| Research Article | ||
Open Vet. J.. 2025; 15(6): 2386-2394 Open Veterinary Journal, (2025), Vol. 15(6): 2386-2394 Research Article Antiulcer activity of Prosopis farcta L. fruits extract in ratsAmer Hakeem Chyad1, Omar Salim Ibrahim2 and Rawaa Saladdin Jumaa3*1Department of Pharmacology, College of Veterinary Medicine, University of Baghdad, Baghdad City, Iraq 2Department of Pharmacology, Faculty of Medicine, University of Anbar, Anbar, Iraq 3Department of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad City, Iraq *Correspondence to: Rawaa Saladdin Jumaa. Department of Microbiology, College of Veterinary Medicine, University of Baghdad, Baghdad City, Iraq. Email: rawaa.saladdin [at] covm.uobaghdad.edu.iq Submitted: 28/12/2024 Revised: 11/05/2025 Accepted: 24/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
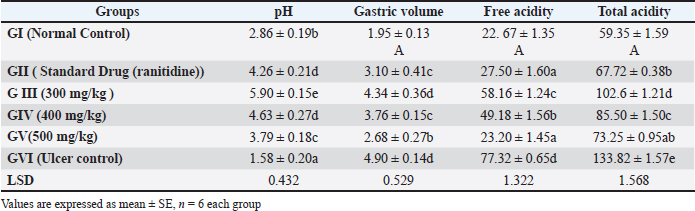
AbstractBackground: Peptic ulcer is a condition where the lining of the stomach is damaged by acid and/or pepsin, often caused by the use of NSAIDs. Previous research has shown that NSAIDs can suppress prostaglandin-E2 synthesis, causing ulceration and delay ulcer healing process. Aim: The researcher had investigated the antiulcer activity of Prosopis farcta (PF). fruits extract against indomethacin-induced ulcers in rats, comparing it to the standard drug, ranitidine. Method: In the study, 36 male albino Wistar rats were used and this study was conducted to assess the safety of the extract. No signs of toxicity were observed in rats given doses up to 5 g/kg body weight, indicating the extract’s safety. To induce ulcers, indomethacin (50 mg/kg p.o.) was administered to the rats. The rats were divided into six groups (n = 6), including a control group, a standard control group receiving ranitidine, and groups receiving different doses of PF extract including (300, 400, and 500mg/kg). The rats were sacrificed after seven days of treatment, and their stomachs were examined for ulcer index, total acidity, free acidity, gastric juice volume, and histopathological changes. Results: The study found that the extract effectively protected against indomethacin-induced gastric mucosal injuries. It showed a significant (p < 0.05) reduced the severity of lesions and increased the percentage protection of ulcers. The extract also decreased gastric acidity and increased mucus production, indicating its cytoprotective properties. Histopathological examination revealed that the extract prevented morphological changes such as inflammation, necrosis, and ulcers caused by indomethacin. Statistical analysis was performed to determine significant differences between groups. Conclusion: Prosopis farcta L. fruit extract special (400 mg /kg) showed potential as a natural treatment for gastric ulcers and can provide cytoprotective effects. Keywords: Antiulcer, Prosopis farcta extract, Rats. IntroductionA peptic ulcer is a complex of various diseases such as viruses or bacterial diseases that are detected by PCR technique (Jumaa et al., 2019; Jbr and Jumaa, 2024; Allmjeed et al., 2024). Also, the peptic ulcer is found as a breach of the lining of the gastric mucosa under the effect of acid and/or pepsin, associated with NSAID consumption causing petechiae, erosions, ulceration, type C gastritis, impediment, and ulcer healing process, also complications from ulcers and damage to the intestines, both large and small (Wallace et al., 2000). Previous research on indomethacin-induced ulcers revealed that it suppresses prostaglandin-E2 (PGE2) synthesis or angiogenesis, promotes free radical formation, increases the expression of cyclooxygenase-2 (COX-2), and produces pro-inflammatory cytokines (Suleyman et al., 2010; Yadav et al., 2012). Furthermore, ulcer healing is delayed by NSAID-induced down-regulation of pro-angiogenic factors such as basic fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor (Tarnawski, 2005; Utsumi et al., 2006). Although there has been a decrease in death and morbidity rates as a result of the use of several pharmaceutical products for the treatment of peptic illnesses and gastroduodenal ulcers, these treatments are not entirely effective and have a number of negative side effects. Some pharmaceutical medicines have been working for the treatment of peptic diseases and stomach ulcers, leading to reduced rates of morbidity and mortality, these treatments are not entirely effective and have a number of adverse effects (Rates, 2001; Jumaa et al., 2020). Additionally, ranitidine inhibits the H2 receptor linked to gastric parietal cells in a competitive manner. Furthermore, due to its anticholinesterase effect, ranitidine elevates the tone of the lower esophageal sphincter and acts as a prokinetic agent, promoting intestinal motility, including colonic motility, and enhancing gastric emptying (Kounenis et al., 1992; Bersenas et al., 2005). Ranitidine does not present a clinical risk in relation to drug interactions linked to suppression of the enzymatic system of the hepatic microsomal cytochrome P-450 (Marshall-Jones et al., 2006). One side effect to be mindful of in cats receiving ranitidine treatment is the brief hypotension that can occur when the medication is given as an IV bolus. In human patients with renal azotemia, dosage decrease is advised (Duran et al., 1991; Jumaa, 2024). Hence, given the adverse effects linked with conventional medication. In recent years, there has been a surge in interest toward alternative therapies and the utilization of natural products, particularly those derived from plants. Plant extracts, in particular, have emerged as highly promising sources of new medications, showing encouraging results in the treatment of gastric ulcers (Luis et al., 2023). Prosopis farcta L. is a widely recognized plant renowned for its medicinal properties, having been traditionally utilized in treating various ailments. Despite being an invasive weed posing significant challenges in control, it has found versatile uses. Beyond its role as a drink or supplementary food for animals such as deer, buffalo, and antelopes, P. farcta L. has been employed in the production of gum, paint, cordage, and other items. Medicinally, it has been documented to effectively address a spectrum of conditions including inflammatory disorders, measles, urinary tract infections, diarrhea, and cold (Asadollahi et al., 2014). The fruits of P. farcta L. have garnered particular attention in medical applications, being valued for their astringent qualities, stomach-toning effects, blood-coagulating properties, and anti-diarrheal benefits (Zughayyar and Gyad, 2023). The researcher had investigated the antiulcer activity of Prosopis farcta L. fruit extract against indomethacin-induced ulcers in rats, with a comparison to ranitidine. Material and MethodsAnimalsMale rats (albino Wister) of 200–250 gms were used in the study. They were maintained in a controlled environmental condition of humidity and temperature alternatively. Also, these animals were fed with a standard pellet diet and water Ad libitum. Plant MaterialThe fruits of P. farcta were collected in November 2022 from Abu Graib region/Baghdad. The classification of this plant was done in the Ministry of Agriculture/ State Board for Seeds Testing and Certification in (Abu Graib-Baghdad). Preparation of extractFruits of Prosopis farcta were dried in a shed and coarsely powdered with a commercial blender. One Hundred (100) g of plant material was extracted with 70 % ethanol in a soxhlet apparatus (continuous hot extraction). After completion of the extraction process, Prosopis farcta extract (PFE) was concentrated by using a rotary evaporator at (40 °C) and the yield value of the extract was 14% and kept at (–20°c) until use (Chyad, 2017; Jumaa et al., 2021; Ibrahim et al., 2023). Acute oral toxicity studyThis test was done on a set of albino rats (male) that were distributed into four groups (six rats in each group). These rats were fastened overnight prior to the experiment. They were observed for any gross behavioral changes and mortality/OECD (Organization for Economic Cooperation and Development) Guidelines 425 (OECD Test Guideline 425, 2001). This extract of PF was found to be a nontoxic fit for the maximum dose of (5 g/kg) body weight. The dose was selected for antiulcer evaluation (300, 400, and 500 mg/kg), respectively (Anupama and Handa, 1990). Indomethacin induced ulcerThirty six rats were divided into six groups of (six animals each). All animals were already fasted for 24 hours before the experiment; however, they were free to access water. The animals had free access to water throughout the experiment. To induce stomach ulcers, all animals were given indomethacin at a dose of 50 mg/kg by oral gavage on an empty stomach. This method ensures effective ulcer formation by maximizing the drug’s irritating effects on the gastric lining (Shahin et al., 2018). Group I served as healthy (control group) which received distilled water without induced ulcer. While group II served as (ulcer control group) which received distilled water with induced ulcer. However, the group III served as (standard control group) which received Ranitidine (20mg/kg, p.o), and animals of Groups IV, V, and VI received extracts of P. farcta at the dose of (300, 400, and 500 mg/kg), orally daily, respectively, for seven days after the induction of ulcer. These rats were sacrificed on the seventh day of treatment subsequently anesthetized with intraperitoneal administration of ketamine (50 mg/ kg) and xylazine (10 mg/kg). After that, the stomachs were detached, and opened along the greater curvature. Later, the stomachs were gently rinsed with water to remove gastric contents and blood. Then, the gastric contents were evaluated using biochemical parameters, and gastric ulceration was assessed by comparing free acidity, total acidity, ulcer index, and the volume of gastric juice, along with histopathological examination. Determination of ulcer indexThe ulcer index was calculated by the method that described previously (Chandann et al., 2013). Also, the severity and the number of lesions were assessed according to the scores including: Normal colored stomach (0); Red coloration (0.5); Spot ulcer (1); Hemorrhagic streak (1.5); Deep ulcers (2); and Perforation (3). Also, the ulcer index = 10/ ×, and the preventive effect was calculated according to the method of Basile et al. (1990) as follow: “Prevention index (%) = UI Control –UI TreatedUI Control × 100”, “UI Control = ulcer index in the negative control group, UI Treated = ulcer index in the group receiving extract”. Determination of total acidityAn aliquot of (1 ml) of gastric juice diluted with (1 ml) of distilled water was taken into a (50 ml) conical flask. Then, two drops of phenolphthalein (indicator) were added to it. After that, titrated with (0.01N NaOH) until observed a permanent pink color. Finally, the volume of (0.01N NaOH) consumed was noted (Ryou et al., 2021). The total acidity is expressed as mEq/L by the following formula: Determination of free acidityTopfer’s reagent was used instead of phenolphthalein indicator. Then, an aliquot of gastric juice was titrated with (0.01N NaOH) until canary yellow color was observed. Also, the volume of (0.01N NaOH) consumed was noted. The free acidity was calculated as the final step using the same formula used to determine total acidity (Nam et al., 2021). Measurement of mucus productionThe generation of bodily gastric fluid was estimated in each rat and submitted to preeminent ethanol prompted gastric mucosal harm. As well, the gastric mucosa of all rats were gently scratched using a glass slide. Finally, the bodily fluid was collected and measured using a precise electronic balance (Tan et al., 2002). Histopathological examinationThe samples of gastric tissue were long-lasting in neutral buffered formalin for 24 hour. These tissues were treated according to the standard procedure (Bancroft and Gamble, 2008). These tissue sections were cut and stained with eosin and hematoxylin stain. As well, these slides were examined microscopically for morphological changes such as oedema, hemorrhage, congestion, and erosions using an arbitrary scale for assessment of the severity of these changes. Statistical analysisFor all parameters, the mean values were calculated ± S.E.M. After that, each parameter was separately analyzed for significant intergroup differences, and ANOVA with a one-way classification was performed. A significant difference was considered at p < 0.05. Ethical approvalThe animal experimental studies were conducted according to the rules of Institutional Animal Ethical Committee of Government College of Veterinary Medicine/University of Baghdad, Iraq (1140 PG 21/6/2023). ResultsAcute oral toxicity studyIn this experiment, acute toxicity was measured in animal subjects administered 2 and 5 g/kg of P. farcta extract orally. The animals were then monitored for two weeks to evaluate any signs of toxicity. Throughout the experimental period, all animals showed no physical signs of adverse reactions after intake at the specified doses. These animals maintained healthy survival with no deaths recorded. There were no abnormal clinical signs, body weight fluctuations, or behavioral changes, nor any gross necropsy findings that could be attributed to treatment with the test substance on any observation day. This leads us to conclude that at these higher doses, there is no acute toxicity to male and female rats, as they survived doses exceeding 5 g/kg body weight. Indomethacin induced ulcerThe study findings reveal that control animals (Group I) did not exhibit any lesions. Additionally, it was observed that administering an oral extract of PF effectively safeguards against indomethacin-induced gastric mucosal injuries, a commonly used measure to assess anti-ulcer and cytoprotective properties. Various biochemical parameters such as ulcer index, total acidity, free acidity and gastric juice volume were evaluated across all six groups, indicating notable variations among them. Measurement of gastric pH, total acidity and free acidityThe administration of indomethcine lead to gastric damages, increase in total acidity (133.82 ± 1.57), highly significant decrease in gastric pH (1.58 ± 0.20), and free acidity (1.81 ± 0.46) in ulcer control group when compared to the normal control group with mean value (59.35 ± 1.59), (2.86 ± 0.19), (22.67 ± 1.35) respectively. While, the treated group with standard drug (Ranitidine) showed significantly increased in the pH (4.26 ± 0.21), significantly reduced the free acidity (27.50 ± 1.60) and total acidity(67.72 ± 0.38) when compared to the ulcer control group. Also, it was noted that the rats treatment with PF extract groups 300 mg/ kg, 400 mg/kg and 500mg/kg led to a marked reduction in free acidity and total acidity and more significantly, especially with a group of 500 mg/kg (23.20 ± 1.45), (73.25 ± 0.95) respectively when compared to the ulcer control group (Table 1) Ulcer indexAdministration of indomethacin-induced gastric ulceration characterized by hemorrhagic lesions in the control group. Treatment with P. farcta extract resulted in a reduction of the severity for these lesions that induced by indomethacin, as evidenced by a moderately significant especially in dose 500mg/kg an increase in the percentage protection of ulcers and decrease in the ulcer index (1.81 ± 0.46) when compared to ulcer control group (3.52 ± 0.60). Similarly, rats treated with Ranitidine, the standard drug of choice, showed a reduction in the severity of indomethacin-induced lesions with mean value (1.45 ± 0.54), as indicated by a significant (p < 0.001) an increase in the percentage protection of ulcers and decrease in the ulcer index when compared to ulcer control group (Table 2). Table 1. The gastric volume, pH, Free acidity and total acidity.
Table 2. Ulcer index and % inhibition of ulcer.
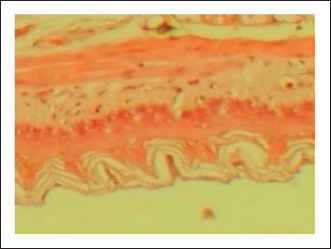
Measurement of mucus productionThe content mucus of gastric mucosa in treated rats with indomethacin (ulcer control group) was reduced significantly with mean value when related to standard control or to the extract groups. The rats treatment of with P. farcta extract showed elevated significantly the mucus content in the indomethacin induced ulcerated rats as showed in (Table 1). Also, the proliferations of mucus production indicated that the P. farcta extract had potential effect to induce the mucus secretion and in this manner ensure the stomach layer from any damage caused. Histopathological examinationThe gastric tissue slides were examined under a microscope for morphological changes such as hyperemia, hemorrhage, edema, necrosis, inflammatory changes, distortion, erosion, and ulcers caused by the destructive effects of indomethacin on stomach tissues. No pathological changes were observed in the normal control group (Fig. 1). Rats in the ulcer control group showed significant damage to the surface epithelium, necrotic areas penetrating the mucosal layer, and severe edema of the submucosal layer. Additionally, there was congestion with infiltration of inflammatory cells (Fig. 2). Histological results revealed that the treated rats with ranitidine had less protection of the gastric lining due to mild infiltration of leucocyte and edema in the submucosal layer, in addition to less disruption in both the superficial and deep mucosal layers. Treatment doses (300, 400 and 500 mg/kg) with P. farcta extract. Groups treated with P. farcta extract (300 and 400 mg/ kg) showed mild infiltration of inflammatory cells, edema, or significant disruption of the deep mucosa (Figs 5 and 6) while, P. farcta extract group (500mg/ kg) prevented histological changes and showed no infiltration of inflammatory cells, edema, or significant disruption of the deep mucosa (Figs 3, 4 and 7). DiscussionIn the present study, the indomethacin, was used to stimulate gastric ulcers in rats. The evaluation result of the acute toxicity for P. farcta extract explained no toxic appearance (mortality rate) effect when given orally in rats up to a dose of 5,000 mg/kg. Therefore, LD50 of the combined extract was measured to be above 5,000 mg/kg (Lorke, 1983). This indicates that the plant extract had a relative safe for used and consumption in ethno-medicine at doses not exceeding 5,000 mg/ kg. Plant extract is the attractive source of new drugs and had shown promising results in the treatment of gastric ulcer. Gastric ulcer or gastrointestinal disorders have been treated using medicinal plants and herbs. The findings of a study by He et al. (2001) displayed that more than (86%) of the infected with gastric ulcers enhanced after oral management of an herbal therapy mixture. Indomethacin is an aryl acetic acid derivative identified as one of the most effective NSAIDs with many alimentary opposing effects due to its inhibitory influence on prostaglandins synthesis. It can injury to stomach tissue by accumulative gastric acid (Suleyman et al., 2010; Sabiu et al., 2015). Gastric ulcer made by Indomethacin which is an anti-inflammatory and non-steroidal drug by reduction the activity of COX and then creation of endogenous prostaglandin levels in the gastric mucosa (Takeeda et al., 2004). Also, it caused increase of gastric motility and affecting the vascular blood vessels, which in stimulates the activating of reactive oxygen species (ROS) production with infiltration of lipid peroxidation and neutrophils (Laine et al., 2008).
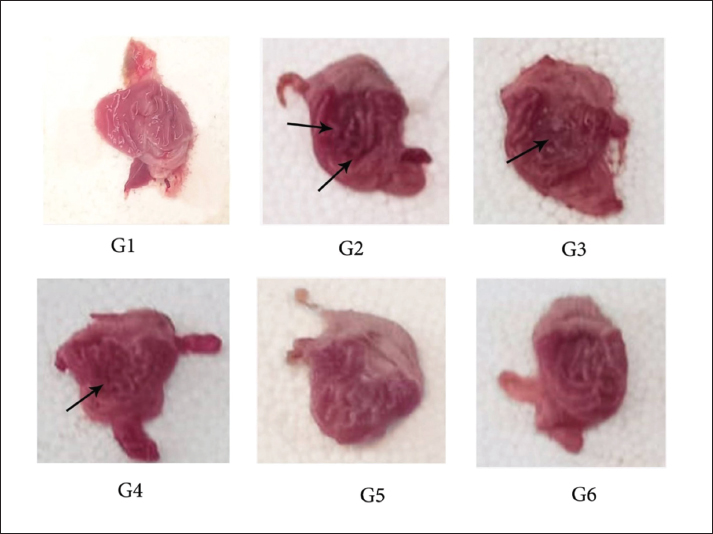
Fig. 1. Gastric mucosa lesion in rats; G1 served as control group; G2 served as ulcer control with indomethacin have sever injuries; G3, G4 and G5 received extract of Prosopis farcta at 300,400 and 500 mg/kg; G6 served as standard control with Ranitidine. The endogenous prostaglandins which regulate the mucosal blood flow, epithelial cell proliferation, epithelial restitution, mucus and bicarbonate secretion, basal acid secretion and mucosal immunocyte function. Indomethacin which prevents the prostaglandin synthesis due to decrements of prostaglandins and lead to ulceration (Khalaf et al., 2022). Hence, this study evaluated the antiulcerative activity of Prosopis farcta extract by measuring ulcer index, total acidity, free acidity and gastric juice volume in addition to examination of histopathological markers and macroscopic features. The repeated dose of oral for indomethacin that induced ulcer model exhibited a significant decrease in ulcer index (p < 0.05) in 5 days treatment with Prosopis farcta at doses 300 mg/kg, 400 mg/kg and 500 mg/kg respectively. Besides this, the ulcer healing percentage has significantly increased in a treatment doses which is dependent manner dose. On the other hand, ranitidine (20 mg/kg bw) exhibited a strong healing effect in indomethacin that induced gastric ulcer. The significant increase in the ulcer index detected in the ulcer group which received water after indomethacin instillation which confirmed the induction of ulcer. Supplementary, the extract revealed a protective action against indomethacin that induced injury of gastric mucosa as demonstrated by the inhibition or reduction of the gastric ulcer area. Moreover, it was revealed that P. farcta extract significantly decreased acid secretion. The treatment group with standard drug (Ranitidine) has significantly increased pH, and significantly reduced the free acidity and total acidity compared to the ulcer control group. As well, the activity of P. farcta extract is dependent on free acidity and gastric pH. Also, the total acidity by acid secretion that may be decreased. This effect was dose related due to administration of the three doses of plant extract. It means that local effect in gastric tissue of plant extract has an significant role in this regard. Resulting the parenteral administration of this extract had a significant effect only at dose (500 mg/kg), and has a lesser potency when compared to oral administration.
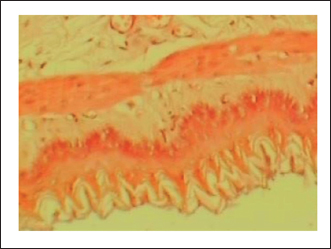
Fig. 2. Normal control group received distilled water, No pathological changes were observed.
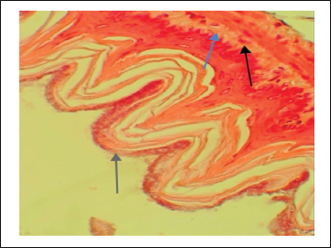
Fig. 3. Ulcer control group damage (↗) to the epithelium, necrotic areas penetrating the mucosal layer, and (↗) severe edema of the sub-mucosal layer with congestion and (↗) infiltration of inflammatory cells
Fig. 4. Standard drug group (ranitidine) mild infiltration of leucocyte and less edema in the sub-mucosal layer.
Fig. 5. Prosopis farcta extract (300mg/kg) mild (↗) filtration of inflammatory cell, (↗) edema or less (↗) disruption in both the superficial and deep mucosal layers
Fig. 6. Prosopis farcta extract (400mg/kg) mild (↗) filtration of inflammatory cell, (↗) edema or less (↗) disruption in both the superficial and deep mucosal layers
Fig. 7. Prosopis farcta extract (500 mg/kg) prevented histological changes, nofiltration of inflammatory cells, edema, or distruption of deepmucosa. The histopathological changes of gastric ulcer induction in the indomethacin (ulcer group) revealed the loss of epithelial layers, mucosal injury, decreased mucosal thickness with inflammatory cells infiltration and the distortion of mucosa and its glands. The ulcer initiation by indomethacin was accomplished on empty stomach via withholding food 12 hour before the induction later it has been revealed that the administration timing lead to gastric mucosal lesions which increased due to food that has buffering capacity against indomethacin effect. Also, the acute ulceration by lowering circulating prostaglandin levels and blocking COX (Jawad, 1976; Byeon et al., 2018). Prosopis farcta have been discovered the presence of resins, carbohydrates, alkaloids, flavonoids, phenols, tannins, saponins, coumarins, terpenes, and glycosides (Sharifi, 2019; Zughayyar and Gyad, 2023) In a previous study of Jain (2016), was reported that the secondary metabolites of plant specifically, terpenoids, flavonoids, and alkaloids have a major effect on treatment of peptic ulcer. On the other hand, the flavonoids are classified to be the most essential of secondary metabolites that have multiple mechanisms for working as an protection and antiulcer agent for gastric mucosa. These mechanisms includes: content increase of mucosal prostaglandin, free radical scavenging, cytoprotection and microcirculation improvement of gastric tissue (Mota et al., 2009; Serafim et al., 2020). Also, the flavonoids which preserves the effect of gastric cytoprotective through nitric oxide synthase and modulating prostaglandins (PG’s) pathways, which serves to mediates gastric blood flow, maintain stomach mucosal integrity, accelerates mucosal healing and inhibits gastric acid secretion (Zhang, 2019). The protective activities of all these saponins are probably due to activation of mucous membrane protective factors rather than due to inhibition of gastric acid secretion (Borrelli and Izzo, 2000). Furthermore, numerous plants that including high amount of saponins that acting as an activator factors of mucus membrane stabilizing due to retain antiulcer effects in several experimental studies (Morikawa et al., 2006). The study of Al-Rehaily et al. (2002) showed the presence of terpenoids, saponins, and tannins were found to possess properties of gastroprotective. Also, the tannins and terpenoids in sativa have the cytoprotective activity in the gastric mucosa. Furthermore, the terpenoids have several pharmacological properties, including antiulcer and anti-inflammatory effects (Arrietta et al., 2003). Tannins have antiulcer effect due to its vasoconstriction and astringency effects. These effects due to deposition of small-scale proteins on the ulcer site. Also the moderate layer was shaped and shields that averts gut and mucosa emissions with poisons and some other aggravations. The previous study, the above dynamic mixes has arranged and had ability to activate mucus, bicarbonate emission, prostaglandin and stimulate with the backsliding effect of responsive oxidants in gastrointestinal tract (Baker, 1994; Sakat and Juvekar, 2009; Abebaw et al., 2017). ConclusionThis study concluded that Prosopis farcta extract shows strong potential as a natural therapeutic option for the prevention and treatment of gastric ulcers. Additionally the presence of active photochemical in this plant lead to cytoprotective effects and regeneration of mucosal tissue. AcknowledgmentThe authors thank College of Veterinary Medicine for supporting. FundingThis research received no specific grant Author’s contributionsAH and OS: study’s concept. AH, OS, and RS: practical work, data analysis, tissue sections preparation and interpretation. AH and RS: writing and editing of the manuscript. All authors have revised the manuscript and prepared it for publication Conflict of interestThe authors declare no conflicts of interest. Data availabilityAll data supporting the current study are available in the manuscript. ReferencesAbebaw, M., Mishra, B. and Gelayee, D.A. 2017. Evaluation of antiulcer activity of the leaf extract of Osyris quadripartita Decne. (Santalaceae) in rats. J. Exp. Pharmacol. 9, 1–11; doi:10.2147/JEP. S125383 Allmjeed, D.I.A., Jumaa, R. S. and Ibrahim, O. M. 2024. Isolation and PCR-based detection of parvovirus in dogs. Open Vet. J. 14(12), 3213; doi: 10.5455/ OVJ.2024.v14.i12.6 Al-Rehaily, A.J., Al-Howiriny, T.A., Al-Sohalbani, M.O. and Rafaullah, S. 2002. Gastroprotective effects of ‘Amla’ Emblica offeinalis on in vivo test models in rats. Phytomedicine 9, 515–522; doi: 10.1078/09447110260573146 Anupama, S. and Handa, S.S. 1990. Hepatoprotective activity of andrographolide from Andrographis paniculata against CCl4. IJMR 92, 276. Available via https://PMID/2228074 Arrietta, J., Benitez, J., Flores, E., Castillo, C. and Navarette, A. 2003. Purification of gastroprotective triterpenoids from the stem bark of Amphipterigium adstringens, role of prostaglandins, sulfhydryls, nitric oxide and capsaicin-sensitive neurons. Planta Med. 69(10), 905–909; doi: 10.1055/s-2003-45098 Asadollahi, A., Sarir, H., Omidi, A. and Torbati, M.B. 2014. Hepatoprotective potential of prosopis farcta beans extracts against acetaminophen-induced hepatotoxicity. Available via https://pmc.ncbi.nlm. nih.gov/articles/PMC4223948/ Baker, M.E. 1994. Licorice and enzymes other than 11 betahydroxysteroid dehydrogenase: an evolutionary perspective. Steroids 59, 136–141; doi: 10.1016/0039-128x(94)90091-4 Bancroft, J.D. and Gamble, M. 2008. Theory and practice of histological techniques, 6th ed. Churchill Livingstone, Elsevier. Available via https://www. sciencedirect.com/book/9780443102790/theory-and-practice-of-histological-techniques Basile, A.C., Sertié, J.A., Panizza, S., Oshiro, T.T. and Azzolini, C.A. 1990. Pharmacological assay of Casearia sylvestrisz. I: preventive anti-ulcer activity and toxicity of the leaf crude extract. J. Ethnopharmacolf. VI. 30, 185–187; doi: 10.1016/0378-8741(90)90007-G Bersenas, A.M., Mathews, K.A. and Allen, D.G. 2005. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am. J. Vet. Res. 66, 425; doi: 10.2460/ajvr.2005.66.425 Borrelli, F. and Izzo, A.A. 2000. The plant kindom as a source of anti-ulcer remedies. Phytother. Res. 14, 581–591;doi:10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s Byeon, S., Oh, J., Lim, J., Lee, J. and Kim, J.S. 2018. Protective effects of Dioscorea batatas flesh and peel extracts against ethanol-induced gastric ulcer in mice. Nutrients 11(10), 1680; doi: 10.1177/0960327120918306 Chandann, G., Tirthankar, D.E.B. and Manju, B. 2013. Evaluation of anti-ulcer activity of Tinospora corifolia in albino rates. Int. J. Pharm. Bio. Sci. 4(2), 78–85; doi: 10.5555/20133289589 Chyad, A.H. 2017. Evaluation of anticancer, analgesic and anti-inflammatory activities of the ethanolic extract of Lepidium draba Linn. leaves. Adv. Anim. Vet. Sci. 5(1), 7–13; doi: 10.14737/journal. aavs/2017/5.1.7.13 Duran, S., Jernigan, A. and Ravis, W. 1991. Pharmacokinetics of oral and intravenous ranitidine in cats. In Proceedings of 9th Annual ACVIM Forum, p 902. He, L.Z., Zhang, Q. and Wang, S.C. 2001. Clinical study on treatment of gastric ulcer with qingwei zhitong pill. Chin. J. Integr. Med. 21(6), 422–423. Available via https://pubmed.ncbi.nlm.nih.gov/12577435/ Ibrahim, O.M.S., Jumaa, R.S. and Yahya, N.Z. 2023. Antimicrobial evaluation of different types of Aurantioideae extracts against some pathogenic bacteria. Adv. Anim. Vet. Sci. 11(10), 1736–1743; doi: 10.17582/journal.aavs/2023/11.10.1736.1743 Jain, P. 2016. Secondary metabolites for antiulcer activity. Nat. Prod. Res. 30(6), 640–656; doi: 10.1080/14786419.2015.1036269 Jawad, F.H.A. 1976. Studies on the origion of tissue histamine in G.P: glasgow. Jbr, A. and Jumaa, R. 2024. Sequencing analysis of the N gene of canine distemper virus from infected dogs in Baghdad City. Iraqi J. Vet. Med. 48(1), 41–47; doi: 10.30539/n4wtde42 Jumaa, R.S. 2024. Sequencing and phylogenetic analysis of P4b gene in pigeon poxvirus. Adv. Anim. Vet. Sci. 12, 173–179; doi: 10.17582/journal. aavs/2024/12.1.173.179 Jumaa, R.S., Abdulmajeed, D.I. and Karim, A.J. 2021. Evaluation of secondary metabolites of herbal plant extracts as an antiviral effect on infectious bursal disease virus isolates in embryonated chicken eggs. Vet. World 14(11), 2971; doi: 10.14202/ vetworld.2021.2971-2978 Jumaa, R.S., Allawe, A.B. and Jabbar, R.N. 2019. Isolation of infectious bursal disease virus and immuno histochemstry of CD4+ and CD8+ for infected Iraqi chickens. Biochem. Cell Arch. 19(2), 3375; doi: 10.35124/bca.2019.19.2.3375 Jumaa, R.S., Allawi, A.B. and Jabbar, R.N. 2020. Genetic analysis of field isolates of infectious bursal disease virus in Iraqi farms. Iraqi J. Vet. Med.44(1), 18–28; doi: 10.30539/ijvm.v44i1.931 Khalaf, H.M., Ahmed, S.M., Welson, N.N. and Abdelzaher, W.Y. 2022. Rivastigmine ameliorates indomethacin experimentally induced gastric mucosal injury via activating α7nAChR with inhibiting oxidative stress and apoptosis. J. Biochem. Mol. Toxicol. 36, e23147; doi: 10.1002/ jbt.23147 Kounenis, G., Koutsoviti-Papadopoulou, M. and Elezoglou, A. 1992. Comparative study of the H2-receptor antagonists cimetidine, ranitidine, famotidine and nizatidine on the rabbit stomach fundus and sigmoid colon. J. Pharmacobiodyn. 15, 561; doi: 10.1248/bpb1978.15.561 Laine, L., Takeuchi, K. and Tarnawski, A. 2008. Reviews in basic and clinical gastroenterology. Gastroenterology 135, 41–60; doi: 10.1053/j. gastro.2010.09.004 Lorke, D. 1983. A new approach to practical acute toxicity testing. Arch. Toxicol. 54(4), 279–287; doi: 10.1007/BF01234480 Luis, V.C., Sabino, D.S., José, D.S.M., Isaias, M.J.A., Maria, O.D., Freitas, F.F., Fioravante, D.S.B., Arunachalam, K. and Tabajara, D.O.M.D. 2023. Antiulcer activity and mechanism of action of the hydroethanolic extract of leaves of Terminalia argentea Mart. In different in vivo and in vitro experimental models. J. Ethnopharmacol. 11, 69–72; doi: 10.1016/j.jep.2023.116972 Marshall-Jones, Z.V., Baillon, M.L. and Croft, J.M. 2006. Effects of Lactobacillus acidophilus DSM13241 as a probiotic in healthy adult cats. Am. J. Vet. Res. 67, 1005; doi:/10.1016/j. jep.2023.116972 Mekonnen, A.N., Asrade, A.S. and Wahab, A.M.A. 2020. Evaluation of antiulcer activity of 80% methanol extract and solvent fractions of the root of Croton macrostachyus Hocsht: Ex Del. (Euphorbiaceae) in rodents. J. Complement. Altern.Med. 2020(1), 2809270; doi: 10.1155/2020/2809270 Morikawa, T., Li, N., Nagamoto, A., Matsuda, H., Li, X. and Yoshikawa, M. 2006. Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis). J. Nat. Prod. 69, 185–190; doi: 10.1021/np058097w Mota, K.S., Dias, G.E., Pinto, M.E., Luiz-Ferreira, A., Souza-Brito, A.R., Hiruma-Lima, C.A., Barbosa-Filho, J.M. and Batista, L.M. 2009. Flavonoids with gastroprotective activity. Molecules 14(3), 979–1012; doi: 10.3390/molecules14030979 Nam, H.H. and Choo, B.K. 2021. Geranium koreanum, a medicinal plant geranii herba, ameliorate the gastric mucosal injury in gastritis-induced mice. J. Ethnopharmacol. 265, 113041; doi: 1016/j. jep.2020.113041 OECD Test Guideline 425. 2001. Guidelines for testing of chemicals. Guidelines 425, 14. Acute oral toxicity-up-and-down procedure. Available via https://www.oecd.org/en/topics/sub-issues/testing-of-chemicals/test-guidelines.html Rates, S.M. 2001. Plants as source of drugs. Toxicology 39(5), 603–613; doi: 10.1016/S0041-0101(00)00154-9 Ryou, S.H., Cho, I.J., Choi, B.-R., Kim, M.B., Kwon, Y.S. and Ku, S.K. 2021. Brassica oleracea Var. Capitata L. alleviates indomethacin-induced acute gastric injury by enhancing anti-inflammatory and antioxidant activity. Processes 9, 372; doi: 10.3390/ pr9020372 Sabiu, S., Garuba, T., Sunmonu, T., Ajani, E., Sulyman, A., Nurain, I. and Balogun, A. 2015. Indomethacin-induced gastric ulceration in rats: protective roles of Spondias mombin and Ficus exasperata. Toxic Rep. 2, 261–267; doi: 10.1016/j.toxrep.2015.01.002 Sakat, S.S. and Juvekar, R.A. 2009. Antiulcer activity of methanol extract of Erythrina indica Lam. leaves in experimental animals. Pharmacogn. Res. 1, 396–401; doi: 10.4103/0974-8490.58026 Serafim, C., Araruna, M.E., Júnior, E.A., Diniz, M., Hiruma-Lima, C. and Batista, L. 2020. A review of the role of flavonoids in peptic ulcer (2010–2020). Molecules 25(22), 5431; doi: 10.3390/ molecules25225431 Shahin, N.N., Abdelkader, N.F. and Safar, M.M. 2018. A novel role of irbesartan in gastroprotection against indomethacin-induced gastric injury in rats: targeting DDAH/ADMA and EGFR/ERK signaling. Sci. Rep. 8(1), 4280; doi: 10.1038/ s41598-018-22727-6 Sharifi-Rad, J. 2019. Prosopis plant chemical composition and pharmacological attributes: targeting clinical studies from preclinical evidence. Biomolecules 9, 777; doi: 10.3390/biom9120777 Suleyman, H., Albayrak, A., Bilici, M., Cadirci, E. and Halici, Z. 2010. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 33, 224–234; doi: 10.1007/ s10753-009-9176-5 Takeeda, M., Hayashi, Y., Yamato, M., Murakami, M. and Takeuchi, K. 2004. Roles of endogenous prostaglandins and cyclooxygenase isozymes in mucosal defense of inflamed rat stomach. J. Physiol. Pharmacol. 55, 193–205 Tan, P.V., Nyasse, B., Dimo, T. and Mezui, C. 2002. Gastric cytoprotective antiulcer effects of a leaf methanol extract of Ocimum suave (Lamiaceae) in rats. J. Ethnopharmacol. 82, 69–74; doi: 10.1016/ s0378-8741(02)00142-3 Tarnawski, A.S. 2005. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 50(Suppl. 1), S24–S33; doi: 10.1007/ s10620-005-2803-6 Utsumi, H., Yasukawa, K., Soeda, T., Yamada, K.I., Shigemi, R., Yao, T. and Tsuneyoshi, M. 2006. Noninvasive mapping of reactive oxygen species by in vivo electron spin resonance spectroscopy in indomethacin-induced gastric ulcers in rats. J. Pharmacol. Exp. Ther. 317, 228–235; doi: 10.1124/ jpet.105.095166 Wallace, J.L., McKnight, W., Reuter, B.K. and Vergnolle, N. 2000. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119, 706–714; doi: 10.1053/gast.2000.16510 Yadav, S.K., Adhikary, B., Chand, S., Maity, B., Bandyopadhyay, S.K. and Chattopadhyay, S. 2012. Molecular mechanism of indomethacin-induced gastropathy. Free Radic. Biol. Med. 52, 1175–1187; doi: 10.1016/j.freeradbiomed.2011.12.023 Zhang, J. 2019. Acute and subacute toxicity assessment of oxyclozanide in wistar rats. Front Vet Sci. 6, 294; doi: 10.3389/fvets.2019.00294 Zughayyar, S.H. and Gyad, A.H. 2023. Antidiarrheal effects of Prosopis farcta l. Fruit extract: an enteropooling and histopathological study in rats. Adv. Anim. Vet. Sci. 11(12), 2038–2044; doi: 10.17582/journal.aavs/2023/11.12.2038.2044 | ||
| How to Cite this Article |
| Pubmed Style Chyad AH, Ibrahim OS, Jumaa RS. Antiulcer activity of Prosopis farcta L. fruits extract in rats. Open Vet. J.. 2025; 15(6): 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 Web Style Chyad AH, Ibrahim OS, Jumaa RS. Antiulcer activity of Prosopis farcta L. fruits extract in rats. https://www.openveterinaryjournal.com/?mno=235086 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.11 AMA (American Medical Association) Style Chyad AH, Ibrahim OS, Jumaa RS. Antiulcer activity of Prosopis farcta L. fruits extract in rats. Open Vet. J.. 2025; 15(6): 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 Vancouver/ICMJE Style Chyad AH, Ibrahim OS, Jumaa RS. Antiulcer activity of Prosopis farcta L. fruits extract in rats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 Harvard Style Chyad, A. H., Ibrahim, . O. S. & Jumaa, . R. S. (2025) Antiulcer activity of Prosopis farcta L. fruits extract in rats. Open Vet. J., 15 (6), 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 Turabian Style Chyad, Amer Hakeem, Omar Salim Ibrahim, and Rawaa Saladdin Jumaa. 2025. Antiulcer activity of Prosopis farcta L. fruits extract in rats. Open Veterinary Journal, 15 (6), 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 Chicago Style Chyad, Amer Hakeem, Omar Salim Ibrahim, and Rawaa Saladdin Jumaa. "Antiulcer activity of Prosopis farcta L. fruits extract in rats." Open Veterinary Journal 15 (2025), 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 MLA (The Modern Language Association) Style Chyad, Amer Hakeem, Omar Salim Ibrahim, and Rawaa Saladdin Jumaa. "Antiulcer activity of Prosopis farcta L. fruits extract in rats." Open Veterinary Journal 15.6 (2025), 2386-2394. Print. doi:10.5455/OVJ.2025.v15.i6.11 APA (American Psychological Association) Style Chyad, A. H., Ibrahim, . O. S. & Jumaa, . R. S. (2025) Antiulcer activity of Prosopis farcta L. fruits extract in rats. Open Veterinary Journal, 15 (6), 2386-2394. doi:10.5455/OVJ.2025.v15.i6.11 |