| Research Article | ||
Open Vet. J.. 2025; 15(1): 339-347 Open Veterinary Journal, (2024), Vol. 15(1): 339-347 Research Article Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organismsPojchanicha Aponrat1,2, Osathee Detkalaya3, Naruemon Phlongtong4, Natnicha Eksatit5, Suppada Kananub6, Sarawan Kaewmongkol7 and Gunn Kaewmongkol2*1Internal Medicine Unit, Kasetsart University Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 2Department of Companion Animal Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 3Urology Unit, Kasetsart University Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 4Intensive Care Unit, Kasetsart University Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 5Critical Care Medicine Unit, Kasetsart University Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 6Department of Veterinary Public Health, Faculty of Veterinary Medicine, Kasetsart University, Nakhon Pathom, Thailand 7Faculty of Veterinary Technology, Kasetsart University, Bangkok, Thailand *Corresponding Author: Gunn Kaewmongkol. Department of Companion Animal Clinical Sciences, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand. Email: gunn_kaew [at] yahoo.com and fvetgunn [at] nontri.ku.ac.th Submitted: 19/11/2024 Accepted: 19/12/2024 Published: 31/01/2025 © 2025 Open Veterinary Journal
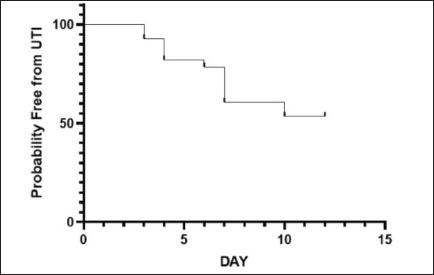
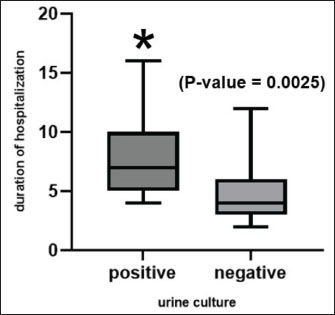
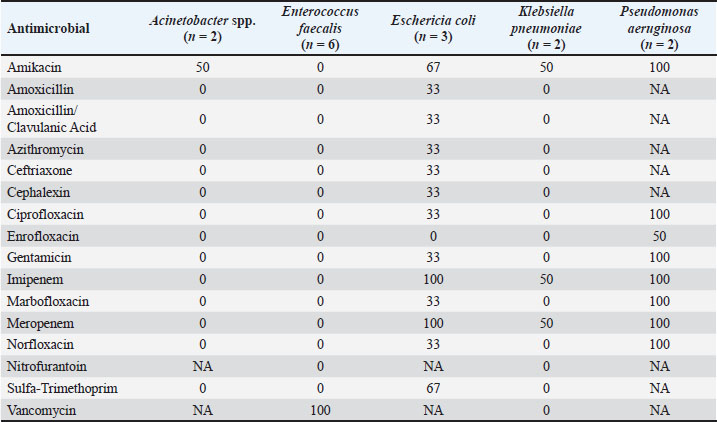
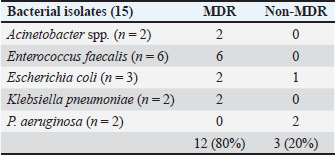
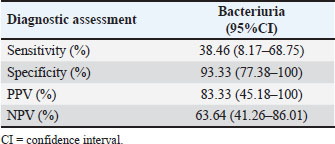
AbstractBackground: Urethral catheterization in the critical care unit often compromises the urinary tract’s defense mechanisms of canine patients and potentially leads to hospital-acquired systemic infection. Clinical signs of hospital-acquired catheter-associated urinary tract infections (CAUTIs) are frequently absent in critical dogs. Aim: This study aimed to evaluate the correlation between urinalysis results and CAUTIs in critical care unit dogs and assess the impact of prior antibiotic treatment for underlying diseases and antibiotic-resistant bacteria. Methods: Twenty-eight dogs underwent urethral catheterization in the critical care unit of Kasetsart University Veterinary Teaching Hospital. Bacterial cultures and drug sensitivity tests were performed immediately after catheter placement (day 0), 3, and 7 and before removal. A positive urine culture was defined as ≥104 CFU/ml. Urinalysis parameters included urine pH, urinary specific gravity, proteinuria, bacteriuria, pyuria, and hematuria. Only dogs with culture-negative results on day 0 were included. Data were analyzed using GraphPad Prism version 10.0.2. A Kaplan–Meier survival analysis was used to assess the probability of being free from CAUTIs over time. Results: No significant association was observed between urine cultures and urinalysis parameters, catheterization duration, breed, sex, neutering status, or age. Dogs pretreated with antibiotics exhibited CAUTI-free periods longer than previously reported. The Kaplan–Meier analysis showed that CAUTI-free probabilities were 92.8% at 3 days, declining to 60.7% by 7 days and 53.6% at 10 days. Alarmingly, 80% of the isolates (12/15) were multidrug-resistant organisms (MDRO) resistant to ≥3 antimicrobials. A high incidence of hospital-acquired CAUTIs was detected in 13 of 28 cases (46.4%). The dogs with CAUTIs stayed longer in the hospital than dogs without CAUTIs. Conclusion: A routine urinalysis is unreliable for predicting hospital-acquired CAUTIs. The high rate of MDRO among critical care dogs underscores the urgent need for judicious antibiotic use and the need for enhanced diagnostic methods in critical care settings. This study proposes that serial bacterial cultures combined with modified urine sediment examinations can better manage CAUTI detection and reduce the growth of MDROs in veterinary practice. Keywords: Hospital-acquired infection, Catheter-associated urinary tract infection, Urinalysis, Canine patients, Critical care unit. IntroductionUrethral catheterization is a common canine procedure. Several natural defense mechanisms prevent urinary tract infection (UTI), normal micturition, anatomical structures, mucosal defense barriers, urine antimicrobial properties, and systemic immunocompetence (Ettinger et al., 2017). However, urinary catheter placement frequently compromises these defense mechanisms, thereby introducing bacteria into the urinary tract during the procedure. This condition could lead to bacterial colonization and systemic infection (sepsis) caused by ascending infection to the kidneys (Segev et al., 2013; Weese et al., 2019). The prevalence of bacteriuria in catheterized dogs and cats is high (Segev et al., 2013; Weese et al., 2019). UTIs are among the most common healthcare-associated infections (HAIs) in humans, resulting in significant morbidity increased health care costs, and, occasionally, mortality. Similarly, catheter-associated UTIs are among the most common HAIs in small animal veterinary medicine, occurring in 10%–32% of hospitalized dogs (Stull and Weese, 2015). The clinical signs of UTI are often absent in catheterized patients, even when urine bacterial culture yields positive results (Schwartz and Barone, 2006). Urinalysis is routinely performed to assess catheter-associated urinary tract inflammation. However, its ability to predict UTIs remains contentious (Schwartz and Barone, 2006). Additionally, in catheterized patients, pyuria is not an indicator of catheter-associated bacteriuria or catheter-associated urinary tract infections (CAUTIs) (Hooton et al., 2010; Saran et al., 2018). Antimicrobials are commonly prescribed to dogs in the critical care unit with suspected infections without confirmed bacterial culture. Blood culture failed to detect bacterial sepsis in critical care unit dogs with pyrexia of unknown origin. There is only 20% yielded bacteria growth from blood culture (Saarenkari et al., 2022). The duration of catheterization is the most significant risk factor for infection. Therefore, catheterization should be as short as possible (Weese et al., 2019). Each additional day of catheterization increases the incidence of UTI by 27% (Bubenik et al., 2007). A previous study reported that placing an indwelling urinary catheter in dogs is associated with a low risk of catheter-associated UTI during the first 3 days after catheter placement (Smarick et al., 2004). Prophylactic antimicrobial therapy for the prevention of catheter-associated bacterial cystitis is not indicated (Bubenik et al., 2007; Weese et al., 2019). One study showed that administering antimicrobials increased the likelihood of UTI by 454% in catheterized dogs (Bubenik et al., 2007). Additionally, other studies have found that administering antimicrobials during catheterization leads to the occurrence of more antimicrobial-resistant bacteria (Lees and Osborne, 1980; Barsanti et al., 1985). The rise of antimicrobial resistance is a growing concern in both small animal medicine and human health. Increasing resistance among canine pathogens complicates treatment and poses a public health risk, especially when the pathogens are zoonotic or when resistance genes can transfer between bacteria of animal and human origin (Guardabassi et al., 2004; Windahl et al., 2014; Wong et al., 2015; Cooke et al., 2022). Conversely, there is debate over whether a single antibiotic dose can delay biofilm development for up to 4 days (Koseoglu et al., 2006). Multidrug-resistant organisms (MDROs) are bacteria that develop resistance to multiple antibiotics, significantly diminishing the effectiveness of antimicrobial treatment. The spread of MDROs is now considered one of the most critical public health threats, contributing to increased rates of illness, death, higher healthcare costs, and excessive antibiotic use (Duin and Paterson, 2016; Serra-Burriel et al., 2020; Antimicrobial Resistance Collaborators, 2022). A retrospective study analyzed 278 patients with community-acquired MDRO-associated UTIs (Bian et al., 2024). The current study found that MDRO-associated UTIs primarily occurred in elderly, frail patients with a history of invasive urinary tract procedures, thereby imposing a more significant economic burden compared with non-MDRO UTIs. There are only a few studies on the occurrence of hospital-acquired CAUTIs in critical care unit dogs. This study aimed to investigate the occurrence of hospital-acquired CAUTIs among dogs in the critical care unit, assess urinalysis parameters for predicting CAUTIs, identify possible associated factors, and identify causative bacteria and patterns of antimicrobial resistance. Materials and MethodsFor the placement of a urinary catheter, the area around the vulva or preputial opening was clipped of hair and then prepared with a chlorhexidine scrub. Subsequently, an appropriately sized silicone-coated latex Foley catheter for female dogs and a silicone Foley catheter for male dogs were inserted into the bladder using sterile gloves and lubricant. Immediately after placement, baseline urine samples (Day 0) were collected via the urinary catheter for urinalysis, aerobic bacterial culture, and drug sensitivity testing. A sterile closed collection system was promptly connected to the catheter following placement. Urinary catheters were regularly inspected to detect any issues that could increase the risk of infection, such as breakage or gross fecal contamination. The exposed portion of the catheter was then cleaned with a chlorhexidine solution, and the vulvar or preputial area was also cleaned and flushed with chlorhexidine solution. Urine was aseptically collected from the drainage port of the urinary catheter on days 3 and 7 postplacement and before removal, and then aseptically transferred via a syringe into a sterile tube containing no preservative. Subsequently, urine samples were subjected to urinalysis, aerobic bacterial culture, and drug sensitivity testing within 1 hour of collection. Urinalysis was performed by dipstick analysis, urine sediment evaluation, and measurement of urinary specific gravity using a refractometer. Pyuria (white blood cell count >5 cells/HPF), hematuria (red blood cell count >5 cells/HPF), proteinuria, and bacteriuria were recorded. Aerobic bacteria from urine were cultured on MacConkey and 5% sheep blood agar plates (Merck, Darmstadt, Germany) and incubated at 37°C for 24–48 hours under aerobic conditions (Quinn et al., 2011). Colony counts were conducted on all urine samples showing growth. A positive urine culture was defined as a culture with isolation of >104 CFU/ml from the urinary catheter of an identified pathogen (Elliott et al., 2017). Bacterial isolates were tested for susceptibility to antimicrobials based on bacterial genera and species. Microbial isolates were identified using routine biochemical and automated systems (VI-TEK 2 COMPACT; Biomerieux). Susceptibility testing for antibacterial agents was performed on Mueller–Hinton agar using antimicrobial disks (MASTDISC® AST; England). The antibiotics included in the testing were amikacin, amoxicillin, amoxicillin/clavulanic acid, azithromycin, ceftriaxone, cephalexin, ciprofloxacin, enrofloxacin, gentamicin, imipenem, marbofloxacin, meropenem, norfloxacin, nitrofurantoin, sulfamethoxazole-trimethoprim, and vancomycin. A bacterial isolate was classified as multidrug-resistant (MDR) if it demonstrated intermediate susceptibility or resistance to three or more antimicrobial classes (Magiorakos et al., 2012). Dogs with a positive urine culture result, pyuria, or bacteriuria prior to catheter placement will be excluded from the study. Data were analyzed using GraphPad Prism version 10.0.2 software (GraphPad Software, Inc.; La Jolla, CA). The Kaplan–Meier survival analysis was used to assess the probability of being free from UTI over time. A dog was considered free from UTI on the day of the first urine sample yielding positive bacterial culture results. The Cox proportional hazard test and Fisher’s exact test were used to examine associations between individual breeds and all breeds, sex, neutering status, age, duration of catheterization, urinalysis parameters, and the rate of UTI. Univariate Cox regression analyses were performed with a p-value ≤0.2. The final model was analyzed by backward stepwise selection. The Mann–Whitney U test was used to compare the duration of hospitalization between the culture-positive and culture-negative groups. The significance level was set at 0.05. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were analyzed between a reference standard tool, urine bacterial cultures, and bacteriuria by microscopic examination of the urine sediment using the NCSS 2023 program version 23.0.1. Ethical approvalThis prospective study was conducted at the Kasetsart University Veterinary Teaching Hospital in Bangkok, Thailand. Dogs hospitalized in the intensive and critical care units between March 2023 and 2024, undergoing urinary catheter placement either upon admission or during hospitalization, will be included in the study. Ethical approval was obtained from the Kasetsart University Institutional Animal Care and Use Committee (ACKU66-VET-016), and owner consent was obtained. Information obtained from each dog enrolled in the study included the dog’s vital signs, disease status, antimicrobials administered, and the duration of urinary catheterization. ResultsThirty-two dogs were initially included in the study. Two dogs were excluded due to positive urine culture results, whereas each one exhibited pyuria and bacteriuria. Subsequently, 28 dogs were enrolled in the study. There were 3 males (2 sexually intact and 1 neutered) and 25 females (6 sexually intact and 19 spayed). The median age was 5 years (range, 2–17 years), and the median weight was 12.05 kg (range, 3.25–36.3 kg). The most common breeds were crossbreed (9/28 [32.1%]), French Bulldog (4/28 [14.3%]), and Thai Bangkaew (3/28 [10.7%]). Other represented breeds included two dogs each of Pomeranian, Poodle, Shih Tzu, Siberian Husky, and Welsh Corgi, and one dog each of Chihuahua and Golden Retriever (Table S1). For disease history, the following conditions were assessed: closed pyometra, vector-borne diseases, pneumonia, pancreatitis, hepatic mass, acute kidney injury, canine distemper virus infection, encephalitis, diabetes mellitus, postoperative cases, lymphoma, cholangiohepatitis, and hemorrhagic gastroenteritis (Table S1). In the history of administration of antimicrobials, nine dogs received a single antibiotic: amoxicillin/clavulanic acid (8 dogs) and imipenem (1 dog). Eighteen dogs received combinations of two antibiotics, imipenem and metronidazole (4 dogs), amoxicillin/clavulanic acid and metronidazole (3 dogs), amoxicillin/clavulanic acid and azithromycin (2 dogs), amoxicillin/clavulanic acid and doxycycline (2 dogs), imipenem and doxycycline (2 dogs), imipenem and sulfamethoxazole-trimethoprim (1 dog), imipenem and azithromycin (1 dogs), doxycycline and clindamycin (1 dog), doxycycline and marbofloxacin (1 dog), and metronidazole and enrofloxacin (1 dog). Additionally, one dog received three antibiotics: imipenem, metronidazole, and tylosin (Table S1). The median duration of urinary catheterization for 28 dogs was 6 days (range, 2–16 days). The bacterial culture of urine samples resulted in the growth of at least one species in 13 of 28 cases (46.4%). Among these, two dogs showed positive urine culture on day 3 (2/13; 15.4%), three dogs on day 4 (3/13; 23%), one dog on day 6 (1/13; 7.7%), five dogs on day 7 (5/13; 38.5%), and two dogs on day 10 (2/13; 15.4%). The median catheterization and hospitalization duration in the urine culture-positive group was 7 days (4–16 days). Fifteen dogs had urine culture-negative results until catheter removal. The median duration of catheterization and hospitalization in the urine culture-negative group was 4 days (2–12 days). According to the Kaplan–Meier survival analysis, the probability of being free from UTI after 3 days was 92.8%, which decreased to 60.7% after 7 days and 53.6% after 10 days (Fig. 1). The Mann–Whitney U test revealed that the duration of hospitalization was significantly longer in the culture-positive group than in the culture-negative group ( p-value=0.0025) (Fig. 2). Twelve dogs had single bacterial growth: 6 had Enterococcus faecalis, 2 had Escherichia coli, 2 had Acinetobacter spp., and one had Klebsiella pneumonia and Pseudomonas aeruginosa. Additionally, one dog showed growth of three bacterial species: E. coli, K. pneumoniae, and P. aeruginosa. The drug sensitivity test results for all samples are shown in Table 1. Fifteen bacterial isolates were obtained from 12 dogs. Eighty percent of the isolates were classified as MDRO, resistant to ≥3 antimicrobials, and the distribution of pathogens resistant to MDR is shown in Table 2. Enterococcus faecalis was the most common MDR isolates. No statistical association was found between positive or negative urine culture results and breed, sex, neutering status, age, duration of catheterization, and all urinalysis parameters, as determined by the Cox proportional hazards test and Fisher’s exact test. In the univariate Cox proportional hazards test, a significant association was found between UTI and bacteriuria and between UTI and age >7 years. However, the multivariate analysis found no significant association between UTI and bacteriuria or age >7 years (p-value=0.087, p-value=0.093, respectively; 95% confidence interval). A comparison of the bacteriuria results with the urine bacterial cultures is presented in Table 3. The sensitivity of bacteriuria was 38.46% (95% CI; 8.17%–68.75%). The specificity of bacteriuria was 93.33% (95% CI; 77.38%–100%). The positive and NPV were 83.33% (95% CI; 45.18%–100%) and 63.64% (95% CI; 41.26–86.01), respectively. DiscussionThe incidence of hospital-acquired CAUTIs in the critical care unit dogs in this study was 46.4% (13 of 28 cases), a figure that requires immediate attention. This high incidence, which is significantly higher than that reported in other studies (Smarick et al., 2004; Ogeer-Gyles et al., 2006), is likely due to the longer median duration of catheterization (6 days; range: 2–16 days). Previous studies have reported that the duration of catheterization is one of the significant risk factors for catheter-associated UTI, which is likely to significantly increase with the duration of catheterization by 27% for each day of catheterization (Bubenik et al., 2007). This retrospective study of human patients with MDRO-associated UTIs found that the independent risk factors for these infections included high white blood cell counts, multiple urinary tract obstructive diseases, use of third-generation cephalosporins, and a history of invasive urologic procedures. The MDRO group also exhibited longer hospital stays, more antibiotic use, and increased bladder catheter use (Bian et al., 2024).
Fig. 1. Kaplan–Meier plot of the probability of being free from CAUTI over time.
Fig. 2. Box and whisker plot showing the duration of hospitalization for dogs in the culture-positive and culturenegative groups. Table 1. Antimicrobial susceptibility (%) of all positive urine culture samples.
Table 2. Distribution of pathogens according to MDR and non-MDR groups.
Table 3. Diagnostic assessment of bacteriuria by microscopic examination of urine sediment compared with urine bacterial culture results.
CAUTIs are among the most common HAIs in small animal veterinary medicine (Stull and Weese, 2015). Patients with these conditions are often caused by MDROs, complicating treatment and potentially leading to prolonged hospitalization, higher costs, and worse outcomes. The duration of hospitalization was significantly longer in dogs with CAUTIs than in the urine culture-negative group. In this study, the probability of remaining free from UTI after 3 days was 92.8%, which decreased to 60.7% after 7 days and 53.6% after 10 days. A longer duration was observed compared with another study in which the probability of remaining free from UTI decreased to 63.3% by day 4 (Smarick et al., 2004), possibly due to antibiotic use in every dog in our study. Another study suggested that a single dose of antibiotic, especially a higher-generation antibiotic (Koseoglu et al., 2006), can delay UTI onset and reduce the likelihood of UTI development compared with dogs that do not receive antimicrobials (Smarick et al., 2004). However, it cannot prevent hospital-associated CAUTIs and may lead to antimicrobial resistance. Additionally, this study found a delay in UTI onset but identified pathogens with MDR in 13 of 15 isolates (80%). The findings from this study provide strong scientific evidence that improves the reliability of the information recommended in the guidelines for diagnosing and managing bacterial UTIs in dogs and cats (Weese et al., 2019). These guidelines state that prophylactic antimicrobial therapy for the prevention of cystitis in catheterized animals is not indicated, and the duration of catheterization should be as short as possible. One possible reason for systemic antimicrobial administration’s failure to prevent catheter-associated UTIs is biofilm formation. After a catheter is placed and comes in contact with urine, a conditioning film forms due to the deposition of host-derived factors. The surface provides binding sites for bacterial colonization, leading to biofilm formation and maturation. Bacteria within biofilms decrease susceptibility to antimicrobials (Trautner and Darouiche, 2004; Koseoglu et al., 2006; Shapur et al., 2012; Segev et al., 2013). Widespread antimicrobial resistance is an emerging problem in small animal medicine and human health. The close contact between pets and humans creates favorable conditions for the transmission of bacteria, either through direct contact or via the domestic environment. The transmission of antimicrobial-resistant bacteria from pets to humans is particularly concerning when strains carry resistance genes relevant to human medicine. There is a risk that resistant bacteria and/or resistance genes could be transferred from pets to humans, including bacterial species and resistance genotypes of clinical significance (Cooke et al., 2002; Guardabassi et al.,2004; Windahl et al., 2014; Wong et al., 2015). In recent years, MDROs have become a significant concern in veterinary practice, mirroring trends observed in human health care. The increasing use of antibiotics in companion animals and livestock has contributed to the development of resistance, particularly in critical care settings where prolonged or broad-spectrum antibiotic use is common. Pathogens, such as E. coli, Staphylococcus pseudintermedius, and P. aeruginosa, have been frequently implicated in infections that are difficult to treat due to resistance to multiple antimicrobial classes (Guardabassi et al., 2004; Weese et al., 2019). In veterinary hospitals, MDROs pose challenges in treating infections such as UTIs, post-surgical wound infections, and respiratory diseases, often requiring advanced diagnostic approaches and limited therapeutic options (Lloyd, 2007). HAIs are a growing concern in veterinary settings, particularly in critical care units where animals are more vulnerable due to compromised immune systems and invasive procedures, such as catheterization, mechanical ventilation, and surgery (Sykes, 2014). Common pathogens in veterinary HAIs include S. pseudintermedius, E. coli, and P. aeruginosa, many of which exhibit MDR (Walther et al., 2017). In critical care units, prolonged patient stay and increased use of medical devices further elevate the risk of infection. Preventing HAIs in these settings requires rigorous infection control protocols, including proper hand hygiene, equipment sterilization, and animal isolation (Sykes, 2014). Despite these measures, HAIs remain a significant issue, leading to prolonged hospitalization, increased costs, and, in severe cases, increased mortality (Stull and Weese , 2015). The rise of MDROs in these settings further complicates treatment, emphasizing the need for ongoing surveillance and antimicrobial stewardship programs to mitigate the risk of HAIs. However, veterinary-specific data on CAUTIs are limited. Research in this area is essential for improving infection control protocols and patient care in critical veterinary care settings. Microscopic examination of urine sediment can generally assist in diagnosing UTIs, but no urinalysis parameters in this study were able to predict catheter-associated UTIs. One study found that light microscopic examination of specimens stained with modified Wright’s stain is more sensitive and specific than examination of routine unstained preparations. The sensitivity and specificity were 93.2%, 99.0% and 82.4%, 76.4%, respectively, compared with urine culture results (Swenson et al., 2004). The prevalence of positive aerobic bacterial urine culture in dogs with inactive urine sediment is low (3.4%) (Strachan et al., 2022). Therefore, urine sediment examination could help identify specimens that are likely to yield positive culture results. Although bacteriuria was likely associated with UTIs in our study, the relationship was not statistically significant. Therefore, modified Wright’s staining may provide a more sensitive and specific prediction of catheter-associated UTI and should be considered. Age may be a risk factor for developing catheter-associated UTIs but this factor was not found to be significant in this study. A previous study reported that each additional year of age increases the risk of developing a UTI by 20% (Bubenik et al., 2007). This increased risk may be attributed to age-related changes in the immune system (Graham et al., 2006). In older dogs, urinary catheterization compromises the normal defense mechanisms, and the altered immune response associated with aging may contribute to the development of UTIs. To the best of our knowledge, there is no effective prevention method for catheter-associated UTIs. However, future research can find a solution. Urinary catheters coated with antibacterial substances, such as silver or chlorhexidine, have shown potential in reducing bacterial colonization and biofilm formation (Shapur et al., 2012; Segev et al., 2013; Gefter Shenderovich et al., 2018; Srisang et al., 2019; Srisang and Nasongkla, 2019a and b; Srisang et al., 2021). Further research is needed to confirm their efficacy. These coatings may offer a promising alternative for prevention in the future. Our study has several limitations that we acknowledge in maintaining transparency and honesty. The small sample size may have affected the power and reliability of the study results. Increasing the sample size could potentially reveal more significant findings. Second, there were fewer male dogs than female dogs in the study. Additionally, differences in the types of urinary catheters used for male and female dogs may have limited the ability to perform comparisons between these groups. Finally, the study did not have specific criteria for antibiotic use, as all dogs received antibiotics for their underlying conditions. The lack of standardized antibiotic criteria could have influenced the urine culture results. However, our results demonstrated a trend toward higher risk associated with pretreated antimicrobials that created unexpected MDRO in catheterized dogs. ConclusionA routine urinalysis is unreliable for predicting CAUTIs in dogs hospitalized in the critical care unit. In this study, dogs that received prior antibiotic treatment exhibited longer CAUTI-free periods following catheter placement than those reported in previous studies. However, a worrying finding emerged from the drug sensitivity tests, which revealed a high prevalence of pathogens with MDR. The acquisition of MDRO created using pretreated antimicrobials without confirmed bacterial culture highlights a growing issue of antibiotic resistance in veterinary medicine, which could complicate treatment and management strategies. Given these concerns, reconsidering prophylactic antibiotics may be advisable, even for dogs at high risk of developing CAUTIs, such as older dogs undergoing short-term urinary catheterization. Implementing serial bacterial culture, drug sensitivity tests, and urine sediment examination using modified Wright’s staining could become the new standard routine. The aim of this study was to prevent and delay the risk of further antibiotic resistance in CAUTIs. Future research should focus on validating this approach and exploring additional strategies to mitigate antibiotic resistance in veterinary practice. AcknowledgmentsThe authors extend their sincere gratitude to the dog owners and the staff at the Veterinary Diagnostic Center, Critical Care Unit, and Intensive Care Unit, Kasetsart University Veterinary Teaching Hospital, for their contributions to the successful completion of this study. Conflicts of interestThe authors declare no conflicts of interest. FundingThis research was supported by research grants for graduate development from the Faculty of Veterinary Medicine at Kasetsart University (VET.KU2023-06). Author contributionsConceptualization, G.K.; methodology, P.A. and S.K.; software, P.A. and S.K.; validation, S.K. and G.K.; formal analysis, P.A., S.K., and G.K.; investigation, P.A.; clinical case handling, N.P., N.E., and P.A.; resources, O.D.; data curation, S.K.; writing—original draft preparation, P.A. and G.K.; writing—review and editing, G.K.; visualization, S.K. and O.D.; supervision, G.K.; project administration, S.K. All authors have read and agreed to the publication of the finale version of the manuscript. Data availabilityThe data used to support the findings of this study are presented in the supplementary files. ReferencesAntimicrobial Resistance Collaborators. 2022. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399(10325), 629–655. Barsanti, J.A., Blue, J. and Edmunds, J. 1985. Urinary tract infection due to indwelling bladder catheters in dogs and cats. J. Am. Vet. Med. Assoc. 187(4), 384–388. Bian, C., Zhu, Y., Fang, X., Ding, R., Hu, X., Lu, J., Mo, C., Zhang, H. and Liu, X. 2024. Risk factors and economic burden for community-acquired multidrug-resistant organism-associated urinary tract infections: a retrospective analysis. Medicine 103(21), e38248. Bubenik, L.J., Hosgood, G.L., Waldron, D.R. and Snow, L.A. 2007. Frequency of urinary tract infection in catheterized dogs and comparison of bacterial culture and susceptibility testing results for catheterized and noncatheterized dogs with urinary tract infections. J. Am. Vet. Med. Assoc. 231, 893–899. Cooke, C.L., Singer, R.S., Jang, S.S. and Hirsh, D.C. 2002. Enrofloxacin resistance in Escherichia coli isolated from dogs with urinary tract infections. J. Am. Vet. Med. Assoc. 220, 190–192. Duin, D.V. and Paterson, D.L. 2016. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect. Dis. Clin. North Am. 30(2), 377–390. Elliott, J., Grauer, G.F. and Westropp, J.L. 2017. BSAVA manual of canine and feline nephrology and urology. Gloucester, UK: British Small Animal Veterinary Association. Ettinger, S.J., Feldman, E.C. and Cóteé, E. 2017. Textbook of veterinary internal medicine: diseases of the dog and the cat, 8th ed. St. Louis, MO: Elsevier. Gefter Shenderovich, J., Zaks, B., Kirmayer, D., Lavy, E., Steinberg, D. and Friedman, M. 2018. Chlorhexidine sustained-release varnishes for catheter coating—dissolution kinetics and antibiofilm properties. Eur. J. Pharm. Sci. 112, 1–7. Graham, J.E., Christian, L.M. and Kiecolt-Glaser, J.K. 2006. Stress, age, and immune function: toward a lifespan approach. J. Behav. Med. 29(4), 389–400. Guardabassi, L., Schwarz, S. and Lloyd, D.H. 2004. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 54(2), 321–332. Hooton, T.M., Bradley, S.F., Cardenas, D.D., Colgan, R., Geerlings, S.E., Rice, J.C., Saint, S., Schaeffer, A.J., Tambayh, P.A., Tenke, P. and Nicolle, L.E. 2010. Diagnosis, prevention, and treatment of catheter associated urinary tract infection in adults: 2009 international clinical practice guidelines from the infectious diseases society of america. Infect. Dis. Soc. Am. 50, 625–663. Koseoglu, H., Aslan, G., Esen, N., Sen, B.H. and Coban, H. 2006. Ultrastructural stages of biofilm development of Escherichia coli on urethral catheters and effects of antibiotics on biofilm formation. Urology 68(5), 942–946. Lees, G.E. and Osborne, C.A. 1980. Urinary tract infections associated with the use and misuse of urinary catheters. Vet. Clin. North Am. Small Anim. Pract. 9(4), 713–727. Lloyd, D.H. 2007. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 45(Suppl 2), S148–S152. Magiorakos, A.P., Srinivasan, A., Carey, R.B., Carmeli, Y., Falagas, M.E., Giske, C.G., Harbarth, S., Hindler, J.F., Kahlmeter, G., Olsson-Liljequist, B., Paterson, D.L., Rice, L.B., Stelling, J., Struelens, M.J., Vatopoulos, A., Weber, J.T. and Monnet, D.L. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18(3), 268–281. Ogeer-Gyles, J., Mathews, K., Weese, J.S., Prescott, J.F. and Boerlin, P. 2006. Evaluation of catheter-associated urinary tract infections and multi–drug-resistant Escherichia coli isolates from the urine of dogs with indwelling urinary catheters. J. Am. Vet. Med. Assoc. 229, 1584–1590. Quinn, P.J., Markey, B.K., Leonard, F.C., Hartigan, P., Fanning, S. and Fitzpatrick, E.S. 2011. Veterinary microbiology and microbial disease, 2nd ed. Hoboken, NJ: John Wiley and Sons. Saarenkari, H.K., Sharp, C.R. and Smart, L. 2022. Retrospective evaluation of the utility of blood cultures in dogs (2009-2018): 45 cases. J. Vet. Emerg. Crit. Care (San Antonio). 32(1), 141–145. Saran, S., Rao, N.S. and Azim, A. 2018. Diagnosing catheter-associated urinary tract infection in critically ill patients: do the guidelines help?. Indian J. Crit. Care Med. 22, 357–360. Schwartz, D.S. and Barone, J.E. 2006. Correlation of urinalysis and dipstick results with catheter-associated urinary tract infections in surgical icu patients. Intensive Care Med. 32(11), 1797–1801. Segev, G., Bankirer, T., Steinberg, D., Duvdevani, M., Shapur, N.K., Friedman, M. and Lavy, E. 2013. Evaluation of urinary catheters coated with sustained-release varnish of chlorhexidine in mitigating biofilm formation on urinary catheters in dogs. J. Vet. Intern. Med. 27(1), 39–46. Serra-Burriel, M., Keys, M., Campillo-Artero, C., Agodi, A., Barchitta, M., Gikas, A., Palos, C. and López-Casasnovas, G. 2020. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: systematic review and metaanalysis. PLoS One 15(1), e0227139. Shapur, N.K., Duvdevani, M., Friedman, M., Zaks, B., Gati, I., Lavy, E., Katz, R., Landau, E.H., Pode, D., Gofrit, O.N. and Steinberg, D. 2012. Sustained release varnish containing chlorhexidine for prevention of biofilm formation on urinary catheter surface: in vitro study. J. Endourol. 26(1), 26–31. Smarick, S.D., Haskins, S.C., Aldrich, J., Foley, J.E., Kass, P.H., Fudge, M. and Ling, G.V. 2004. Incidence of catheter-associated urinary tract infection among dogs in a small animal intensive care unit. J. Am. Vet. Med. Assoc. 224, 1936−1940. Srisang, S., Boongird, A., Ungsurungsie, M., Wanasawas, P. and Nasongkla, N. 2021. In vivo catheterization study of chlorhexidine-loaded nanoparticle coated foley urinary catheters in male New Zealand white rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 109, 1836−1843. Srisang, S. and Nasongkla, N. 2019a. Layer-by-layer dip coating of foley urinary catheters by chlorhexidine-loaded micelles. J. Drug Deliv. Sci. Technol. 49, 235−242. Srisang, S. and Nasongkla, N. 2019b. Spray coating of foley urinary catheter by chlorhexidine-loaded poly(ε-caprolactone) nanospheres: effect of lyoprotectants, characteristics, and antibacterial activity evaluation. Pharm. Dev. Technol. 24, 402−409. Srisang, S., Wongsuwan, N., Boongird, A., Ungsurungsie, M., Wanasawas, P. and Nasongkla, N. 2019. Multilayer nanocoating of foley urinary catheter by chlorhexidine-loaded nanoparticles for prolonged release and anti-infection of urinary tract. Int. J. Polym. Mater. Polym. Biomater. 69, 1081−1089. Strachan, N.A., Hales, E.N. and Fischer, J.R. 2022. Prevalence of positive urine culture in the presence of inactive urine sediment in 1049 urine samples from dogs. J. Vet. Intern. Med. 36(2), 629–633. Stull, J.W. and Weese, J.S. 2015. Hospital-associated infections in small animal practice. Vet. Clin. North Am. Small Anim. Pract. 45(2), 217–233. Swenson, C.L., Boisvert, A.M., Kruger, J.M. and Gibbons-Burgener, S.N. 2004. Evaluation of modified wright-staining of urine sediment as a method for accurate detection of bacteriuria in dogs. J. Am. Vet. Med. Assoc. 224, 1282–1289. Sykes, J.E. 2014. Canine and feline infectious diseases. St. Louis, MO: Elsevier/Saunders. Trautner, B.W. and Darouiche, R.O. 2004. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 164, 842−850. Walther, B., Tedin, K. and Lübke-Becker, A. 2017. Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet. Microbiol. 200, 71−78. Weese, J.S., Blondeau, J., Boothe, D., Guardabassi, L.G., Gumley, N., Papich, M., Jessen, L.R., Lappin, M., Rankin, S., Westropp, J.L. and Sykes, J. 2019. International society for companion animal infectious diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 247, 8−25. Windahl, U., Ström Holst, B., Nyman, A., Grönlund, U. and Bengtsson, B. 2014. Characterisation of bacterial growth and antimicrobial susceptibility patterns in canine urinary tract infections. BMC Vet. Res. 10, 217. Wong, C., Epstein, S.E. and Westropp, J.L. 2015. Antimicrobial susceptibility patterns in urinary tract infections in dogs (2010-2013). J. Vet. Intern. Med. 29(4), 1045−1052. Table S1. Signalment and history.
| ||
| How to Cite this Article |
| Pubmed Style Aponrat P, Detkalaya O, Phlongtong N, Eksatit N, Kananub S, Kaewmongkol S, Kaewmongkol G. Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. Open Vet. J.. 2025; 15(1): 339-347. doi:10.5455/OVJ.2025.v15.i1.32 Web Style Aponrat P, Detkalaya O, Phlongtong N, Eksatit N, Kananub S, Kaewmongkol S, Kaewmongkol G. Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. https://www.openveterinaryjournal.com/?mno=229366 [Access: January 15, 2026]. doi:10.5455/OVJ.2025.v15.i1.32 AMA (American Medical Association) Style Aponrat P, Detkalaya O, Phlongtong N, Eksatit N, Kananub S, Kaewmongkol S, Kaewmongkol G. Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. Open Vet. J.. 2025; 15(1): 339-347. doi:10.5455/OVJ.2025.v15.i1.32 Vancouver/ICMJE Style Aponrat P, Detkalaya O, Phlongtong N, Eksatit N, Kananub S, Kaewmongkol S, Kaewmongkol G. Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. Open Vet. J.. (2025), [cited January 15, 2026]; 15(1): 339-347. doi:10.5455/OVJ.2025.v15.i1.32 Harvard Style Aponrat, P., Detkalaya, . O., Phlongtong, . N., Eksatit, . N., Kananub, . S., Kaewmongkol, . S. & Kaewmongkol, . G. (2025) Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. Open Vet. J., 15 (1), 339-347. doi:10.5455/OVJ.2025.v15.i1.32 Turabian Style Aponrat, Pojchanicha, Osathee Detkalaya, Naruemon Phlongtong, Natnicha Eksatit, Suppada Kananub, Sarawan Kaewmongkol, and Gunn Kaewmongkol. 2025. Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. Open Veterinary Journal, 15 (1), 339-347. doi:10.5455/OVJ.2025.v15.i1.32 Chicago Style Aponrat, Pojchanicha, Osathee Detkalaya, Naruemon Phlongtong, Natnicha Eksatit, Suppada Kananub, Sarawan Kaewmongkol, and Gunn Kaewmongkol. "Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms." Open Veterinary Journal 15 (2025), 339-347. doi:10.5455/OVJ.2025.v15.i1.32 MLA (The Modern Language Association) Style Aponrat, Pojchanicha, Osathee Detkalaya, Naruemon Phlongtong, Natnicha Eksatit, Suppada Kananub, Sarawan Kaewmongkol, and Gunn Kaewmongkol. "Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms." Open Veterinary Journal 15.1 (2025), 339-347. Print. doi:10.5455/OVJ.2025.v15.i1.32 APA (American Psychological Association) Style Aponrat, P., Detkalaya, . O., Phlongtong, . N., Eksatit, . N., Kananub, . S., Kaewmongkol, . S. & Kaewmongkol, . G. (2025) Hospital-acquired catheter-associated urinary tract infections in critical care unit dogs with high rates of multidrug-resistant organisms. Open Veterinary Journal, 15 (1), 339-347. doi:10.5455/OVJ.2025.v15.i1.32 |