| Research Article | ||
Open Vet. J.. 2025; 15(1): 402-415 Open Veterinary Journal, (2025), Vol. 15(1): 402-415 Research Article Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in ratsBarakat M. Alrashdi1*, Mahmoud Ashry2, Mousa O. Germoush1, Maged Fouda1, Ibrahim Abdel-Farid1, Diaa Massoud1, Fayez Shaldoum1, Ahmed E. Abdel Moneim3, Ali G. Gadel-Rab2, Mohamed Mahrous4, Mohamed H.A. Gadelmawla5 and Hussam Askar21Biology Department, College of Science, Jouf University, Sakaka, Saudi Arabia 2Zoology Department, Faculty of Science, Al-Azhar University, 71524 Assuit, Egypt 3Zoology and Entomology Department, Faculty of Science, Helwan University, Cairo, Egypt 4Department of Biochemistry, Faculty of Pharmacy, Port Said University, Port Said, Egypt 5Department of Histology, Faculty of Dentistry, Sinai University, Kantara, Egypt *Corresponding Author: Barakat M. Alrashdi. Biology Department, College of Science, Jouf University, Sakaka, Saudi Arabia. Email: bmalrashdi [at] ju.edu.sa Submitted: 28/10/2024 Accepted: 31/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
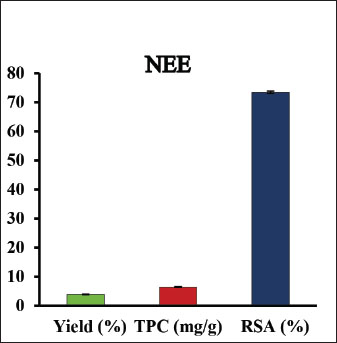
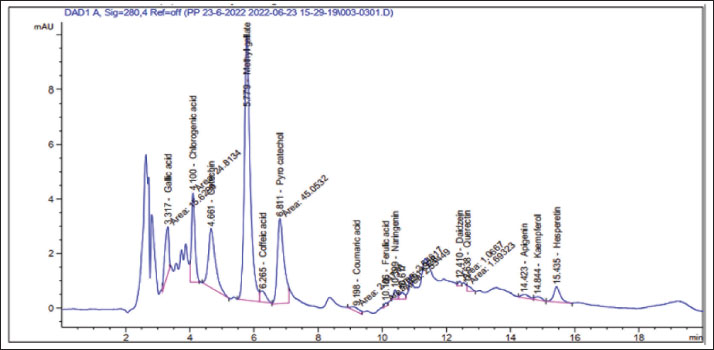
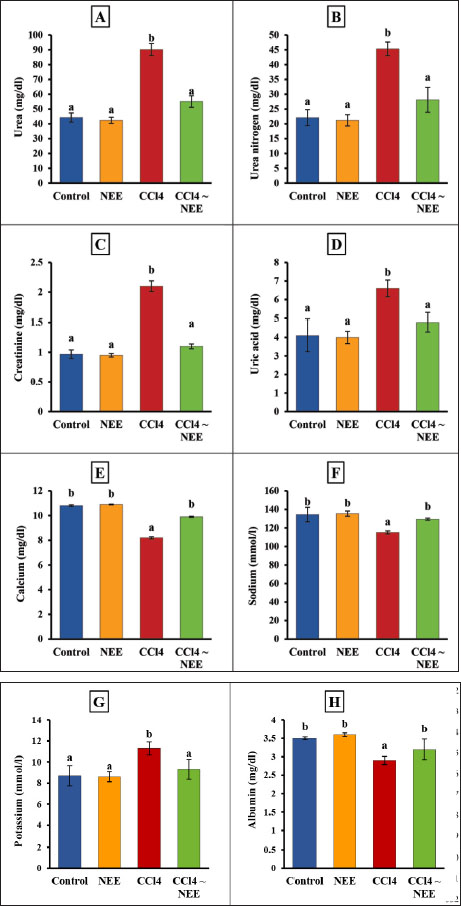
AbstractBackground: Exposure to carbon tetrachloride (CCl4) induces acute and chronic kidney damage alongside oxidative stress in rats. Aim: This study examines Nigella sativa ethanolic extract’s (NEE) potential barriers against CCl4-induced nephrotoxicity. Methods: Wistar albino male rats weighing between 150 and 200 g were acclimatized and randomly divided into four groups, each comprising 10 animals. The control group consisted of healthy rats; the second group received oral administration of 200 mg/kg NEE for six weeks; the third group received intraperitoneal injections of CCl4 at a dose of 0.5 mg/kg twice weekly for six weeks; and the fourth group received both oral NEE and CCl4. Results: Results indicate that NEE significantly mitigated renal degeneration induced by CCl4, evidenced by notable reductions in creatinine, urea, urea nitrogen, uric acid, potassium, IL-1β, IL-4, IL-6, IL-10, TNF-α, renal NO, MDA, and DNA fragmentation, coupled with substantial increases in kidney SOD, GPx, GSH, and CAT levels. Additionally, CD4, Albumin, Sodium, Calcium, Immunohistochemistry, and Histopathological analyses revealed marked regenerative effects. Conclusion: In conclusion, NEE exhibits anti-nephrotoxic, anti-inflammatory, and antioxidant properties, with a likely mediation by its antioxidant constituents. The radical scavenging activity, particularly the high phenolic content of its active component, suggests NEE’s potential efficacy as a nephroprotective supplement. Keywords: Nephrotoxicity, CCl4, Nigella sativa, Anti-inflammatory, Antioxidant, Rat. IntroductionThrough filtration and excretion, the kidney is crucial in eliminating harmful compounds from the body. Because of its high blood flow and complex cellular transport networks, which lead to a buildup of these substances within nephron epithelial cells, it is particularly susceptible to toxic effects from medications and poisons (Kumar et al., 2013; Adewale and Orhue, 2015). Renal failure, which is defined by a high rate of morbidity and death and a loss of the kidney’s ability to remove waste, collect urine, preserve electrolytes, and maintain fluid balance, is becoming more common at an alarming rate (Komail and Narendra, 2017; Sabra et al., 2023). To treat liver and kidney issues, several synthetic drugs are employed; however, they frequently have unfavorable side effects. Considering these restrictions, researchers are examining the safety and effectiveness of new medications made from natural sources (Delgado-Montemayor et al., 2015; Joshy et al., 2016; Gogoi et al., 2017). Solvents, cleaning products, and chlorofluorocarbons are all synthesized using the organic molecule carbon tetrachloride (CCl4) (Karabulut et al., 2021). Moreover, oxidative stress and free radicals contribute to CCl4-induced nephrotoxicity, which is regulated by cytochrome P450 (Nomier et al., 2022). Immune response, cellular migration, adhesion, and proliferation are all significantly influenced by the proinflammatory cytokines. The escalation of inflammatory responses is promoted by interleukins and tumor necrosis factors (TNFR) (Ashry et al., 2021). The chemical compound CCl4 has also been linked to cell apoptosis, resulting in several modifications to cellular morphology, including mRNA degradation, DNA fragmentation, and cell shrinkage (Safhi, 2018). Caspase 3 and 9 activation starts cellular apoptosis, which breaks down proteins and causes cell death (Rudel, 1999). Recently, plants have been used as an important source of many biologically active compounds for medication discovery (Abdel-Wahhab et al., 2021). Herbal extracts have been shown to mitigate the oxidative stress brought on by CCl4 by increasing the reduction of lipid peroxidation levels and antioxidant enzyme activity. Ranunculaceae includes the green plant N. sativa, also known as the black seed. Flavonoids, alkaloids, polyphenols, saponins, 36%–38% settling oils, and 0.4%–2.5% of essential oil are present in N. sativa seeds. Thymoquinone (TQ) and its derivatives thymoquinone, thymohydroquinone, and thymol, make up between 30 and 48 percent of N. sativa’s pharmacologically active compounds. According to previous studies, N. sativa possesses preventive, anti-hypertensive, anti-diabetic, and cardiovascular properties (Leong et al., 2013; Enayatfard et al., 2019) and anti-hyperlipidemic (Hosseini et al., 2015) and also attenuates endothelial dysfunction (Abbasnezhad et al., 2019; Enayatfard et al., 2019). Previous research suggested that endothelial dysfunction and hypertension are both encouraged by systemic oxidative stress (Taşar et al., 2012). Additionally, N. sativa’s ability to act as an antioxidant has a long history of research. TQ is thought to work as a redox equilibrium enhancer and free radical scavenger (Abbasnezhad et al., 2016; Abbasnezhad et al., 2019; Atwa et al., 2023). N. sativa ethanolic extract lessens the functional renal damage caused by doxorubicin in rats, increases glomerular filtration rate, and lowers glucosuria (El-Saleh et al., 2004). Despite all of this, there is not enough knowledge about how N. sativa impacts renal tissue. Thus, the current investigation aimed to evaluate the N. sativa ethanolic extract as a reducing agent to oxidative stress, DNA damage, apoptosis, and inflammation in the rats’ kidney tissues that had undergone renal cellular damage caused by CCl4. MethodsChemicalsWe bought from Sigma Aldrich (St. Louis, MO, USA), CCl4 with olive oil. According to (Dehpour et al. 2022), twice weekly intraperitoneal (IP) injections of 0.5 mg/kg CCl4 in olive oil were given to rats. Plant materials and extractionN. sativa was obtained from the Agricultural Research Center, Giza, Egypt. The ethanolic extraction method of dry powdered seeds of N. sativa is described in (Horwitz et al. 1970) with slight modifications. At room temperature, 800 g of N. sativa powder was steeped in 2 liters of absolute ethanol under continuous stirring for 24 hours; then, the sterile filter paper was used to filter the mixture (Whatman number 42, England). A rotary evaporator was used to remove the solvent, and the extract was then kept at −20 °C until use. With some minor adjustments, ethanol was used to extract the dry powdered roots of N. sativa. As mentioned earlier, the capacity of NEE to scavenge 1,1-diphenyl-2-picrylhydrazyl radicals was evaluated according to a previous method (Sethiya et al., 2014). The total phenolic content of NEE was determined, using established biochemical method (Nogala-Kalucka et al., 2005). The extract yield was calculated according to Ashry et al. (2021). HPLC analysis of phenolic constituentsA high-performance liquid chromatography (HPLC) analysis was performed using an Agilent 1,260 series. 4.6 mm × 250 mm id, 5 m Kromasil C18 column was used for the separation. Chromatograph the extract samples at a flow rate of 1 ml/min with solvents A (water) and B (acetonitrile, 0.05% trifluoroacetic acid). The linear gradient was used to program the mobile phase as follows: 0 minute (82% A), 0 to 5 minutes (80% A), 5–8 minutes to 6 minutes, 5–8 minutes to 12 minutes, 85% A, and 15 minutes to 16 minutes (82% A). Each sample was added to the column in 10 l at a temperature of 35°C while the detector multi-wavelength was watched at 280 nm. Animals and experimental designFrom the National Research Center’s Animal Colony in Giza, Egypt, albino male rats weighing 150–200g were obtained. Rats were handled by humans in compliance with the ethical guidelines established by the Faculty of Science at Al-Azhar University in Assiut, Egypt, to maintain and employ animals in experiments. To give the animals time to adjust and have unrestricted access to food and water, they were kept in adequate plastic cages for a week before the experiment. The accustomed animals were divided at random among four groups of ten animals. In the first group, which served as the control group, there were only healthy rats; The second group of rats received a six-week oral administration of 200 mg/kg NEE; for six weeks (twice weekly), rats in the third group received CCl4 intraperitoneally at a dose of 0.5 mg/kg and for six weeks, rats in the fourth group received NEE/CCl4 orally. All experiments in the study were approved by the Ethical Committee of the Faculty of Science at Al-Azhar University in Assiut, Egypt, under the reference number Al-Azhar 13/2023 (approval number: Al-Azhar 13/2023). Blood and tissue samplingAll rats were weighed and fasted overnight at the conclusion of the treatment period (6 weeks). After administering sodium pentobarbital (9.1 mg/kg in a sterile 0.9% NaCl solution) intramuscularly, Heparinized and sterilized glass capillaries were used to obtain blood samples from the retro-orbital plexus. All blood samples were centrifuged at 1,000 rpm for 10 minutes while being cooled to separate the sera, which were then divided into aliquots and stored at −80°C. After the animals’ blood was drawn, their kidneys were removed, and they were then put to death. A section of the kidney was cleaned in saline, dried, and covered with aluminum foil for biochemical testing. For histopathological and immunohistochemistry processing investigation, a different kidney segment was immersed in a 10% formalin-saline buffer. 10% homogenate (w/v) was produced by ultrasonically homogenizing a sample of kidney tissue in an ice-cold phosphate buffer (50 mM, pH 7.4). The homogenate was centrifuged for 20 minutes at 5,000 rpm to separate the nuclear and mitochondrial components. After being separated, aliquots of the supernatant were stored at –80°C until the biochemical analyses. Biochemical determinationsFor all biochemical measurements, a Shimadzu spectrophotometer (UV-vis 1201, Japan) was used. Utilizing instruments from the German company DiaSys Diagnostic Systems GmbH, serum concentrations of urea, urea nitrogen, uric acid, creatinine, potassium, sodium, calcium, and albumin were measured. Oxidative stress markers of kidney tissueAn ELISA method was used to test the kidney for SOD, GPx, GSH, CAT, NO, and MDA (Dynatech Microplate Reader Model MR 5000, 478 Bay Street, Suite A213 Midland, ON, Canada). Rat ELISA kits are available from SinoGeneClon Biotech Co., Ltd., No. 9 BoYuan Road, YuHang District 311112, Hang Zhou, China. Pro-inflammatory cytokine and apoptotic biomarkerThe blood levels of CD4, TNF-α, IL-1β, IL-4, IL-6, and IL-10 were analyzed using an ELISA technique. Rat ELIZA kits (Dynatech Microplate Reader Model MR 5000, 478 Bay Street, Suite A213 Midland, ON, Canada) are available from SinoGeneClon Biotech Co., Ltd., No. 9 BoYuan Road, YuHang District 311112, Hang Zhou, China. Renal DNA fragmentation percentageDNA fragmentation in kidney samples was assessed using the quantitative approach. HistopathologyBefore being sectioned and embedded in paraffin, the removed kidneys were preserved for 24 hours in 10% neutral buffered formalin. Other procedures included running water washings, ethanol dehydration, xylene clearing, and paraffin embedding. To examine the slides under a light microscope later, they were first stained with hematoxylin and eosin stains (Suvarna et al., 2018). ImmunohistochemistryImmunostaining was performed on paraffin-embedded kidney tissues for all groups; Sections 5 µm thick were immunostained for 90 minutes using anti-Caspase-3 as the main antibody, and the immunoperoxidase method was used as the secondary antibody as described by (Suvarna et al., 2018; Gadelmawla et al., 2022). Statistical analysisThe statistical analysis was carried out using SAS computer software, which is copyrighted (c) 1998 by SAS Institute Inc., Cary, North Carolina, USA. According to ( Douglas Steel and Torrie, 1986), the means were compared using the post-hock (Tukey) multiple comparisons test at p ≤ 0.05 following the one-way analysis of variance (ANOVA). ResultsHPLC analysis of phenolic constituentsFigure 1 shows the ethanolic extract of N. sativa’s phenolic content (yield, total) and radical scavenging activity (RSA). Sixteen phenolic compounds were principally discovered in NEE using HPLC analysis. High concentrations of chlorogenic acid, catechin, methyl gallate, and pyrocatechol were discovered among the components (Fig. 2 and Table 1). Biochemical determinationsCompared to the control group, CCl4 intoxication resulted in significantly higher serum concentrations of creatinine, urea, uric acid, urea nitrogen, and potassium and significantly lower concentrations of calcium, sodium, and albumin. It is fascinating to observe that administration of NEE to CCl4-intoxicated rats improved renal profile parameters, as shown by the marked rise in calcium, sodium, and albumin levels compared to CCl4-treated animals and the marked decline in urea, urea nitrogen, creatinine, uric acid, and potassium levels (Fig. 3A–H)..
Fig. 1. Three trials were conducted to measure the yield (%), total phenolic content (%), and radical scavenging activity (%) of an ethanolic extract of dry, powdered Nigella sativa roots.
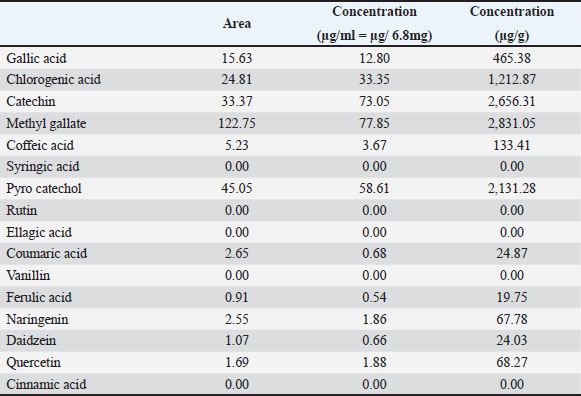
Fig. 2. HPLC analysis of phenolic constituents of Nigella sativa ethanolic extract. Table 1. Using HPLC analysis, phenolic components of the ethanolic extract of Nigella sativa.
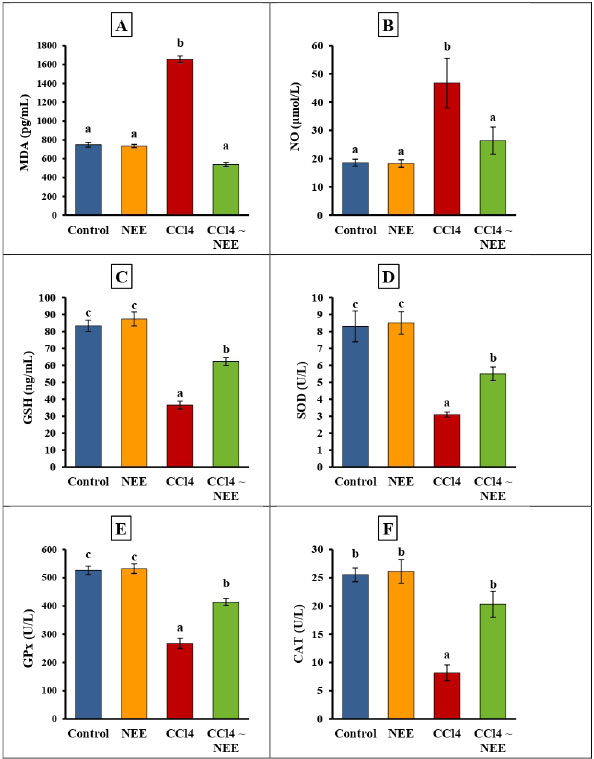
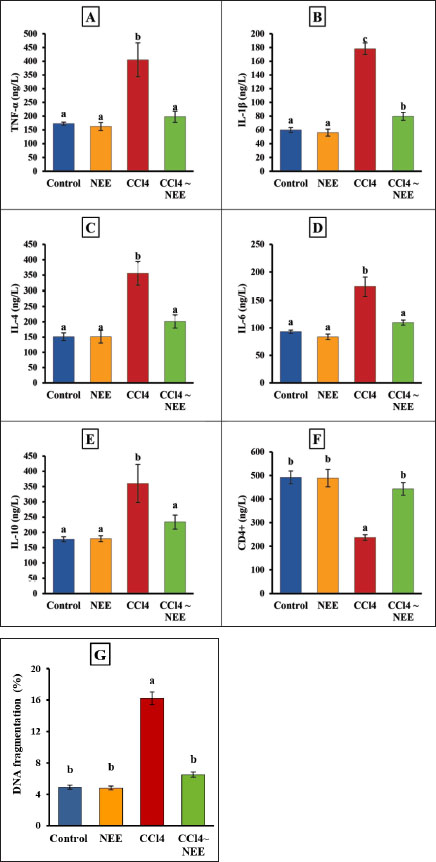
Determination of Oxidative stress markers of kidney tissueKidney oxidative stress was dramatically exacerbated by CCl4 poisoning as seen by the kidneys’ much higher levels of MDA and NO as well as their significantly lower levels of CAT, SOD, and GPx activity as well as GSH. Compared to the CCl4-intoxicated group, animals fed NEE displayed significantly lower kidney MDA and NO levels and significantly higher levels of GSH, CAT, SOD, and GPx activity (Fig. 4–F). Determination of pro-inflammatory cytokine and apoptotic biomarker and renal DNA fragmentation %The data collected showed that the CCl4 group had significantly higher levels of TNF-α, IL-1β, IL-4, IL-6, IL-10, and DNA fermentation than the control group, while the CD4 level had decreased considerably. Remarkably, NEE considerably decreased TNF-α, IL1β, IL-4, IL-6, and IL-10 DNA damage levels and greatly increased CD4 compared to CCl4 animal levels, improving all inflammatory cytokines, apoptotic markers, and renal DNA damage within normal ranges (Fig. 5–G).
Fig. 3. Effect of CCl4 and NEE on blood urea (A), urea nitrogen (B), creatinine (C), uric acid (D), calcium (E), sodium (F), potassium (G), and albumin (H). Data are presented as Mean ± SD using one-way ANOVA and a post hoc test (Duncan) at p ≤ 0.05. Columns sharing the same symbol are not significant.
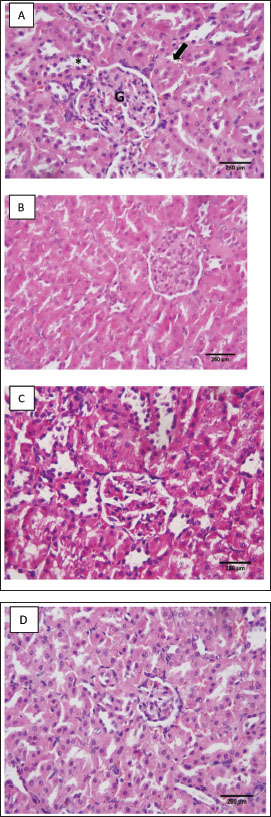
Fig. 4. Effect of CCl4 and NEE on kidney MDA (A), NO (B), GSH (C), SOD (D), GPx (E) and CAT (F). Data are presented as Mean ± SD using one-way ANOVA and a post hoc test (Duncan) at p ≤ 0.05. Columns sharing the same symbol are not significant. HistopathologyThe control group’s evaluation of the renal tissues revealed a normal renal cortex with proximal and distal convoluted tubules (PCTs) and renal corpuscles. The glomerulus of the renal corpuscles, which was comprised of a lobulated tuft of capillaries, was ringed and bordered by the Bowman’s capsule. The PCTs were seen to be more abundant, to have a narrow lumen, and to be bordered by pyramidal cells with indistinct cell borders. They had spherical vesicular nuclei and highly acidophilic cytoplasm (Fig. 6a). The NEE-treated group showed an approximately normal histological pattern of renal tissues (Fig. 6b). In several regions, the tubular architecture of the group that received CCl4 revealed disruption. Some tubules were recognized as basophilic aggregates with poorly defined nuclei, while others were seen as a few dilated tubules with flattened epithelium. Some tubules showed sloughing off parts of their epithelium inside their lumen with the obliterated tubular lumen. It appeared that some tubular cells had acidophilic cytoplasm. A congested glomerular arteriole was detected (Fig. 6c); while the structure of the glomeruli and renal cortical tubules in the CCl4 and NEE-treated group was almost identical to that of the control group. The intact epithelial lining was visible in closely packed cortical tubules (Fig. 6d).
Fig. 5. Serum TNF-α, IL1β, IL-4, IL-6, IL-10 and CD4 levels as well as kidney DNA fragmentation of control, CCl4-intoxicated and NEEtreated male albino rats A–G. Data are presented as Mean ± SD using one-way ANOVA and a post hoc test (Duncan) at p ≤ 0.05. Columns sharing the same symbol are not significant.
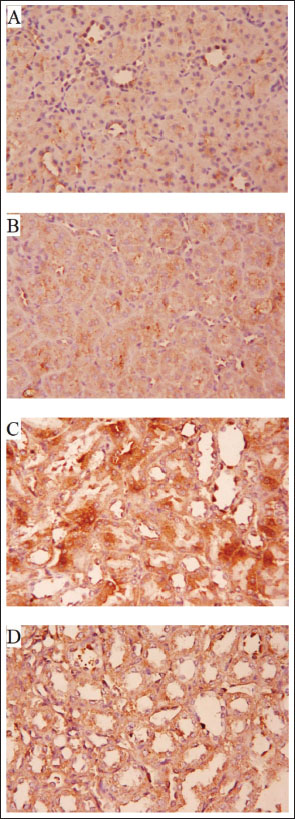
Fig. 6. (A–D). A histopathological analysis of the groups’ renal tissues. Photomicrograph of the control group’s renal tissue in (A) demonstrates the healthy renal cortical structure with glomeruli (*), proximal convoluted tubules (arrow), and distal convoluted tubules (long arrow); (B) Approximately typical renal cortical structure with glomeruli, proximal convoluted tubules, and distal convoluted tubules can be seen in the photomicrograph of renal tissue taken from the NEE group; (C) photomicrograph of the treated group’s renal tissue reveals aberrant renal cortical structure, including atrophied glomeruli, obliterated proximal convoluted tubules in some locations, and others with atypical architecture (D) A photomicrograph of the CCl4 and NEE group’s renal tissue reveals enhanced cortical nephron structure, including glomeruli, PCT, and DCT, as well as modest arteriolar congestion. Immunohistochemistry:Caspase-3’s sporadic expression was found in the renal tissue of the control group (Fig. 7a) and NEE group (Fig. 7b). In the CCl4 group, caspase-3 displayed a strong expression (Fig. 7c). The CCl4 and NEE – treated group showed moderate Caspase-3 expression when compared with CCl4 group (Fig. 7d). DiscussionOne of the many environmental toxins linked to various types of harmful cellular damage in various human organs is CCl4 (Marie et al., 2022). CCl4 is utilized to cause organ toxicity in experimental animal models, including hepatotoxicity, nephrotoxicity, and cardiotoxicity (Ashry et al., 2021). The toxicity of CCl4 was attributed to its metabolic activation by the P450 system, which produces the extremely reactive free radicals trichloromethyl and chloride radicals. These radicals damage DNA and cause protein peroxidation because of their strong affinity to bind to organ tissue electrons (Alkreathy et al., 2014). It was once believed that renal impairment brought on by CCl4 medication might occur without being influenced by the functional condition of the liver (Rincón et al., 1999). Furthermore, it has been observed that renal tissue disperses CCl4 far more than hepatic tissue (Sanzgiri and Bruckner, 1997). In a prior study, oxidative stress brought on by CCl4 was blamed for the nephrotoxicity in rats (Abraham et al., 1999). It was also asserted that exposure to CCl4 led to kidney damage by creating reactive oxygen species (Ganie et al., 2011). The current study’s primary goal was to investigate if NEE could protect rats from CCl4-induced nephrotoxicity. Our results revealed that injection of CCl4 significantly elevated serum urea, creatinine, uric acid, K+, and urea nitrogen matched with decreased Na+ and Ca+, which is the kidney removing them from the blood as a nitrogenous product of metabolism. After receiving CCl4, this function was compromised, and the amount of creatinine excreted from the blood was decreased, as shown by the noticeably elevated quantity in the blood. The results are consistent with past research written about in the literature (Marie et al., 2022; Nomier et al., 2022; Habashy and Abu-Serie, 2024). One potential reason is nephron structural integrity degradation (Marie et al., 2022). The CCl4 group also displayed significant cytoplasmic vacuolation and pyknosis of their tiny, very basophilic nuclei, and had a measurable enlargement of the epithelial lining of the renal tubules. Serum urea, creatinine, uric acid, and urea nitrogen levels were significantly reduced as a result of the concurrent delivery of NEE. N. sativa’s antioxidant activity may be responsible for this reduction, which lowers the ROS burden by quenching free radicals. It has been discovered that CCl4 increases the production of free radicals in the tissues of the liver, kidney, brain, and lungs (Alkreathy et al., 2014). lipid peroxidation, protein denaturation, and cell death are all caused by these free radicals (Hismiogullari et al., 2015). Important oxidative stress evaluation indicators include lipid peroxidation and GSH (Shahsavari et al., 2017). Glutathione, a naturally occurring antioxidant, guards against cellular harm by removing free radicals and lipid peroxides (Jaswal et al., 2003).
Fig. 7. (A–D) Photomicrographs of Caspase-3- immunostained renal tissues from several groups (x400). (A): The typical, modest cytoplasmic Caspase-3 IHC positivity was present in the control group of rats. (B): NEE group displaying cytoplasmic light Positive Caspase-3 IHC result. (C) Caspase-3 immuno-reactivity was severely exhibited in the CCl4 treated group. (D): Moderate Caspase-3 IHC positivity in the CCl4 and NEE-treated group.
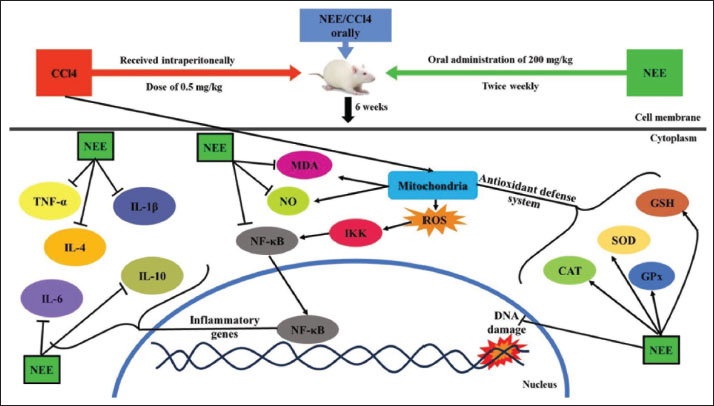
Fig. 8. Schematic diagram depicting the anti-nephrotoxic, antioxidant, and anti-inflammatory efficacy of NEE against CCl4-induced nephrotoxicity in rats. Our results demonstrated that treatment of CCl4 significantly increased renal MDA and NO levels and significantly decreased renal SOD, CAT, GPx, GSH, and DNA fragmentation, as previously reported (Suzuki et al., 2015). The considerable reduction in enzymatic antioxidants was attributed to the renal cortex and medulla’s elevated hydrogen peroxide levels, which are linked to oxidative stress (Gomes et al., 2009). MDA and NO levels were significantly reduced after taking NEE orally, and antioxidant enzymes (renal SOD, CAT, GPx, and GSH) and renal DNA fragmentation were noticeably elevated. This could explain the improvement in catalase, SOD, GSH, and GPx activities, the reported decrease in lipid peroxidation, and the DNA fragmentation in the kidney. Additionally, phytochemical analysis of NEE revealed that it contains advantageous phytochemicals such flavonoids and polyphenols. Gallic acid, amentoflavone, quercetin, apigenin, pcoumaric acid and caffeic acid are polyphenols and flavonoids found in NEE that may contribute to its significant antioxidant capacity and ability to prevent kidney damage from CCl4-induced oxidative stress. Some of these substances, such as CCl4, oxidative stress-related pathways that have been changed by environmental toxicants have been demonstrated to be impacted (An et al., 2016; Akinwumi et al., 2020). Since CCl4 injection caused a considerable increase in inflammatory cytokines (IL-1β, IL-4, IL-6, IL-10, and TNF-α), the new study found a relationship between oxidative stress and inflammatory cytokines. Our results agree with (Marie et al., 2022; Nomier et al., 2022; Noroozi et al., 2024). This connection might be explained by the production of trichloromethyl peroxyl radicals and trichloromethyl that CCl4 causes. Through various processes, the rise in oxidative stress may promote the production of inflammatory cytokines. When oxygen molecules activated NF-jB and activator protein-1 (AP-1), genes encoding inflammatory cytokines were transcriptionally activated. NF-jB significantly impacts the mesangial cell activation that results in renal injury (Wilkins et al., 2011). According to our research, NEE considerably decreased the elevated inflammatory cytokines, most likely due to the suppression of oxidative stress. Through NF-κB suppression, N. sativa is thought to concurrently alter oxidative stress and the inflammatory process (Wilkins et al., 2011). Thymoquinone inhibits the nuclear expression of the NF-B p65 subunit and the in vivo binding of the p50 subunit to the TNF-α promoter (El Gazzar et al., 2007). Numerous additional cytokines, such as TNF-α, IL-1β, and IL-6, act as NF-κB activators in addition to being up-regulated by NF-κB, prolonging the pro-inflammatory status (Ahn and Aggarwal, 2005). Another mechanism, suppression of NF-κB by N. sativa may interrupt these interactions and play a significant impact through its anti-oxidant and anti-inflammatory properties (Woo et al., 2012). The renal histology studies related to the biochemical data. In fact, our findings showed that CCl4 significantly damaged the renal structure, causing obvious glomeruli and tubular damage that was likely brought on by the production of reactive radicals and the subsequent lipid peroxidation brought on by its metabolites. Therefore, the buildup of hydroperoxides in the kidney could result in cytotoxicity linked to the peroxidation of membrane phospholipids, which is the cause of renal cellular damage and necrotic renal cells. Additionally, apoptosis is the second phenomenon that may result from exposure to CCl4 and was evident in the current study by a large rise in Caspase-3, an apoptotic marker, following CCl4-induced nephrotoxicity. Previous investigations showed that exposure to CCl4 caused renal tissues to undergo apoptosis (the cortex and medulla were impacted) (Fang et al., 2021). Similarly, other in-vivo and in-vitro studies (Almeer et al., 2019; Gong et al., 2019; Omotoso et al., 2020) confirmed that CCl4 exposure causes cell death in various human tissues, including the kidney, liver, and testis. The destruction of mitochondrial membranes, significant ROS production, the release of apoptotic factors, activation of caspase-3, disruption of calcium homeostasis caused by stressing the endoplasmic reticulum, increased calcium ion concentration, and activation of numerous apoptosis induction pathways are just a few of the underlying mechanisms. The intrinsic mitochondrial-mediated apoptosis pathway is mostly mediated by caspase-3 (Charlton et al., 2020). The combination of N. sativa medication and exposure to CCl4 had a significant ameliorative impact, thereby protecting the kidney, as shown by the statistically lower blood levels of creatinine, urea, and MDA in the tissues’ histomorphological architecture of the studied kidney. N. sativa was thought to possess anti-inflammatory, anti-apoptotic, and antioxidant qualities that supported its protective effects and made it a potential therapeutic choice for the treatment of induced nephrotoxicity (Charlton et al., 2020). The caspase enzyme family includes caspase-3, a key player in the execution of apoptosis. Although it was mostly detected in injured tubules, certain glomerular and interstitial cells also contained caspase-3 (Hropot et al., 2001). The current findings were consistent with the earlier findings that the CCl4 group had significant Caspase3 immunoreactivity, reflecting the damage caused by CCl4. In contrast, the CCl4 and NEE combined group had significant anti-apoptotic effects and renal protective properties, evident from the decreased Caspase3 expression. The modest Caspase3 immunoreactivity in the NEE-treated rats suggests that NEE is safe for renal tissue. ConclusionIn a rat model of CCl4-induced nephrotoxicity, N. sativa extract improved kidney functioning and decreased oxidative stress, inflammation, and apoptosis. N. sativa administration showed a significant recovery effect on kidney tissue through histopathological and immunohistochemical studies. Overall, the N. sativa extract may reduce oxidative stress, decrease inflammatory cytokines, and deactivate caspase-3 to provide antioxidant, anti-inflammatory, and anti-apoptotic actions, which may help to alleviate CCl4-induced nephrotoxicity (Fig. 8). As a result, N. sativa extract may be taken into consideration as a viable therapeutic approach for the treatment of nephrotoxicity. Ethics approval and consent to participateAll experiments in the study were approved by the Ethical Committee of the Faculty of Science at Al-Azhar University in Assiut, Egypt, under the reference number Al-Azhar 13/2023 (approval number: Al-Azhar 13/2023). AcknowledgmentThe authors extend their appreciation to the Deanship of Scientific Research at Jouf University, Saudi Arabia for funding this work through research grant number (DSR2022-RG-0117). Conflicts of interestThe authors declare no conflict of interest. Consent to participateAll the authors agree to participate in this paper. Consent for publicationAll the authors agree to the publication of this paper. FundingThis work was funded by the Deanship of Scientific Research at Jouf University under grant number (DSR2022-RG-0117). Author’s contributionB. Alrashdi: Resources, Data curation, Funding acquisition, Project administration, writing-review and editing. Mahmoud Ashry: Data curation, Formal analysis, Writing-review and editing M. Germoush: Data curation, Formal analysis. M. Fouda: Writing-review and editing. I. Abdel-Farid: Formal analysis, Writing-review and editing. D. Massoud: Data curation, Formal analysis, F. Shaldoum: Writing-review and editing. A. E. Abdel Moneim: Data curation, Formal analysis. A. G. Gadel-Rab: writing original draft. M. Mahrous: Data curation, Formal analysis. M. Gadelmawla: Data curation, Formal analysis. H. Askar: Conceptualization, Data curation, Formal analysis, Supervision, Writing-review and editing. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data generated or analyzed during this study are included in this article. ReferencesAbbasnezhad, A., Niazmand, S., Mahmoudabady, M., Rezaee, S.A., Soukhtanloo, M., Mosallanejad, R. and Hayatdavoudi, P. 2019. Nigella sativa L. seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J. Ethnopharmacol. 228, 142–147; doi:10.1016/j.jep.2018.09.021. Abbasnezhad, A., Niazmand, S., Mahmoudabady, M., Soukhtanloo, M., Rezaee, S.A. and Mousavi, S.M. 2016. Nigella sativa improve redox homeostasis in heart and aorta of diabetic rat. Curr. Nutr. Food Sci. 12, 35–41; doi:10.2174/1573401311666150804213742 Abdel-Wahhab, K.G., Mannaa, F.A., Ashry, M., Khaled, D.M., Hassan, L.K. and Gomaa, H.F. 2021. Chenopodium quinoa ethanolic extract ameliorates cyclophosphamide®-induced hepatotoxicity in male rats. Comp. Clin. Pathol. 30, 267–276; doi:10.1007/s00580-021-03199-z Abraham, P., Wilfred, G. and Cathrine. 1999. Oxidative damage to the lipids and proteins pf the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin. Chim. Acta. 289, 177–179; doi:10.1016/s0009-8981(99)00140-0. Adewale, O.B., and Orhue, N.E.J. 2015. Protective effect of Xylopia aethiopica fruits extracts on carbon tetrachloride-induced nephrotoxicity in rats. J. Exp. Integr. Med. 5(2), 105; doi:10.5455/jeim.120515.cr.131 Ahn, K.S., and Aggarwal, B.B. 2005. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 1056, 218–233; doi:10.1196/annals.1352.026. Akinwumi, K.A., Jubril, A.J., Olaniyan, O.O. and Umar, Y.Y. 2020. Ethanol extract of Nigella sativa has antioxidant and ameliorative effect against nickel chloride-induced hepato-renal injury in rats. Clin. Phytosci. 6, 64; doi:10.1186/s40816-020-00205-9 Alkreathy, H.M., Khan, R.A., Khan, M.R., and Sahreen, S. 2014. CCl4 induced genotoxicity and DNA oxidative damages in rats: hepatoprotective effect of Sonchus arvensis. BMC Complement. Altern. Med. 14, 452; doi:10.1186/1472-6882-14-452. Almeer, R.S., AlBasher, G.I., Alarifi, S., Alkahtani, S., Ali, D. and Abdel Moneim, A.E. 2019. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 9, 5825; doi:10.1038/s41598-019-42368-7. An, X., Zhou, A., Yang, Y., Wang, Y., Xin, R., Tian, C. and Wu, Y. 2016. Protective effects of gallic acid against NiSO4-induced toxicity through down-regulation of the Ras/ERK signaling pathway in Beas-2B Cells. Med. Sci. Monit. 22, 3446–3454; doi:10.12659/msm.900460. Ashry, M., Galal El-Sahra, D., Gaber, D.A., M.A.M. and Abdel-Wahhab, K.G. 2021. Nephroprotective effect of costus (Saussurea costus) ethanolic extract on oxaliplatin(®)-induced nephrotoxicity in adult male wistar rats. Pak. J. Biol. Sci. 24, 830–839; doi:10.3923/pjbs.2021.830.839. Atwa, A., Sofy, M.R., Fakhrelden, S.M., Darwish, O., Mehany, A.B.M., Sofy, A.R. and Bakry, S. 2023. Biodegradable materials from natural origin for tissue engineering and stem cells technologies. in Handbook of Biodegradable Materials. Eds., Ali, G.A.M. and Makhlouf, A.S.H. Cham: Springer International Publishing, pp: 1–40; doi:10.1007/978-3-031-09710-2_63 Charlton, A., Garzarella, J., Jandeleit-Dahm, K.A. and Jha, J.C. 2020. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 10(1), 18; doi:10.3390/biology10010018. Dehpour, A.R., Yousefi-Manesh, H., Sheibani, M., Sadeghi, M.A., Hemmati, S., Noori, T. and Shirooie, S. 2022. Evaluation of anti-inflammatory and antioxidant effects of sumatriptan on carbon tetrachloride-induced hepatotoxicity in rats. Drug Res. 72(1), 41–46; doi: 10.1055/a-1589-5395. Delgado-Montemayor, C., Cordero-Pérez, P., Salazar-Aranda, R. and Waksman-Minsky, N2015. Models of hepatoprotective activity assessment. Med. Univ. 17, 222–228; doi:10.1016/ j.rmu. 2015.10.002 Douglas Steel, R.G., and Torrie, J.H. 1986. Principles and procedures of statistics: a biometrical approach. New York, NY: McGraw-Hill. El Gazzar, M.A., El Mezayen, R., Nicolls, M.R. and Dreskin, S.C. 2007. Thymoquinone attenuates proinflammatory responses in lipopolysaccharide-activated mast cells by modulating NF-kappaB nuclear transactivation. Biochim. Biophys. Acta. 1770, 556–564; doi:10.1016/j.bbagen.2007.01.002. El-Saleh, S.C., Al-Sagair, O.A. and Al-Khalaf, M.I. 2004. Thymoquinone and Nigella sativa oil protection against methionine-induced hyperhomocysteinemia in rats. Int. J. Cardiol. 93(1):19–23; doi:10.1016/s0167-5273(03)00108-6. Enayatfard, L., Mohebbati, R., Niazmand, S., Hosseini, M. and Shafei, M.N. 2019. The standardized extract of Nigella sativa and its major ingredient, thymoquinone, ameliorates angiotensin II-induced hypertension in rats. J. Basic Clin. Physiol. Pharmacol. 30(1):51–58; doi:10.1515/jbcpp-2018-0074. Fang, J., Xie, S., Chen, Z., Wang, F., Chen, K., Zuo, Z., Cui, H., Guo, H., Ouyang, P. and Chen, Z. 2021. Protective effect of vitamin e on cadmium-induced renal oxidative damage and apoptosis in rats. Biol. Trace Elem. Res. 199(12), 4675–4687; doi:10.1007/s12011-021-02606-4. Gadelmawla, M.H., Alazzouni, A.S., Farag, A.H., Gabri, M.S. and Hassan, B.N. 2022. Enhanced effects of ferulic acid against the harmful side effects of chemotherapy in colon cancer: docking and in vivo study. JoBAZ 83(28), 1–11; doi:10.1186/s41936-022-00293-8 Ganie, S.A., Haq, E., Hamid, A., Qurishi, Y., Mahmood, Z., Zargar, B.A., Masood, A. and Zargar, M.A. 2011. Carbon tetrachloride induced kidney and lung tissue damages and antioxidant activities of the aqueous rhizome extract of Podophyllum hexandrum. BMC Complement. Altern. Med. 11(17), 1–10; doi:10.1186/1472-6882-11-17. Gogoi, N., Gogoi, A., Neog, B., Baruah, D. and Singh, K.D. 2017. Evaluation of antioxidant and hepatoprotective activity of fruit rind extract of Garcinia dulcis (Roxburgh) Kurz. Pharmacognosy Res. 9(3):266–272; doi:10.4103/0974-8490.210330. Gomes, P., Simão, S., Silva, E., Pinto, V., Amaral, J.S., Afonso, J., Serrão, M.P., Pinho, M.J. and Soares-da-Silva, P. 2009. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid. Med. Cell Longev. 2(3), 138–145; doi:10.4161/oxim.2.3.8819. Gong, Z.-G., Wang, X.-Y., Wang, J.-H., Fan, R.-F. and Wang, L. 2019. Trehalose prevents cadmium-induced hepatotoxicity by blocking Nrf2 pathway, restoring autophagy and inhibiting apoptosis. J. Inorg. Biochem. 192, 62–71; doi:10.1016/j.jinorgbio.2018.12.008. Habashy, N.H., and Abu-Serie, M.M. 2024. Attenuation of carbon tetrachloride-induced nephrotoxicity by gum Arabic extract via modulating cellular redox state, NF-kappaB pathway, and KIM-1. Biomed. Pharmacother. 173, 116340; doi:10.1016/j.biopha.2024.116340. Hismiogullari, A.A., Hismiogullari, S.E., Karaca, O., Sunay, F.B., Paksoy, S., Can, M., Kus, I., Seyrek, K. and Yavuz, O. 2015. The protective effect of curcumin administration on carbon tetrachloride (CCl4)-induced nephrotoxicity in rats. Pharmacol. Rep. 67(3):410–416; doi:10.1016/j.pharep.2014. 10.021. Horwitz, W., Chichilo, P. and Reynolds, H. 1970. Official methods of analysis of the association of official analytical chemists. Washington, DC: The Association. Hosseini, M., Mohammadpour, T., Karami, R., Rajaei, Z., Sadeghnia, H.R. and Soukhtanloo, M. 2015. Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin. J. Integr. Med. 21(6), 438–444; doi:10.1007/s11655-014-1742-5. Hropot, M., Juretschke, H.-P., Langer, K.H. and Schwark, J.-R. 2001. S3226, a novel NHE3 inhibitor, attenuates ischemia-induced acute renal failure in rats. Kidney Int. 60(6):2283–2289; doi:10.1046/j.1523-1755.2001.00058.x. Jaswal, S., Mehta, H.C., Sood, A.K. and Kaur, J. 2003. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin. Chim. Acta. 338(1–2), 123–129; doi:10.1016/j.cccn.2003.08.011. Joshy, C., Thahimon, P., Kumar, R.A., Carla, B. and Sunil, C. 2016. Hepatoprotective, anti-inflammatory and antioxidant activities of Flacourtia montana J. Grah leaf extract in male Wistar rats. Bull. Fac. Pharm. Cairo Univ. 54(2), 209–217; doi:10.1016/ j.bfopcu.2016.06.001 Karabulut, D., Akin, A.T., Unsal, M., Lekesizcan, A., Ozyazgan, T.M., Keti, D.B., Yakan, B. and Ekebas, G. 2021. L-Carnitine ameliorates the liver by regulating alpha-SMA, iNOS, HSP90, HIF-1alpha, and RIP1 expressions of CCL4-toxic rats. Iran J. Basic Med. Sci. 24(2), 184–190; doi:10.22038/IJBMS.2020.47711.10990. Komail, M., and Narendra, B. 2017. Nephroprotective effect of Jatropha curcas fruit extracts against carbon tetrachloride induced nephrotoxicity in rats. Int. J. Pharmacog. Phytochem. Res. 9(7):943–946; doi:10.25258/phyto.v9i07.11160 Kumar, A., D’Souza, P. and Bhargavan, D. 2013. Evaluation of renal protective activity of Adhatoda zeylanica (medic) leaves extract in wistar rats. J. Health Allied Sci. NU 3, 45–56; doi:10.1055/s-0040-1703701 Leong, X.-F., D’Souza, P. and Bhargavan, D. 2013. Nigella sativa and its protective role in oxidative stress and hypertension. Evid. Based Complement. Alternat. Med. 2013, 120732; doi:10.1155/2013/120732. Marie, O., Elfakhrany, A.T., Dessouki, A.A. and Omar, M.S. 2022. Renal protective effect of Cinnamon ethanolic extract against induced renal damage by carbon tetrachloride administration in rats. Res. Sq; doi:10.21203/rs.3.rs-1425027/v1. Nogala-Kalucka, M., Korczak, J., Dratwia, M., Lampart Szczapa, E., Siger, A., Buchowski, M. 2005. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycer ols during accelerated tests. Food Chem. 93(2):227–235. Nomier, Y.A., Alshahrani, S., Elsabahy, M., Asaad, G.F., Hassan, A. and El-Dakroury, W.A. 2022. Ameliorative effect of chitosan nanoparticles against carbon tetrachloride-induced nephrotoxicity in Wistar rats. Pharm. Biol. 60(1):2134–2144; doi:10.1080/13880209.2022. 2136208. Noroozi, F., Asle-Rousta, M., Amini, R. and Sahraeian, Z. 2024. Alpha-pinene alleviates CCl(4)-induced renal and testicular injury in rats by targeting oxidative stress, inflammation, and apoptosis. Iran J. Basic Med. Sci. 27(6), 678–684; doi:10.22038/IJBMS.2024.73116.15890. Omotoso, D.R., Owonikoko, W.M. and Ehiemere, W.P. 2020. Comparative amelioration of renal histomorphology by ascorbic acid and Camellia sinensis extract in Wistar rats exposed to Lead-induced nephropathy. Ann. Med. Res. 27(8), 2161–2165; doi:10.5455/annalsmedres.2020.02.105 Rincón, A.R., Covarrubias, A., Pedraza-Chaverrí, J., Poo, J.L., Armendáriz-Borunda, J. and Panduro, A. 1999. Differential effect of CCl4 on renal function in cirrhotic and non-cirrhotic rats. Exp. Toxicol. Pathol. 51(3), 199–205; doi:10.1016/S0940-2993(99)80094-3. Rudel, T. 1999. Caspase inhibitors in prevention of apoptosis. Herz 24(3), 236–241; doi:10.1007/BF030 44967. Sabra, M.S., Hemida, F.K. and Allam, E.A.H. 2023. Adenine model of chronic renal failure in rats to determine whether MCC950, an NLRP3 inflammasome inhibitor, is a renopreventive. BMC Nephrol. 24(1):377; doi:10.1186/s12882-023-03427-4. Safhi, M.M. 2018. Nephroprotective effect of Zingerone against CCl4-induced renal toxicity in Swiss albino mice: molecular mechanism. Oxid. Med. Cell Longev. 2018, 2474831; doi:10.1155/2018/2474831. Sanzgiri, U., and Bruckner, J. 1997. Effect of Emulphor, an emulsifier, on the pharmacokinetics and hepatotoxicity of oral carbon tetrachloride in the rat. Fundam. Appl. Toxicol. 36(1):54–61; doi:10.1006/faat.1997.2290. Sethiya, NK., Trivedi, A., Mishra, S. 2014. The total antioxidant con tent and radical scavenging investigation on 17 phytochemi cals from dietary plant sources used globally as functional. Food Biomed Prev Nutr. 4(3):439–444. Shahsavari, G., Firouzi, M., Mahdavifard, S., Joudaki, A. and Birjandi, M. 2017. Phototherapy motivates protein and lipid oxidation in jaundiced term and late term neonates. Caspian J. Pediatr. 3(2), 248–252. Available from: http://caspianjp.ir/article-1-72-en.html Suvarna, K.S., Layton, C. and Bancroft, J.D. 2018. Bancroft’s theory and practice of histological techniques. Amsterdam, Netherlands: Elsevier health sciences. Suzuki, M., Bandoski, C. and Bartlett, J.D. 2015. Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic. Biol. Med. 89, 369–378; doi:10.1016/j.freeradbiomed.2015.08.015. Taşar, N., Şehirli, Ö., Yiğiner, Ö., Süleymanoğlu, S., Yüksel, M., Yeğen, B. and Şener, G. 2012. Protective effects of Nigella sativa against hypertension-induced oxidative stress and cardiovascular dysfunction in rats. Marmara Pharm. J. 16(2), 141–149; doi:10.12991/201216412 Wilkins, R., Tucci, M. and Benghuzzi, H. 2011. Role of plant-derived antioxidants on NF-kb expression in LPS-stimulated macrophages-biomed 2011. Biomed. Sci. Instrum. 47, 222–227. Woo, C.C., Kumar, A.P., Sethi, G. and Tan, K.H.B. 2012. Thymoquinone: potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 83(4), 443–451; doi:10.1016/j.bcp.2011.09.029. | ||
| How to Cite this Article |
| Pubmed Style Alrashdi BM, Ashry M, Germoush MO, Fouda M, Abdel-farid I, Massoud D, Shaldoum F, Moneim AEA, Gadel-rab AG, Mahrous M, Gadelmawla MH, Askar H. Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Vet. J.. 2025; 15(1): 402-415. doi:10.5455/OVJ.2025.v15.i1.36 Web Style Alrashdi BM, Ashry M, Germoush MO, Fouda M, Abdel-farid I, Massoud D, Shaldoum F, Moneim AEA, Gadel-rab AG, Mahrous M, Gadelmawla MH, Askar H. Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. https://www.openveterinaryjournal.com/?mno=226481 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.36 AMA (American Medical Association) Style Alrashdi BM, Ashry M, Germoush MO, Fouda M, Abdel-farid I, Massoud D, Shaldoum F, Moneim AEA, Gadel-rab AG, Mahrous M, Gadelmawla MH, Askar H. Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Vet. J.. 2025; 15(1): 402-415. doi:10.5455/OVJ.2025.v15.i1.36 Vancouver/ICMJE Style Alrashdi BM, Ashry M, Germoush MO, Fouda M, Abdel-farid I, Massoud D, Shaldoum F, Moneim AEA, Gadel-rab AG, Mahrous M, Gadelmawla MH, Askar H. Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 402-415. doi:10.5455/OVJ.2025.v15.i1.36 Harvard Style Alrashdi, B. M., Ashry, . M., Germoush, . M. O., Fouda, . M., Abdel-farid, . I., Massoud, . D., Shaldoum, . F., Moneim, . A. E. A., Gadel-rab, . A. G., Mahrous, . M., Gadelmawla, . M. H. & Askar, . H. (2025) Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Vet. J., 15 (1), 402-415. doi:10.5455/OVJ.2025.v15.i1.36 Turabian Style Alrashdi, Barakat M., Mahmoud Ashry, Mousa O. Germoush, Maged Fouda, Ibrahim Abdel-farid, Diaa Massoud, Fayez Shaldoum, Ahmed E. Abdel Moneim, Ali G. Gadel-rab, Mohamed Mahrous, Mohamed H.a. Gadelmawla, and Hussam Askar. 2025. Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Veterinary Journal, 15 (1), 402-415. doi:10.5455/OVJ.2025.v15.i1.36 Chicago Style Alrashdi, Barakat M., Mahmoud Ashry, Mousa O. Germoush, Maged Fouda, Ibrahim Abdel-farid, Diaa Massoud, Fayez Shaldoum, Ahmed E. Abdel Moneim, Ali G. Gadel-rab, Mohamed Mahrous, Mohamed H.a. Gadelmawla, and Hussam Askar. "Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats." Open Veterinary Journal 15 (2025), 402-415. doi:10.5455/OVJ.2025.v15.i1.36 MLA (The Modern Language Association) Style Alrashdi, Barakat M., Mahmoud Ashry, Mousa O. Germoush, Maged Fouda, Ibrahim Abdel-farid, Diaa Massoud, Fayez Shaldoum, Ahmed E. Abdel Moneim, Ali G. Gadel-rab, Mohamed Mahrous, Mohamed H.a. Gadelmawla, and Hussam Askar. "Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats." Open Veterinary Journal 15.1 (2025), 402-415. Print. doi:10.5455/OVJ.2025.v15.i1.36 APA (American Psychological Association) Style Alrashdi, B. M., Ashry, . M., Germoush, . M. O., Fouda, . M., Abdel-farid, . I., Massoud, . D., Shaldoum, . F., Moneim, . A. E. A., Gadel-rab, . A. G., Mahrous, . M., Gadelmawla, . M. H. & Askar, . H. (2025) Anti-nephrotoxic, antioxidant and anti-inflammatory efficiency of Nigella sativa ethanolic extract against CCl4-induced nephrotoxicity in rats. Open Veterinary Journal, 15 (1), 402-415. doi:10.5455/OVJ.2025.v15.i1.36 |