1), 87–100. Gudipaty, S.A., Conner, C.M., Rosenblatt, J. and Montell, D.J. 2018. Unconventional ways to live and die: cell death and survival in development, homeostasis, and disease. Annu. Rev. Cell Dev. Biol. 34(1), 311–332. Huang, H., Wang, M., Hou, L., Lin, X., Pan, S., Zheng, P. and Zhao, Q. 2021. A potential mechanism associated with lead-induced spermatogonia and Leydig cell toxicity and the mitigative effect of selenium in chicken. Ecotoxicol. Environ. Saf. 209(1), 111671. Ibrahem, S., Hassan, M., Ibraheem, Q. and Arif, K. 2020. Genotoxic effects of lead and cadmium on workers at wastewater plants in Iraq. J. Environ. Public Health 2020(1), 9171027. Ighodaro, O.M. and Akinloye, O.A. 2018. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defense grid. Alexandria J. Med. 54(4), 287–293. Irawati, Y., Kusnoputranto, H., Achmadi, U.F., Safrudin, A., Sitorus, A., Risandi, R., Wangsamuda, S., Permana, D.H., Syahrani, L., Dewayanti, F.K., Asih, P.B.S. and Syafruddin, D. 2022. Aminolevulinic acid dehydratase allelic frequency and lead toxicity in children under 5 years old in a previously used lead-acid battery area. Public Health J. 17(1), 66–73. Istiqomah, N.A. and Marleni, N.N.N. 2020. Particulate air pollution in Indonesia: quality index, characteristics, and source identification. IOP Conf. Ser. Earth Environ. Sci. 599(1), 012084. Laoung-On, J., Saenphet, K., Jaikang, C. and Sudwan, P. 2021. Effects of Moringa oleifera Lam. leaf tea effects on sexual behavior and reproductive function in male rats. Plants 10(10), 2019. Lemoine, M., Ferraretto, X., Royer, M.L., Benammar, A., Darolles, J., Epelboin, S., Eustache, F. and Patrat, C. 2018. Sperm concentration measurement using a disposable counting chamber. Asian J. Androl. 20(5), 525–526. Makita, C., Chimuka, L., Steenkamp, P., Cukrowska, E. and Madala, E. 2016. Comparative analyses of flavonoid Content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting. S. Afr. J. Bot. 105(1), 116–122. Malmir, M., Mehranjani, M.S., Faraji, T. and Noreini, S.N. 2021. Antioxidant effect of vitamin E on the male rat reproductive system by a high oral dose of bisphenol-A. Toxicol. Res. Appl. 5(1), 23978473211005562. Mardatillah, M., Wurlina, West, Yudaniayanti, I.S., Plumeriastuti, H., Primarizky, H. and Hamid, I.S. 2022. Moringa oleifera leaf extract restored the diameter and epithelial thickness of the seminiferous tubules of rat (Rattus norvegicus) injected with gentamicin. Ovozoa J. Anim. Reprod. 11(1), 15–21. Manna, P.R., Stetson, C.L., Slominski, A.T. and Pruitt, K. 2016. Role of acute steroidogenic regulatory protein in health and disease. Endocrine 51(1), 7–21. Martemucci, G., Costagliola, C., Mariano, M., D’andrea, L., Napolitano, P. and D’Alessandro, A.G. 2022. Free radical properties, source and targets, antioxidant consumption, and health. Oxygen 2(2), 48–78. Meles, D.K., Rachmawati, K., Hamid, I.S., Mustofa, I., Wurlina, W., Suwasanti, N., Putri, D.K.S.C. and Utama, S. 2022. α-Tocopherol prevents sperm apoptosis and necrosis in rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Vet. Med. Int. 2022(1), 3685686. Mohlala, K., Offor, U., Monageng, E., Takalani, N.B. and Opuwari, C.S. 2023. Overview of the effects of Moringa oleifera leaf extract on oxidative stress and male infertility: a review. Appl. Sci. 13(7), 4387. Murphy, M.P., Bayir, H., Belousov, V., Chang, C.J., Davies, K.J.A., Davies, M.J., Dick, T.P., Finkel, T., Forman, H.J., Janssen-Heininger, Y., Gems, D., Kagan, V.E., Kalyanaraman, B., Larsson, N.G., Milne, G.L., Nyström, T., Poulsen, H.E., Radi, R., Van Remmen, H., Schumacker, P.T., Thornalley, P.J., Toyokuni, S., Winterbourn, C.C., Yin, H. and Halliwell, B. 2022. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 4(6), 651–662. Ngizzah, N., Wurlina, W., Hastutiek, P., Hamid, I.S., Hestianah, E.P., Mafruchati, M. and Yustinasari, L.R. 2023. Effect of the ethanolic extract of Moringa oleifera leaves on the number of spermatogenic and Leydig cells in gentamicin-induced rats. Ovozoa J. Anim. Reprod. 12(2), 93–100. Obeng-Gyasi, E. 2019. Sources of lead exposure in various countries. Health 34(1), 25–34. Ochi, H. and Takeda, S.. 2015. Two sides of vitamin E supplementation. Gerontology 61(4), 319–326. Octaviani, N.A.A., Utomo, B., Sunarso, A., Hamid, I.S., Madyawati, S.P. and Suprayogi, T.W. 2021. Effect of Moringa (Moringa oleifera Lam.) leaf extract on the motility and viability of heat-exposed spermatozoa (Rattus norvegicus). Ovozoa J. Anim. Reprod. 10(3), 65–71. Oduwole, O.O., Peltoketo, H. and Huhtaniemi, I.T. 2018. Role of follicle-stimulating hormone in spermatogenesis. Front. Endocrinol. 9(1), 763. Offor, S.J., Mbagwu, H.O. and Orisakwe, O.E. 2019. Improvement of lead acetate-induced testicular injury and sperm quality deterioration by Solanum anomalum Thonn. Ex. Schumach fruit extracts in albino rats. J. Family Reprod. Health 13(2), 98–108. Ogunlade, B., Jeje, S.O., Adelakun, S.A. and Akingbade, G.T. 2022. Moringa oleifera restores semen quality, hormonal profile, and testicular morphology against highly active antiretroviral therapy-induced toxicity in adult male wistar rats. JBRA Assist. Reprod. 26(1), 3–12. Olufemi, A.C., Mji, A. and Mukhola, M.S. 2022. Potential health risks of lead exposure from early life to later life: implications for public health education. Public Health 19(23), 16006. Oluwakemi, O. and Olufeyisipe, A. 2016. DNA fragmentation and oxidative stress compromise sperm motility, and survival in late pregnancy exposure to omega-9 fatty acid in rats. Iran. J. Basic Med. Sci. 19(5), 511–520. Osowski, A., Fedoniuk, L., Bilyk, Y., Fedchyshyn, O., Sas, M., Kramar, S., Lomakina, Y., Fik, V., Chorniy, S. and Wojtkiewicz, J. 2023. Lead exposure assessment and its impact on the structural organization and morphological peculiarities of rat ovaries. Toxics 11(9), 769. Pal, M., Sachdeva, M., Gupta, N., Mishra, P., Yadav, M. and Tiwari, A. 2015. Lead exposure in different organs of mammals and prevention by curcumin-nanocurcumin: a review. Biol. Trace Elem. Res. 168(2), 380–391. Panggalih, A., Susilowati, S., Maslachah, L., Ratnani, H. and Suprayogi, T.W. 2021. Effect of watermelon (Citrullus lanatus) rind ethanolic extract on the number of Leydig, Sertoli, and spermatogenic cells of rats (Rattus novergicus) exposed to heat. Ovozoa J. Anim. Reprod. 10(1), 7–11. Paramita, V.D. 2023. Effect of drying methods on vitamin C levels and antioxidant activity of Moringa oleifera Leaves. J. Agritechno 16(1), 29–35. Patra, R.C., Rautray, A.K. and Swarup, D. 2011. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011(1), 457327. Peñalver, R., Martínez-Zamora, L., Lorenzo, J.M., Ros, G. and Nieto, G., 2022. Nutritional and antioxidant properties of Moringa oleifera leaves in functional foods. Foods 11(8), 1107. Pereira, R., Sá, R., Barros, A. and Sousa, M. 2017. The major regulatory mechanisms involved in sperm motility. Asian J. Androl. 19(1), 5–14. Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D. and Bitto, A. 2017. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017(1), 8416763. Qu, D., Jiang, M., Huang, D., Zhang, H., Feng, L., Chen, Y., Zhu, X., Wang, S. and Han, J. 2019. Synergistic effects of taspartame and potassium sorbate enhancements onmitochondrial ROS, p53 activation,, and apoptosis in HepG2 cells. Molecules 24(3), 457. Rabeta, M.S. and Faraniza, N. 2013. Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of Garcania atroviridis and Cynometra cauliflora. Int. Food Res. J. 20(4), 1691–1696. Raj, K. and Das, A.P. 2023. Lead pollution: impact on environment and human health and approach for a sustainable solution. J. Environ. Chem. Ecotoxicol. 5(1), 79–85. Ramaswamy, S. and Weinbauer, G.F. 2014. Endocrine control of spermatogenesis: role of FSH and LH/testosterone. Spermatogenesis 4(2), e996025. Rarinca, V., Nicoara, M.N., Ureche, D. and Ciobica, A. 2023. Exploitation of quercetin’s antioxidative properties as a potential alternative therapeutic option for neurodegenerative diseases. Antioxidants 12(7), 1418. Redza-Dutordoir, M. and Averill-Bates, D.A. 2016. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta 1863(12), 2977–2992. Sari, N.M., Yuliani, G.A., Yimer, N., Hernawati, T., Herupradoto, E.B.A. and Hidayatik, N. 2023. Reddragon (Hylocereus polyrhizus) fruit peel extract increased the motility and viability of spermatozoa of hypercholesterolemic rats (Rattus norvegicus). Ovozoa J. Anim. Reprod. 12(1), 33–41. Septiani, R.A., Hamid, I.S., Sabdoningrum, E.K., Ma’ruf, A., Hestianah, E.P. and Mafruchati, M. 2022. Tomato (Lycopersicon esculentum Mill.) juice restored the number of Leydig cells and diameter of the seminiferous tubules of mice (Mus musculus) exposed to lead acetate. Ovozoa J. Anim. Reprod. 11(3), 123–129. Shastak, Y., Obermueller-Jevic, U. and Pelletier, W. 2023. A century of vitamin E: early milestones and future directions in animal nutrition. Agriculture 13(8), 1526. Silviani, M.S., Sukmanadi, M., Kurnijasanti, R., Madyawati, S.P. and Luqman, E.M.. 2022. Green tea (Camellia sinensis) leaf extract maintains spermatozoaplasma membrane integrity, viability, and motility in mice (Mus musculus) exposed to cigarette smoke. Ovozoa J. Anim. Reprod. 11(3), 130–136. Steffensen, I.L., Dirven, H., Couderq, S., David, A., D’Cruz, S.C., Fernández, M.F., Mustieles, V., Rodríguez-Carrillo, A. and Hofer, T. 2020. Bisphenols and oxidative stress biomarker associations found in human studies, evaluation of methods used, and strengths, and weaknesses of the biomarkers. Int. J. Environ. Res. Public Health 17(10), 3609. Traber, M.G. and Stevens, J.F. 2011. Vitamin C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 51(5), 1000–1013. Vergara-Jimenez, M., Almatrafi, M.M. and Fernandez, M.L. 2017. Bioactive components of Moringa oleifera leaves protect against chronic disease. Antioxidants 6(4), 91. Wang, H.Q., Zhang, W.D., Yuan, B. and Zhang, J.B. 2021. Advances in the regulation of mammalian follicle-stimulating hormone secretion. Animals 11(4), 1134. Weydert, C.J. and Cullen, J.J. 2010. Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 5(1), 51–66. Wurlina, W., Mustofa, I., Meles, D.K., Safitri, E., Susilowati, S., Mulyati, S., Utomo, B. and Utama, S. 2022. α-Tocopherol restores semen quality in rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Vet. World 15(2), 316–323. Zirkin, B.R. and Papadopoulos, V. 2018. Leydig cells: formation, function, and regulation. Biol. Reprod. 99(1), 101–111. | ||
| How to Cite this Article |
| Pubmed Style Wurlina W, Mustofa I, Meles DK, Khairullah AR, Akintunde AO, Rachmawati K, Suwasanti N, Putra DMS, Mulyati S, Utama S, Khoiriyah U, Defvyanto BRT, Heriana SF, Riwu KHP, Ahmad RZ, Riwu AG. Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. Open Vet. J.. 2025; 15(1): 416-427. doi:10.5455/OVJ.2025.v15.i1.37 Web Style Wurlina W, Mustofa I, Meles DK, Khairullah AR, Akintunde AO, Rachmawati K, Suwasanti N, Putra DMS, Mulyati S, Utama S, Khoiriyah U, Defvyanto BRT, Heriana SF, Riwu KHP, Ahmad RZ, Riwu AG. Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. https://www.openveterinaryjournal.com/?mno=226188 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.37 AMA (American Medical Association) Style Wurlina W, Mustofa I, Meles DK, Khairullah AR, Akintunde AO, Rachmawati K, Suwasanti N, Putra DMS, Mulyati S, Utama S, Khoiriyah U, Defvyanto BRT, Heriana SF, Riwu KHP, Ahmad RZ, Riwu AG. Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. Open Vet. J.. 2025; 15(1): 416-427. doi:10.5455/OVJ.2025.v15.i1.37 Vancouver/ICMJE Style Wurlina W, Mustofa I, Meles DK, Khairullah AR, Akintunde AO, Rachmawati K, Suwasanti N, Putra DMS, Mulyati S, Utama S, Khoiriyah U, Defvyanto BRT, Heriana SF, Riwu KHP, Ahmad RZ, Riwu AG. Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 416-427. doi:10.5455/OVJ.2025.v15.i1.37 Harvard Style Wurlina, W., Mustofa, . I., Meles, . D. K., Khairullah, . A. R., Akintunde, . A. O., Rachmawati, . K., Suwasanti, . N., Putra, . D. M. S., Mulyati, . S., Utama, . S., Khoiriyah, . U., Defvyanto, . B. R. T., Heriana, . S. F., Riwu, . K. H. P., Ahmad, . R. Z. & Riwu, . A. G. (2025) Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. Open Vet. J., 15 (1), 416-427. doi:10.5455/OVJ.2025.v15.i1.37 Turabian Style Wurlina, Wurlina, Imam Mustofa, Dewa Ketut Meles, Aswin Rafif Khairullah, Adeyinka Oye Akintunde, Kadek Rachmawati, Niluh Suwasanti, Dewa Made Sucipta Putra, Sri Mulyati, Suzanita Utama, Ulul Khoiriyah, Baich R. Tyarraushananda Defvyanto, Sila Faredy Heriana, Katty Hendriana Priscilia Riwu, Riza Zainuddin Ahmad, and Audrey Gracelia Riwu. 2025. Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. Open Veterinary Journal, 15 (1), 416-427. doi:10.5455/OVJ.2025.v15.i1.37 Chicago Style Wurlina, Wurlina, Imam Mustofa, Dewa Ketut Meles, Aswin Rafif Khairullah, Adeyinka Oye Akintunde, Kadek Rachmawati, Niluh Suwasanti, Dewa Made Sucipta Putra, Sri Mulyati, Suzanita Utama, Ulul Khoiriyah, Baich R. Tyarraushananda Defvyanto, Sila Faredy Heriana, Katty Hendriana Priscilia Riwu, Riza Zainuddin Ahmad, and Audrey Gracelia Riwu. "Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract." Open Veterinary Journal 15 (2025), 416-427. doi:10.5455/OVJ.2025.v15.i1.37 MLA (The Modern Language Association) Style Wurlina, Wurlina, Imam Mustofa, Dewa Ketut Meles, Aswin Rafif Khairullah, Adeyinka Oye Akintunde, Kadek Rachmawati, Niluh Suwasanti, Dewa Made Sucipta Putra, Sri Mulyati, Suzanita Utama, Ulul Khoiriyah, Baich R. Tyarraushananda Defvyanto, Sila Faredy Heriana, Katty Hendriana Priscilia Riwu, Riza Zainuddin Ahmad, and Audrey Gracelia Riwu. "Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract." Open Veterinary Journal 15.1 (2025), 416-427. Print. doi:10.5455/OVJ.2025.v15.i1.37 APA (American Psychological Association) Style Wurlina, W., Mustofa, . I., Meles, . D. K., Khairullah, . A. R., Akintunde, . A. O., Rachmawati, . K., Suwasanti, . N., Putra, . D. M. S., Mulyati, . S., Utama, . S., Khoiriyah, . U., Defvyanto, . B. R. T., Heriana, . S. F., Riwu, . K. H. P., Ahmad, . R. Z. & Riwu, . A. G. (2025) Restoration of sperm quality in lead acetate-induced rats via treatment with Moringa oleifera leaf extract. Open Veterinary Journal, 15 (1), 416-427. doi:10.5455/OVJ.2025.v15.i1.37 |