| Research Article | ||
Open Vet. J.. 2024; 14(12): 3487-3497 Open Veterinary Journal, (2024), Vol. 14(12): 3487-3497 Research Article Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imagingMohamed Aref1*, Mustafa Abd El Raouf2, Walaa O. M. Youssef2, Ahmed Abdelbaset-Ismail2, Gamal A. Salem3*, Mohamed A. Nassan4, Catrin S. Rutland5 and Eman A. A. Mahdy11Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Surgery, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Clinical Laboratory Sciences, Turabah University College, Taif University, Taif, Saudi Arabia 5School of Veterinary Medicine and Science, University of Nottingham, Nottingham, UK *Corresponding Author: Gamal A. Salem. Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: gamal_vet_85 [at] yahoo.com Submitted: 29/10/2024 Accepted: 21/11/2024 Published: 31/12/2024 © 2024 Open Veterinary Journal
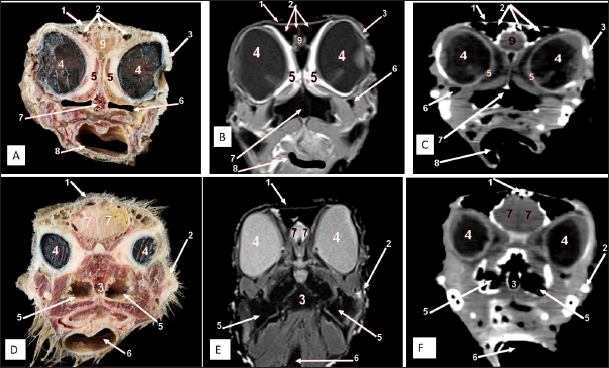
AbstractBackground: The significance of the ostrich (Struthio camelus) has increased recently due to the growth of the global ostrich farming industry. Morphological and diagnostic imaging of the ostrich head presents challenges for enhancing clinical treatment and veterinary care, particularly concerning surgical disorders in the head and paranasal sinuses. Aim: This study aims to guide veterinarians in improving the accuracy of clinical diagnoses and treatment for upper respiratory tract and cranial conditions, particularly in surgical cases involving the head and paranasal sinuses. Methods: Ten healthy adult ostrich heads (Struthio camelus) were collected for anatomical examination. This sample consisted of 5 males (average age: 1.84 ± 0.32 years) and 5 age-matched females (average age: 2.02 ± 0.311 years). The study focused on the cranial, orbital, nasal, and oropharyngeal cavities, along with their contents and paranasal sinuses. The examination included the analysis of bony and cartilaginous structures, as well as soft tissues and cavities, using median, four sagittal, and five cross-anatomical sections. Subsequently, the specimens underwent diagnostic screening using CT and MRI. Results: Here, we found that the ostrich has two oval featherless nostrils covered by a characteristic operculum at its entrance. The nasal septum separates the nasal cavity, which is supported by the rostral cartilaginous part. There were three features of nasal conchae: rostral (T-shape), middle (coiled bullae), and caudal (triangular), which differ from other bird species. Two paranasal sinuses were detected including triangular-shaped infraorbital and two identical frontal sinuses. The maxillary rhamphotheca had a median culmen and lateral tomium, while the mandibular rhamphotheca also had a median gonys and lateral tomium. The brain was divided into the hindbrain (consisting of medulla oblongata and cerebellum), the midbrain (peduncles of the cerebrum and optic lobes), and the forebrain (thalamus, pineal body, hypophysis, optic tracts and chiasm, cerebral hemispheres, and olfactory lobes). All last structures were compared and verified by CT and MRI. Conclusion: This study provides an atlas of anatomical cross-sections, CT, and MRI scans of the ostrich head, which can serve as valuable guidance for veterinarians to improve diagnoses and treatments, ultimately enhancing health outcomes for these birds. Keywords: Computed tomography, Cranium, Magnetic resonance imaging, Ostrich, Paranasal sinuses. IntroductionOstriches, scientifically known as Struthio camelus, are the world’s largest and fastest birds, and the only ones that cannot fly. Adult ostriches typically weigh between 63.5 and 155 kg and lay the largest eggs of any terrestrial animal, making them distinct creatures (Blem and Blem, 1994). The ostrich farming industry has grown globally, producing feathers, low-fat eggs, and meat (Horbańczuk et al., 2008) in addition to several studies determining growth, body characteristics, and hematological parameters of ostrich (Karimi-Kivi and Dadashbeiki, 2015). The increased interest in ostrich anatomy, particularly its morphological structures, represents an exciting potential for further research and discovery (Jamroz, 2000; Peng et al., 2010; Ali, 2015a; Mahdy and Abd El Raouf, 2020). Improving clinical management and care for ostriches can be difficult, especially for surgical problems in the head and paranasal sinuses (Hillyer, 1997; Pye et al., 2000). Thus, studying the anatomical characteristics of the ostrich head and paranasal sinuses is beneficial to avian experts and doctors as earlier studies that make a descriptive anatomical study of the parts of the axial skeleton in the ostrich (Struthio camelus) in addition to a morphometric analysis of each bone (Kassem et al., 2024). Dissection and X-rays were formerly employed to study the morphology of the axial skeleton, including the skull. Some ex vivo 3D computed tomography (CT) models of the skull have also been created without the surrounding soft tissues to compare dinosaur skulls to those of modern reptiles and birds. Various biomedical scanning devices, such as radiography, CT, and MRI, have been used to analyze the ostrich’s body. CT is an excellent tool for imaging the ostrich’s head, particularly in the nasal cavity and quadratomandibular joint (Ali, 2015b); however, the results are limited to anatomical dissection. CT can assist in locating lumps and fluid within birds’ sinuses and may have several studies (Krautwald-Junghanns et al., 1993; Hillyer, 1997; Krautwald-Junghanns, 1998) suggest opportunities for healthcare improvement. MRI, a noninvasive imaging technology, offers precise anatomical multiplanar images with high soft tissue resolution (Krautwald-Junghanns et al., 1993; Krautwald-Junghanns, 1998). Although there has been minimal research on MRI use in avian medicine, particularly in the sinuses and respiratory system (Orosz, 1997; Pye et al., 2000), it has proven to be an effective tool for examining general ostrich anatomy. Materials and MethodsBird’s specimensThe present research was conducted on 10 healthy adult ostriches (Struthio camelus) of both sexes taken from ostrich farms in Bilbies, Sharkia governorate, Egypt. Five males (1.84 ± 0.32 years) and five age-matched females (2.02 ± 0.311 years) were used to examine all structures of the ostrich head, including nasal contents, paranasal sinuses, oropharyngeal, and cranium, were studied. Following slaughter at a commercial slaughterhouse (for non-research purposes), the heads were transported using an ice box and retrieved for anatomical examination, then subjected to CT and MRI scanning. Anatomical sectionsFive ostrich heads were photographed for head exterior features, then dissected and cut into different median, four sagittal, and five cross sectionsrostro-caudally by an electrical saw; all of them were cleaned with tape water to remove debris before being photographed with a Sony® digital camera with 12.1 MP and 4X zoom. No fixatives were utilized to keep the tissues’ original color. CT and MRI scanningThe other five fresh ostrich heads were scanned using a helical CT scanner, the Hispeed NX/I Dual Slice CT (GE, Japan), with the head positioned ventrally to acquire transverse plane images. The scanning parameters were 120 kV for anode voltage and 200 mA for electrical current intensity. The window width and level were modified appropriately to improve bone and soft tissue exposure. Additionally, MRI scanning of the nasal cavity, paranasal sinuses, oropharyngeal cavity, and cranium was performed on the same heads using a 1-tesla MRI machine (Philips, Intra, USA) equipped with an internal magnetom and human body coil. The T1 (longitudinal relaxation time)-weighted transverse plane was created with a short time to echo (TE) and repetition time (TR). Ethical approvalThe Zagazig University Ethical Committee for Animal Care and Use has approved all techniques used in the current study, which followed the worldwide criteria outlined in the approved Institutional Animal Care and Use Committee (IACUC) protocol (ZU IACUC/2/F/443/2023). ResultsNostrils and nasal cavityTwo featherless oval nostrils were found on either side of the premaxilla and covered by a characteristic operculum at the entrance. The upper beak’s dorsal ridge (culmen) was located between both nostrils (Fig. 1A and B). The nasal cavity was cone-shaped, extending to reach the choanae. Dorsolateral bony boundaries of the nasal cavity are the frontal process of the premaxilla, nasal, and dorsal horizontal plate of ethmoidal and nasal bone’s lateral process, while the ventral boundary created by the maxillary and palatine processes of the premaxilla, maxilla, and rostral part of the pterygoid and vomer bones. The vomer bone was pneumatic. The nasal septum divides the nasal cavity into two halves and consists of caudal bony and rostral cartilaginous parts (Fig. 2). There are three types of nasal conchae: rostral, middle, and caudal (Figs. 3–5). The rostral nasal concha extended to reach the middle third of the nasal cavity (Fig. 3), and it was partially visible through the nostrils (Fig. 1B). The cross-section showed a T-shaped projection linked to the nasal cavity’s lateral wall, forming dorsal and ventral recesses. The rostral concha extends caudally beneath the rostral section of the middle nasal concha, forming the middle nasal meatus. At the floor of the nasal cavity, there is a feature mucous membrane fold connecting the rostral concha to the nasal septum (Fig. 6). The middle nasal concha was more prominent than the rostral concha. It was situated on the caudo-dorsally to rostral one and resembled a coiled or bulla-shaped structure. The middle nasal concha was located in the middle of the nasal cavity, between the dorsal and middle nasal meatus (Figs. 3,4,5,6D, and 7A).
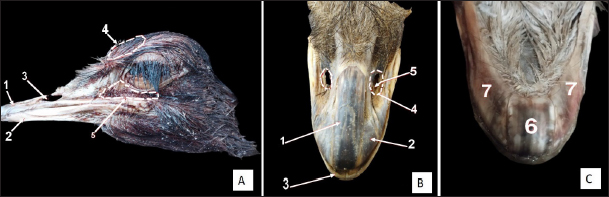
Fig. 1. External views of the adult ostrich head. A) Photograph (dorsolateral view) showing: 1- Upper beak, 2- Lower beak, 3- Nostril, 4- Frontal sinus, 5- Infraorbital sinus. B) Photograph of the maxillary ramphotheca (dorsal view) showing: 1- Culmen, 2- Tomium (maxillary part), 3- Gonys (mandibular rhamphotheca), 4- Nostril, 5- Rostral nasal concha. C) Photograph of the mandibular ramphotheca (ventral view) showing: 6- Gonys and 7- Tomium (mandibular part).
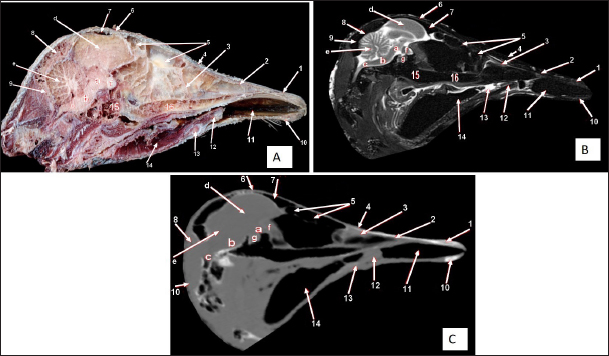
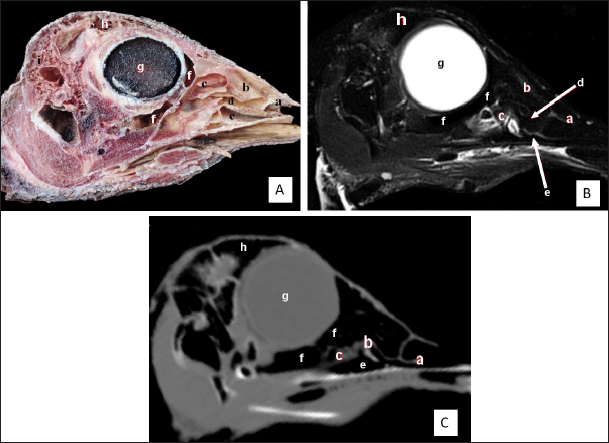
Fig. 2. Sagittal cross section of the adult ostrich head—level 1. A) Photograph of median sagittal anatomical section. B) MRI and C) computed tomography images showing: 1- Os premaxillare, 2- Culmen, 3- Nasal septum, 4- Os nasale, 5- Frontal sinus, 6- Frontal bone, 7- Cranial cavity, 8- Os parietale, 9- Os supraccipitale, 10- Os dentale, 11- Oral cavity, 12- Tongue, 13- Basihyoid, 14- Larynx, 15- Os basisphenoidale, 16- Vomer, and a- Optic lobe, b- Pons, c- Medulla oblongata, d- Cerebral hemisphere, e- Cerebellum, f- Optic chiasma and g- Pituitary gland. The caudal nasal concha is the smallest triangular one. In cross-section, it appeared scroll-like, attached rostrally to the middle one, making it difficult to differentiate between them. While caudally, the ventral nasal meatus was connected to the infraorbital sinus and the oropharyngeal cavity by two slits (or holes) in the palate called choanae. Paranasal sinusesTwo paranasal sinuses were detected, triangular-shaped infraorbital and two identical frontal sinuses as the infraorbital paranasal sinus was identified as a triangular chamber rostroventral to the orbit (Figs. 3 and 8A), while the frontal paranasal sinuses were positioned dorsally, separating the cranial and nasal cavities. There were two frontal sinuses, one on the right and one on the left, separated by a bone septum in the center. The frontal sinuses were found between the frontal and nasal bones, dorsally and laterally, between the lacrimal and vertical plates of the ethmoidal bone (Figs. 2, 3, 4, 7D, 8, and 9A). All last structures were compared and verified by CT and MRI, to guarantee precise identification and labeling; the anatomical section structures for each cross-section level were matched to their corresponding MRI and CT images. In CT images, the bones appeared white (high CT density), the gaseous parts were black (the lowest attenuation), and the soft tissue was grey (low-to-intermediate CT density). In MR images, bones appeared white (high signal intensity), the gaseous parts were black (no signal intensity), and the soft tissue was grey (low-to-intermediate signal intensity).
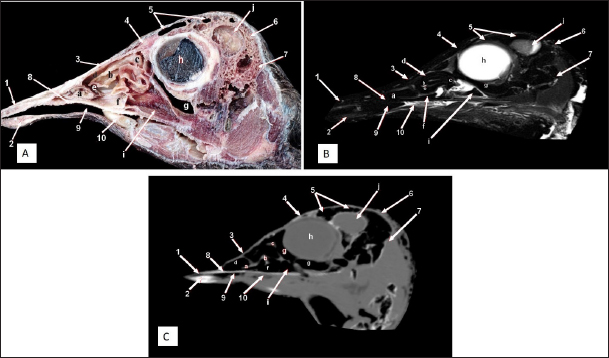
Fig. 3. Sagittal cross section of the adult ostrich head—level 2. A) Photograph of a paramedian sagittal anatomical section (medial view). B) MRI and C) computed tomography images showing: 1- Os premaxillare, 2- Os dentale, 3- Os nasale, 4- Processus. frontalis, 5- Frontal sinus, 6- Os parietale, 7- Os temporalis, 8-Palate, 9- Oral cavity, 10- Tongue, a- Rostral nasal concha, b- Middle nasal concha, c- Caudal nasal concha, d- Dorsal nasal meatus, e- Middle nasal meatus, f- Ventral nasal meatus, g- Infraorbital sinus, h- Orbit containing eye ball, i- Choanal opening and j-Cranial cavity.
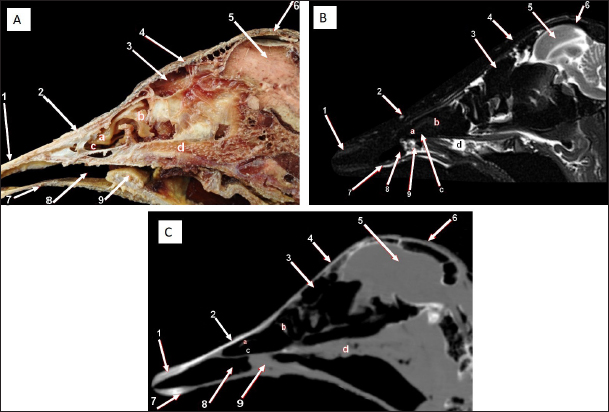
Fig. 4. Sagittal cross section of the adult ostrich head—level 3. A) Photograph of a paramedian sagittal anatomical section (medial view). B) MRI and C) computed tomography images showing: 1- Os premaxillare, 2- Os nasale, 3- Frontal sinus, 4- Processus.frontalis, 5- Cerebral hemisphere, 6- Os frontalis, 7- Os dentale, 8- Oral cavity, 9- Tongue, a- Middle nasal concha (rostral part), b- Middle nasal concha (caudal part), c- Ventral nasal meatus and d- Vomer.
Fig. 5. Sagittal cross section of the adult ostrich head—level 4. A) Photograph of paramedian sagittal anatomical section (medial view). B) MRI and C) computed tomography images showing: a- Rostral nasal concha, b- Middle nasal concha, c- Caudal nasal concha, d- Middle nasal meatus, e- Ventral nasal meatus, f- Infraorbital sinus, g- Orbit containing eye ball, h- Frontal sinus and i- Os temporalis. The nasal conchae exhibited in the MRI images as bright (high or hyperintense signal intensity) to gray (low or hypointense signal intensity) structures that could be divided into three parts: rostral, middle, and caudal conchae, which correlated to the morphological examinations. In contrast, the nasal conchae could not be recognized clearly on CT images compared to those obtained after MRI (Figs. 2–5 B and C). The paranasal sinuses with other structures could be identified using both CT and MRI. The nasal septum and paranasal sinuses revealed a white bone border (high signal intensity), soft tissue was grey (low-to-intermediate signal intensity), and they contained black gas, so both the CT and MRI scans showed little signal intensity (Figs. 1–5B and C and 7–9B, C, E, F). Oropharyngeal cavityThe characteristic ostrich beak was made up of the top jaw (premaxilla) and lower jaw (mandibular symphysis and dentary bones), as well as their keratinized covers (maxillary and mandibular rhamphotheca) (Fig. 1). The beak had a triangular form with a thin rostral apex. The maxillary rhamphotheca had a median culmen and lateral tomium, whereas the mandibular rhamphotheca had a median gonys and lateral tomium (Fig. 1B and C). The oropharyngeal cavity appeared compressed dorsoventrally, similar to the shape of a bell, and supported dorsally by the palate and ventrally by the mandible. The ostrich’s tongue was tiny and located on the floor of the oropharynx (Figs. 2–4 and 6D). Oropharyngeal CT and MRI studies confirmed the anatomical findings. MRI provided a greater appreciation and visualization of the various soft tissue structures than CT scanning (Figs. 2–4 and 6E and F).
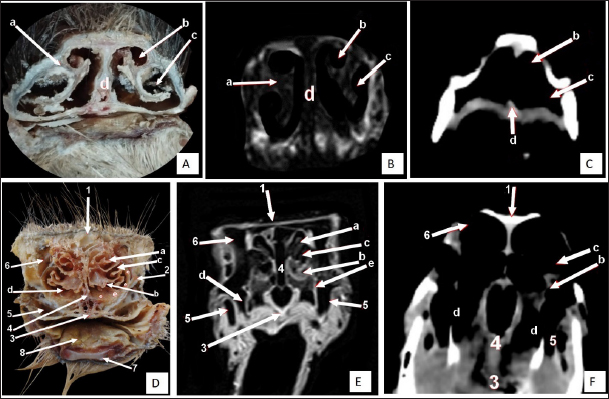
Fig. 6. Rostral-caudal cross sections of the adult ostrich head—level 1. Rostral views of A) photograph of an anatomical cross section, B) MRI and C) computed tomography images (rostral view) showing: a- Rostral nasal concha, b- Dorsal nasal recess, c- Ventral nasal recess, d- Nasal septum. Caudal views of D) an anatomical section photograph, E) MRI and F) computed tomography images showing: 1- Os nasale, 2- Os maxillare, 3- Vomer, 4- Nasal septum, 5- Infra orbital sinus, 6- Frontal sinus, 7- Mandible, 8- Tongue, a- Middle nasal concha (bulla), b- Middle nasal concha, c- Middle nasal meatus, d- Ventral nasal meatus, and e- Rostral nasal concha mucosal fold. Cranium and brainThe ostrich’s cranial cavity was bounded by frontal bone dorsally, basioccipital and basisphenoid bones ventrally, supraoccipital and parietal bones caudally, interorbital septum rostrally, and temporal bone laterally. The parietal, sphenoid, and temporal bones were pneumatic (Figs. 2, 3, 9, and 10). The brain was divided into three parts: the hindbrain (medulla oblongata and cerebellum), the midbrain (cerebrum’s peduncles and optic lobes), and the forebrain (thalamus, pineal body, hypophysis, optic tracts, and chiasm, cerebral hemispheres, and olfactory lobes) (Figs. 2, 9, and 10). The sagittal section of the brain includes the olfactory bulb, cerebrum, cerebellum, medulla oblongata, optic lobe, optic chiasm, and hypophysis (Fig. 2). The dorsal median longitudinal fissure separates the cerebrum into two hemispheres. The sagittal prominence (west) was present on both sides of this fissure (Figs. 9 and 10). The CT pictures revealed the skull as a readily discernible white structure due to its high CT density, while the brain appeared as a grey structure due to its intermediate density. However, the settings utilized made it impossible to identify different areas of the brain in the CT scans (Figs. 9 and 10C, F). The MRI pictures showed the brain as a whitish structure with a high signal intensity. This technique was used to identify various brain structures, including the hindbrain (medulla oblongata, and cerebellum), the midbrain (peduncles of the cerebrum, optic lobes), and the forebrain (thalamus, pineal body, hypophysis, optic tracts and chiasm, cerebral hemispheres, and olfactory lobes) (Figs. 2B, 9 E, and 10E). Additionally, the olfactory bulb, cerebrum, cerebellum, medulla oblongata, optic lobe, optic chiasm, thalamus, and hypophysis. DiscussionThe standard external anatomical features of the ostrich head in this study could effectively help veterinarians diagnose various head disorders in ostriches. Diphtheritic lesions produce pharyngitis and tracheitis, which affect feeding and respiration and cause mortalities in ostriches (ElAbasy et al., 2017; Waziri, M. I. and Saidu, 2022). Also, diseases of the eyes and their muscles adversely influence homeostasis, leading to reproductive disorders, loss in weight, and egg production (Klećkowska-Nawrot et al., 2018). This investigation provides essential anatomical, CT, and MRI data about the nasal cavity of ostrich that will be required in detecting mycotic rhinitis in ostrich. The nasal passages revealed Aspergillus granuloma that was suggested to be occurring owing to inhalation of plant material (Papkoff et al., 1982).
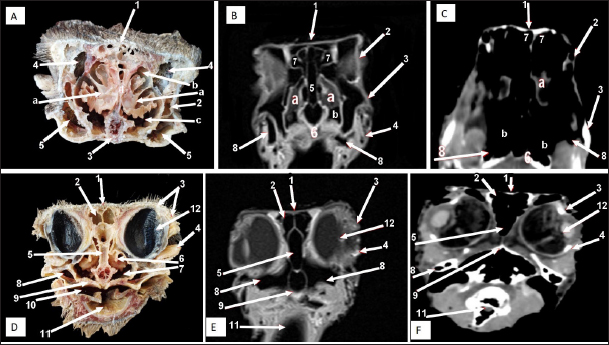
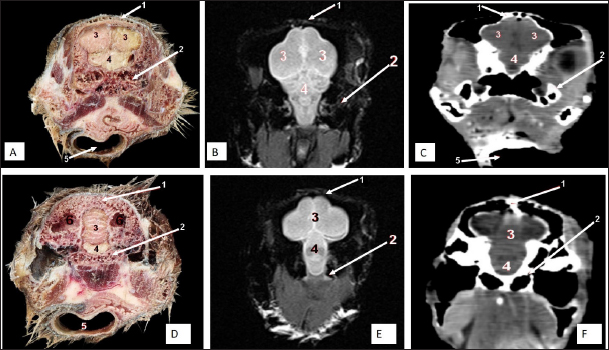
Fig. 7. Rostral-caudal cross sections of the adult ostrich head—level 2. Rostral views of A) photograph of an anatomical cross section, B) MRI and C) computed tomography images showing: 1- Processus.frontalis, 2- Os maxillare, 3- Vomer 4- beginning of the orbit, 5- Infra orbital sinus, a- Middle nasal concha, b- Dorsal nasal meatus, c- Ventral nasal meatus, d- Nasal septum. Caudal views of D) anatomical cross section, E) MRI, and F) computed tomography images showing: 1- Processus. frontalis, 2- Frontal sinus (separated by the median septum), 3- Lateral nasal process, 4- Processus.jugalis, 5- Nasal septum (two ridges separated by an empty groove), 6- Caudal nasal concha, 7- Ventral nasal meatus, 8- Infra orbital sinus, 9- Palate, 10- Oropharynx, 11- Laryngeal cavity, and 12- Eye ball.
Fig. 8. Rostral-caudal cross sections of the adult ostrich head—level 3. Rostral views of A) photograph of an anatomical cross section, B) MRI and computed tomography images showing: 1- Os nasalis, 2- Frontal sinus (divided by septea), 3- Lateral nasal process, 4-Os jugalis, 5- Vomer (pneumatic), 6- Nasal septum (two ridges separated by an empty groove), 7- Laryngeal cartilage, 8- Laryngeal cavity, 9- Eye ball, 10- Infra orbital sinus, 11- Choanae. Caudal view of D) an anatomical section, E) MRI and F) computed tomography images showing: 1- Os nasalis, 2- Frontal sinus, 3- Lateral nasal process, 4- Eye ball, 5- Os jugalis, 6- Pars verticalis ethmoidale, 7- Vomer, 8- Infra orbital sinus, 9-Latyngeal cartilage, 10- Tracheal lumen surrounded by tracheal cartilaginous rings.
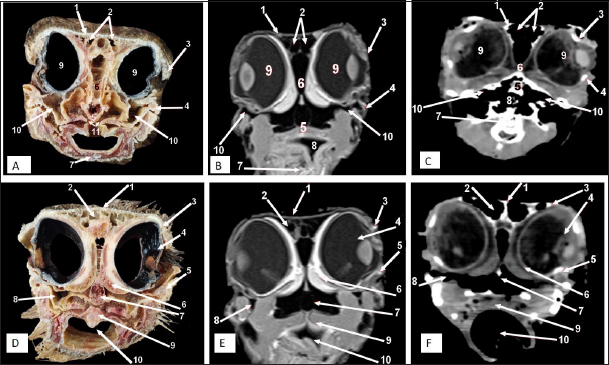
Fig. 9. Rostral-caudal cross sections of the adult ostrich head—level 4. Rostral views of A) photograph of an anatomical cross section, B) MRI and C) computed tomography images showing: 1- Os frontalis, 2- Frontal sinus, 3- Processus quadratojugale, 4- Eye ball, 5- Pars verticalis ethmoidale, 6- Infra orbital sinus, 7- Vomer, 8- Tracheal lumen surrounded by tracheal cartilaginous rings, 9- Sagittal eminence (wulst) of the cerebrum. Caudal views of D) an anatomical cross section, E) MRI and F) computed tomography images showing: 1- Os frontalis, 2- Processus. quadratojugale, 3- Vomer, 4- Eye ball, 5- Infra orbital sinus, 6- Trachea, 7- Cerebral hemispheres of the brain.
Fig. 10. Rostral-caudal cross sections of the adult ostrich head—level 5. Rostral view of A) photograph of an anatomical cross section, B) MRI and C) computed tomography images showing: 1- Os parietale, 2- Os basisphenoidale, 3- Cerebral hemispheres, 4- Mid brain, 5- Trachea Plate. Caudal views of D) an anatomical section, E) MRI and F) computed tomography images showing: 1- Os parietale, 2- Os basioccipitale, 3- Cerebellum, 4- Pons, 5- Trachea, 6- Os temporalis. As in the earlier study, our anatomical examinations of the head ostrich clarify all bony boundaries of the nasal, oropharyngeal, and cranial cavities (Moselhy et al., 2018). Accordingly, the nasal cavity was entirely separated by the median septum into two separate halves(Ali, 2015b). On the contrary, the nasal cavity of Wanxi white geese and ducks was communicated through a long, narrow opening (Mushi et al., 1998). As in the previous studies, the nasal septum consisted of the rostral cartilaginous and caudal bony parts (Baumel et al., 1993; Ali, 2015b). The nasal conchae within the nasal cavity help improve air ventilation and circulation within the nasal cavity and increase the cavity’s surface area (Deeming and Angel, 1996; Geist, 2000). There are three ostrich nasal conchae and meatuses (Ali, 2015b; Peng et al., 2010) as three nasal conchae were also reported in ducks, geese, and quails (Peng et al., 2010), moorhens (Hanafy, 2021), and broad-breasted white turkey (Schumacher and Tellhelm, 2022) but only two nasal conchae (rostral and caudal) in hooded crow (Hassan, 2012), dove (Madkour, 2019), and common moorhen (Khazaal Waad et al., 2020) while the absence of nasal conchae was reported in the parrot (Schmidt et al., 2015). Previous work that described the rostral nasal concha cross-section as C-shaped in the ostrich also assessed the shapes and relative sizes of the conchae. In contrast, the rostral concha in the present ostrich study was a T-shaped projection attached to the lateral wall of the nasal cavity and showed dorsal and ventral recesses. The structure also prolonged caudally underneath the rostral portion of the middle nasal concha, forming the middle nasal meatus (Mohamed, 2008). In the same line, the middle concha was also the largest of the three conchae and occupied a large nasal cavity area that appeared coiled and curled, ending in a sac-like dilation in the cross-sections that those reported (Peng et al., 2010; Ali, 2015b) in ostrich and (Bang, BG, Wenzel, 1985; Bang, 1971) in the domestic chicken. The ostrich has a higher olfactory ability than the chicken (Ali, 2015a), as scrolling of the nasal conchae allows better humidification of inhaled air, this hypothesized explanation in the same line with previous publications confirmed the middle nasal concha was scrolled rostrally and caudally in duck and goose (Madkour, 2019), moorhen (Hanafy, 2021), nd broad-breasted white turkey (Madkour, 2022). The nasal conchae in the present study could be observed in both the CT and MRI images but with superior clarity in the MRI images. Consistently, there is more differentiation of nasal mucosa than other soft tissue in MRI images compared to CT images in dogs (De Rycke et al., 2003). A human study of the nasal sinuses also reported the superiority of MRI to CT in detecting incidental nasal abnormalities (Nazri et al., 2013). On the contrary, the middle concha of the ostrich could be observed by anatomical examination and CT images but did not conduct MRI. As in other research, two paranasal sinuses were observed in the ostrich head via gross anatomical examination and diagnostic imaging using CT and MRI as the infraorbital and frontal sinuses (Mohamed, 2008), but in contrast, other publications noted that only the frontal sinus identified (Ali, 2015a; Moselhy et al., 2018). Although all three previous studies were also on the ostrich, they used limited methods, such as dissection and/or X-ray. The infraorbital sinus was observed as a triangular-shaped cavity beneath the orbit, which extended rostroventrally as in the quail, dove, duck, and goose (Madkour, 2019) but as a round-shaped structure in both the moorhen and the broad-breasted white turkey (Hanafy, 2021). Due to the gaseous content of the paranasal sinuses, their boundaries were readily distinguished in CT and MRI scans. Ostriches can ingest anything, such as fruit, plants, roots, insects, small rodents, or Lizards (Abbas et al., 2017). This may be attributed to a strong beak and a large oropharyngeal cavity of the ostrich. The available information about the ostrich oropharyngeal cavity is helpful in the identification of bilateral granulomatous inflammation of the pharyngeal tonsils in a South African ostrich caused by avian mycobacteriosis. Continued growth of these tonsils will interfere with proper drinking, feeding, and respiration (Crole et al., 2013). The boundaries and components of the oropharyngeal cavity in the ostrich align with earlier descriptions established through gross anatomical studies (Deeming and Angel, 1996; Pasand et al., 2011; Tivane et al., 2011). This investigation further corroborates the previously documented gross anatomical characteristics of the ostrich tongue (Pasand et al., 2011). In this study, the intricate structures of the oropharynx were effectively visualized using MRI technology, whereas CT scanning provided comparatively limited detail. The brain’s morphology depicted in the current sagittal and cross-sectional images was consistent with findings from prior research on ostriches (Pasand et al., 2011). Moreover, both CT and MRI modalities facilitated the identification of brain structures, with MRI offering enhanced clarity. The anatomical sections also revealed the cranial structures, allowing for precise identification of features and facilitating topographical analysis. The sagittal section of the ostrich head and MRI scanning revealed that the olfactory bulbs of the ostrich brain are small, so the olfaction is ill-developed. As our study shows, all complicated physiological functions of the ostrich are controlled by the cerebellum, which is more prominent with more complex physiological functions. Previous experiments display that dyskinesia, hypomotility, involuntary movement, and disequilibrium occur as a result of injuring the cerebellum (Peng et al., 2010). Ostrichs depend on their leg movement (kicking) as a defense mechanism against enemies. This characteristic function is closely correlated to the large ostrich cerebellum (Karkoura et al., 2015). Ostrich’s neck moves in rolling and lever patterns during the feeding behavior. The pituitary glands of the ostrich regulate most of the body functions by secretion of several hormones such as growth hormone, prolactin, follicle-stimulating hormone, luteinizing hormone, and thyrotropin. Further studies into the pituitary glands of the ostrich and its function are warranted. A preceding study confirmed that by resecting the cerebellum, the cervical and leg muscles cramp, and the head shakes (Papkoff et al., 1982; Nakano et al., 2023). Insufficient sample size due to a limited number of available ostrich farms in Egypt, in addition to a lack of previous research studies specially on MRI scanning of ostrich head are the main limitations for this research. Meanwhile, the implication of MRI scans of ostrich head in this work strengthens the findings since it is considered the best tool to image soft tissue structures enabling accurate features of head investigation. ConclusionThis study presents a tri-method atlas that compares serial anatomical sections with various diagnostic imaging techniques, including CT and MRI, taken at regular intervals throughout the ostrich head. The atlas aims to guide veterinarians in improving the accuracy of clinical diagnoses and treatment for upper respiratory tract and cranial conditions, particularly in surgical cases involving the head and paranasal sinuses. This approach is suggested to lead to better health outcomes for these unique birds. AcknowledgmentsThe authors express their gratitude to the staff members of the Anatomy and Embryology and Surgery, Anesthesiology and Radiology Departments, Faculty of Veterinary Medicine at Zagazig University, Egypt, for their support during our work. Conflict of interestThe authors confirm that we do not have any conflicts of interest to declare. FundingThe authors did not receive any external funds for this study. Authors’ contributionsM.A., AAI, M.A.E, M.N., E.E., W.Y., and E.A.A.M. executed the experiments, validated the results, shared in writing the original manuscript, and performed data curation and analysis. M.A., G.A.S., and C.S.R. conceptualized the study, abstracted the work, and wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript. Data availabilityData not already included in the publications is available from the authors following reasonable requests. ReferencesAbbas, G., Mahmood, S., Sajid, M. and Yaser.A. 2017. Ostrich farming: a new turn in poultry industry of Pakistan. Adv. Zool. Bot. 5, 33–38. Ali, S. 2015a. Gross Anatomical Studies on The Nasal Cavity of The Ostrich. Benha. Vet. Med. J. 29, 326–332. Ali, S. 2015b. Some morphological studies on the quadratomandibular joint of ostrich (Struthio camelus). Benha. Vet. Med. J. 29, 319–325. Bang, B.G. and Wenzel, B. 1985. Nasal cavity and olfactory system. Form. Funct. Birds. 3, 195–225. Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E. and Vanden Berge, J.C.I.J.J. 1993. Nomina Anatomica Avium. Handbook of Avian Anatomy. 2nd ed. World Assoc. Vet. Anat. Internatonal Comm. Avian Anat. Nomencl, pp: 45–132. Blem, C.R. and Blem, C.R. 1994. Handbook of the Birds of the World. Volume 1: Ostrich to ducks. Eds., del Hoyo J, Elliott A, Sargatal J. Wilson Bull. Lynx Edicions, The University of Chicago Press, 106, 575. De Rycke, L.M., Saunders, J.H., Gielen, I.M., van Bree, H.J. and Simoens, P.J. 2003. Magnetic resonance imaging, computed tomography, and cross-sectional views of the anatomy of normal nasal cavities and paranasal sinuses in mesaticephalic dogs. Am. J. Vet. Res. 64, 1093–1098. Deeming, D.C. and Angel, C. 1996. Introduction to the ratites and farming operations around the world. Improving our understanding of ratites in a farming environment, in Ratite Conference. Oxfordshire, UK, pp: 1–4. Geist, N.R. 2000. Nasal respiratory turbinate function in birds. Physiol. Biochem. Zool. 73, 581–589. Hanafy, B. 2021. Structural adaption of the nasal conchae of Eurasian common moorhen (Gallinula chloropus chloropus, Linnaeus, 1758) Histomorphological study. Microscopy Res. Tech. 84, 2195–2202. Hassan, S. 2012. Gross anatomical features of the nasal cavity of the hooded crow (Corvuscornix). Suez. Canal. Univ. Med. J.17, 119–127. Hillyer, E.V. 1997. Clinical manifestations of respiratory disorders. In Eds., Altman R.B., Clubb S.L., Dorrestein G.M. and Quesenberry K. Avian Med. Surg. Philadelphia, PA: WB Saunders, pp: 394–411. Horbańczuk, J.O., Tomasik, C. and Cooper, R.G. 2008. Ostrich farming in Poland—Its history and current situation after accession to the European Union. Avian. Biol. Res. 1, 65–71. Jamroz, D. 2000. Feeding ostriches and emus-physiological basis and nutritional requirements-a review. Prace. Materiały. Zootechniczne. 56, 51–73. Karimi-Kivi, R. and Dadashbeiki, M.S.A. 2015. Growth, body characteristics and blood parameters of ostrich chickens receiving commercial probiotics. Spanish. J. Agric. Res. 13(1), 604–615. Karkoura, A., Alsafy, M. and Elgendy, S. E.F. 2015. Morphological investigation of the brain of the African Ostrich (Struthio camelus). Int. J. Morphol. 33, 1468–1475. Kassem, M.A.M., Tahon, R.R. and El-Ayat, M.A. 2024. Anatomical and morphometric studies on the axial skeleton of ostrich (Struthio camelus). Zoomorphology. 143, 231–248. Khazaal Waad, S., Sajid Hashim, W. and Adeeb Mohamed, A. 2020. Morphological and histological study of concha in moorhen (Gallinula). Int. J. Psychosoc. Rehabil. 24, 2020. Klećkowska-Nawrot, J., Goździewska-Harłajczuk, K., Barszcz, K. and Janeczek, M. 2018. Morphology of the extraocular muscles (m. bulbi) in the pre-hatchling and post-hatchling african black ostriches ( Struthio camelus domesticus l., 1758) (aves: Struthioniformes). Acta Biol. Hung. 69, 42–57. Krautwald-Junghanns, M.E.V.K. 1998. Comparative studies on the diagnostic value of conventional radiography and computed tomography in evaluating the heads of psittacine and raptorial birds. J. Avian Med. Surg. 12, 149–157. Krautwald-Junghanns, M.E., Schumacher, F. and Tellhelm, B. 1993. Evaluation of the lower respiratory tract in psittacines using radiology and computed tomography. Vet. Radiol. Ultrasound 34, 382–390. Madkour, F.A. 2019. Anatomical descriptions of the nasal cavity of the Aquatic and Non-aquatic birds. Int. J. Vet. Sci. 2, 101–110. Mahdy, E. and Abd El Raouf, M. 2020. Normal anatomical and diagnostic imaging techniques of the musculotendinous structures of the ostrich (Struthio camelus) foot. J. Adv. Vet. Anim. Res. 7, 242–252. Mohamed, S. 2008. Some morphological studies of the nasal cavity of ostrich (Struthio camelus). Zagazig Univ. J. (Doctoral dissertation, Zagazig University), Egypt. Mushi, E.Z., Isa, J.F.W., Chabo, R.G. and Segaise, T.T. 1998. Growth rate of ostrich (Struthio camelus) chicks under intensive management in Botswana. Trop. Anim. Health Prod. 30, 197–203. Nakano, K., Gunji, M., Ikeda, M., Keung,O.,Mitsuhito, A. and Katsuma, I. 2023. “RobOstrich” manipulator: a novel mechanical design and control based on the anatomy and behavior of an ostrich neck. IEEE Robot. Autom. Lett. 8, 3062–3069. Nazri, M., Bux, S.I., Tengku-Kamalden, T.F., Ng, K.H. and Sun, Z. 2013. Incidental detection of sinus mucosal abnormalities on CT and MRI i maging of the head. Quant. Imaging Med. Surg. 3, 82–88. Orosz, S. 1997. Anatomy of the respiratory system In Altman, R.B., Clubb, S.L., Dorrestein, G.M. and Quesenberry, K. Avian Med. Surg. 1,3–17. Papkoff, H., Licht, P., Bona-Gallo, A., MacKenzie D.S., Oelofsen.W. and Oosthuizen, M.M.J. 1982. Biochemical and immunological characterization of pituitary hormones from the ostrich (Struthio camelus). Gen. Comp. Endocrinol. 48, 181–195. Pasand, A., Tadjalli, M. and Parto, P. 2011. Gross anatomy of the tongue in male ostrich. World J. Zool. 6, 346–349. Peng, K., Feng, Y., Zhang, G., Liu, H. and Song, H. 2010. Anatomical study of the brain of the African ostrich. Turkish J. Vet. Anim. Sci. 34, 235–241. Pye, G., Bennett, R., Newell, S., Kindred, J. and Johns, R. 2000. Magnetic resonance imaging in psittacine birds with chronic sinusitis. J. Avian. Med. Surg. 14, 243–256. Schmidt, R., Struthers, J. and Phalen, D. 2015. Pathology of pet and aviary birds. Hoboken, NJ: John Wiley Sons. Schumacher, F. and Tellhelm, B. 2022. Beak, Oropharyngeal and nasal cavities of broad breasted white Turkey (Meleagris gallopavo): gross anatomical and Morphometrical study. J. Adv. Vet. Res. 12, 99–106. Tivane, C., Rodrigues M.N., Soley, J.T. and Groenwald, H.B. 2011. Gross anatomical features of the oropharyngeal cavity of the ostrich (Struthio camelus). Pesqui. Veterinária. Bras. 31, 543–550. | ||
| How to Cite this Article |
| Pubmed Style Aref M, Raouf MAE, Abdelbaset-ismail A, Salem GA, Youssef WOM, Nassan MA, Rutland CS, Mahdy EAA. Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. Open Vet. J.. 2024; 14(12): 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 Web Style Aref M, Raouf MAE, Abdelbaset-ismail A, Salem GA, Youssef WOM, Nassan MA, Rutland CS, Mahdy EAA. Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. https://www.openveterinaryjournal.com/?mno=226478 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i12.32 AMA (American Medical Association) Style Aref M, Raouf MAE, Abdelbaset-ismail A, Salem GA, Youssef WOM, Nassan MA, Rutland CS, Mahdy EAA. Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. Open Vet. J.. 2024; 14(12): 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 Vancouver/ICMJE Style Aref M, Raouf MAE, Abdelbaset-ismail A, Salem GA, Youssef WOM, Nassan MA, Rutland CS, Mahdy EAA. Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. Open Vet. J.. (2024), [cited January 12, 2026]; 14(12): 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 Harvard Style Aref, M., Raouf, . M. A. E., Abdelbaset-ismail, . A., Salem, . G. A., Youssef, . W. O. M., Nassan, . M. A., Rutland, . C. S. & Mahdy, . E. A. A. (2024) Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. Open Vet. J., 14 (12), 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 Turabian Style Aref, Mohamed, Mustafa Abd El Raouf, Ahmed Abdelbaset-ismail, Gamal A. Salem, Walaa O. M. Youssef, Mohamed A. Nassan, Catrin S. Rutland, and Eman A. A. Mahdy. 2024. Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. Open Veterinary Journal, 14 (12), 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 Chicago Style Aref, Mohamed, Mustafa Abd El Raouf, Ahmed Abdelbaset-ismail, Gamal A. Salem, Walaa O. M. Youssef, Mohamed A. Nassan, Catrin S. Rutland, and Eman A. A. Mahdy. "Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging." Open Veterinary Journal 14 (2024), 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 MLA (The Modern Language Association) Style Aref, Mohamed, Mustafa Abd El Raouf, Ahmed Abdelbaset-ismail, Gamal A. Salem, Walaa O. M. Youssef, Mohamed A. Nassan, Catrin S. Rutland, and Eman A. A. Mahdy. "Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging." Open Veterinary Journal 14.12 (2024), 3487-3497. Print. doi:10.5455/OVJ.2024.v14.i12.32 APA (American Psychological Association) Style Aref, M., Raouf, . M. A. E., Abdelbaset-ismail, . A., Salem, . G. A., Youssef, . W. O. M., Nassan, . M. A., Rutland, . C. S. & Mahdy, . E. A. A. (2024) Structural investigations of the normal ostrich head using anatomical sections, computed tomography, and magnetic resonance imaging. Open Veterinary Journal, 14 (12), 3487-3497. doi:10.5455/OVJ.2024.v14.i12.32 |