| Research Article | ||
Open Vet. J.. 2025; 15(7): 3044-3053 Open Veterinary Journal, (2025), Vol. 15(7): 3044-3053 Research Article Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western LibyaAhmad A. A. Saad1, Mohammed A. Murshid2, Marium M. Hussein3*, Tarek A. Zahmol2, Najwa A. I. Abdulsalam1, Esmaiel I. F. Saad4, Osama N. Elwaer5, Esmahan M. Ismaeil5 and Abderrahman. J. Jbeel51Department of Preventive Medicine and Public Health, Faculty of Veterinary Medicine Omar El-Mokhtar University, Albida, Libya 2Department of Veterinary Medicine, Faculty of Veterinary Medicine and Agricultural Sciences, University of Zawia, Zawiya, Libya 3Department of Zoology, Faculty of Science, Omar El-Mokhtar University, Albida, Libya 4Department of Microbiology, Faculty of Science, Omar El-Mokhtar University, Albida, Libya 5National Center for Animal Health, Tripoli, Libya *Corresponding Author: Marium M. Hussein. Department of Zoology, Faculty of Science, Omar El-Mokhtar University, Albida, Libya. Email: marium.hussein [at] omu.edu.ly Submitted: 30/12/2024 Revised: 21/05/2025 Accepted: 04/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
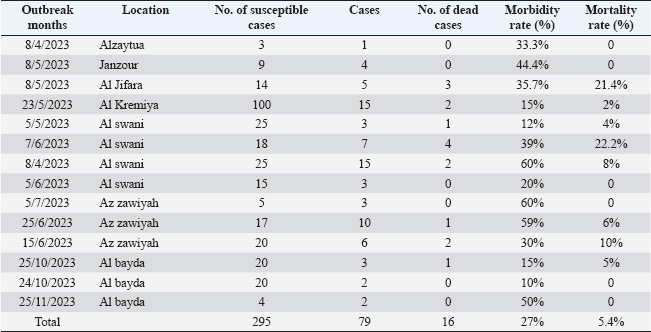
ABSTRACTBackground: Cattle are naturally susceptible to the viral disease lumpy skin disease (LSD), which has become a significant concern for the livestock industry in Libya since its first emergence in 2023. Originating from Africa, it rapidly spread across the country, facilitated by the movement of animals both within Libya and from neighboring regions. Aim: This investigation determined the molecular features and the phylogenetic analysis of LSD virus (LSDV) isolated from cattle from Al-Zawiya, Western Libya. Methods: Polymerase chain reaction (PCR) was performed on 14 collected samples. Real-time PCR was employed to detect and amplify specific viral DNA sequences, confirming the presence and identifying the genetic profile of LSDV. Results: Results confirmed the presence of LSDV in 13 of 14 samples, with subsequent sequencing revealing distinct genetic markers characteristic of the virus strains in Libya. The findings also revealed that the LSDV responsible for these outbreaks is closely related to LSDV Sudan/06-Obied_GU119938, LSDV Egypt/89-Ismalia_GU119947, and LSDV Serbia/Buj/2016_KY702007. Additionally, genetic analysis revealed that the Libyan LSDV strain is unique among all LSDV isolates due to an additional single -nucleotide polymorphism at position 111 (C→A), which may affect the efficacy of immunization efforts to control the disease. Conclusion: This study serves as a valuable resource for understanding the genetic characteristics of LSDV in Libya and may assist in selecting the most appropriate vaccine strain for disease control. Keywords: Lumpy skin disease, PCR, Sequence, Outbreak, Libya. IntroductionLumpy skin disease (LSD) is a cross-border viral disease that primarily affects cattle and buffaloes. It is transmitted by insects, leading to significant economic impacts on livestock production and trade (Gari et al., 2011; Alkhamis and VanderWaal, 2016). The causative agent is a DNA virus belonging to the genus of Capripox virus (CaPV) and family Poxviridae (Abutarbush et al., 2015). Despite the fact that all ages and breeds of cattle may be at high risk, the degree of symptoms differs from asymptomatic infection to death according to the cattle breed’s susceptibility and virulence of the strains type, vector number, or host’s immune condition (Tuppurainen et al., 2017). Various clinical symptoms, such as swollen superficial lymph nodes, fever, watery eyes, nasal discharge, skin edema, firm, flat-topped papules and nodules throughout the body, including the buccal mucosa, head, neck, scrotum, and udder, may appear in infected animals (Babiuk et al., 2008; Abutarbush et al., 2015; Molla et al., 2017). Furthermore, LSD can lead to a decrease in milk yield in dairy cows and, in some cases, death due to secondary bacterial infections. (Chihota et al., 2003). Moreover, it affects the commerce of cows and their products in countries where the disease is endemic (Babiuk et al., 2008). LSD generally causes a morbidity rate ranging from 3% to 100% and a mortality rate of less than 20% (Abutarbush et al., 2015; Sprygin et al., 2018). In fact, as a result of the substantial hazard to the cattle herd and its massive economic effect, LSD is recorded as a bovine notifiable disease by the World Organization for Animal Health (WOAH) (OIE, 2010). Animal movements, immunization levels, and insect activity influenced by climatic patterns are among the risk variables that contribute to LSD onset (Ochwo et al., 2020). In the past, LSDV was initially identified in Zambia in 1929. Since then, becoming endemic in several African nations. Additionally, LSDV has quickly expanded across southeast Europe, the Middle East, and South Asia, Lately, there have been reports of early LSD onset in Egypt, Sudan, and Chad (FAO, 2013). Early diagnosis and verification of suspected cases are crucial steps to effectively control and eliminate LSD, especially in non-endemic regions. Practically, the most accurate methods used to diagnose the disease include electron microscope, serological methods like enzyme-linked immunosorbent assays (ELISAs) and polymerase chain reaction (PCR) (Lamien et al., 2011a,b; Elhaig et al., 2017). Even though most of these methods are precise and sensitive, some serological methods cannot distinguish between Parapoxvirus and Capripoxvirus (Tian et al., 2010; Selim et al., 2020). Consequently, the PCR approach is the most dependable, sensitive, and effective method for differentiating the virus (Body et al., 2012; Selim et al., 2020). Molecular epidemiological and genetic characterization studies of LSDV are based on the analysis of various genomic regions, such as the G-protein-coupled receptor (GPCR), the RNA polymerase 30 kDa subunit (RPO30), the CaPV homolog of the variola virus B22R gene, and the EEV glycoprotein genes (Le Goff et al., 2009; Lamien et al., 2011; Gelaye et al., 2013; Menasherow et al., 2016). The first notification of the disease in Libya was reported to the OIE. Beginning in the northwest (coastal district) of the nation in May 2023, the disease rapidly spread over the entire nation. The Molecular Diagnostic unit at the Veterinary Laboratory Center of the National Center for Animal Health used real-time PCR to confirm that the samples from the suspected cases tested positive for LSD. Verifying the presence of LSDV in Libyan cattle and providing a molecular characterization of field LSDV isolates circulating in Libya are the goals of the current investigation. Materials and MethodsEpidemiological dataData concerning the epidemiological aspects of LSD outbreaks were sourced from reports of disease onset submitted to the National Center for Animal Health. The initial notification was recorded on April 8, 2023, with laboratory confirmation following on June 8, 2023, in the Qasr Bin Ghasir, located in the south of Tripoli, in 2023. This delay in confirmation was attributed to a lack of diagnostic resources and appropriate materials. The reports thoroughly detailed the clinical manifestations of the disease, including skin nodules distributed across the body, excessive salivation, lacrimation, and nasal discharge. Unfortunately, the outbreak spread rapidly across the country. As a result, follow-up reports were submitted, with the seventh report issued on November 30, 2023. Although not all disease outbreaks were officially reported to the National Center for Animal Health, the collected reports provided comprehensive coverage of the entire country, offering a reasonably accurate depiction of the disease’s spread and severity. The initial outbreak report and subsequent reports up to the eighth submission dated November 30, 2023, were thoroughly examined. It is important to note that reporting continued beyond this date. The samples accompanying these reports were analyzed sequentially in the central laboratory of the National Center for Animal Health using either ELISA or real-time PCR. This process aligns with the reporting protocols submitted periodically to the WOAH regarding the epidemiological situation. Table 1, which aims to understand the outbreak dynamics, shows the reported mortalities and morbidities in the seventh regions as well as the overall number of susceptible cattle based on reporting records from the National Center for Animal Health, starting from the immediate notification up to follow-up report 7 (WOAH, 2025). Study areaThis study investigated the molecular characteristics of LSDV in cattle in Libya. Tissue samples of 14 suspected cattle were collected from the Zawia region in northwest Libya, located at 32◦75′N (Fig. 1), following the initial onset of the disease in Libya. The research area is geographically situated adjacent to agricultural areas where animals are raised intensively. Furthermore, high summer temperatures (above 31°C) in the research area are ideal conditions for LSDV vector proliferation and animal-to-animal transmission. The most observed clinical symptoms were high fever and nodular lesions on different body parts. Transportation and collection of samplesAccording to OIE (2010) guidelines, tissue samples for virus isolation (VI) and molecular characterization were collected from suspected cattle. Fourteen samples of skin nodules were taken from suspected animals exhibiting significant clinical indications for the disease. In the presence of a veterinarian, tissue samples from nodular lesions of the skin were obtained aseptically by cleaning and rinsing the affected area, cutting any hair with a sterile scalpel blade, and making an incision that penetrated the epidermis and dermis. The 14 samples were subjected to real-time PCR testing at the central laboratory upon confirmation of positivity. The collected tissue samples were then placed in sterile universal bottles containing virus transport medium, which was maintained at −20°C, and transported to the Animal Production and Health Laboratory, Joint FAO/IAEA Center of Nuclear Techniques in Food and Agriculture, Seibersdorf, Austria. Table 1. Morbidity and mortality rates of LSD in cattle from affected farms during the 2023 outbreak in Libya.
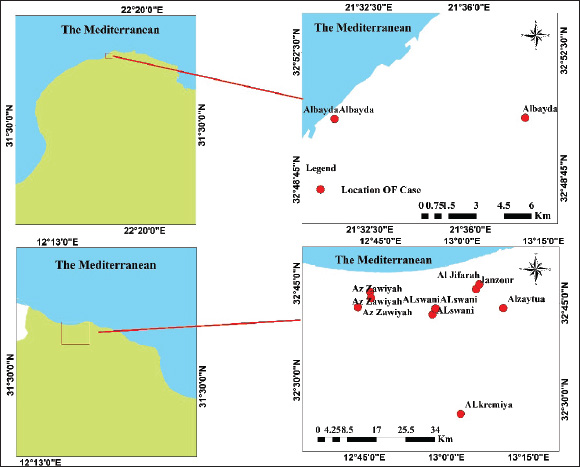
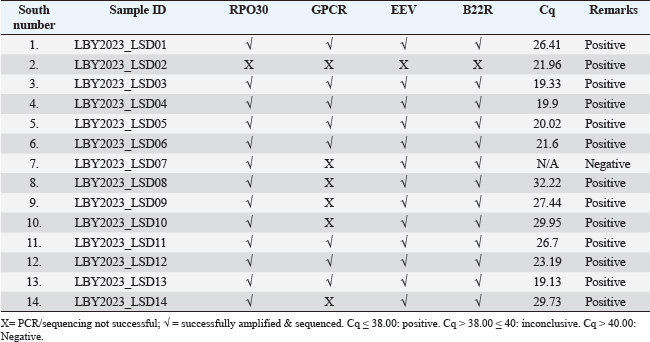
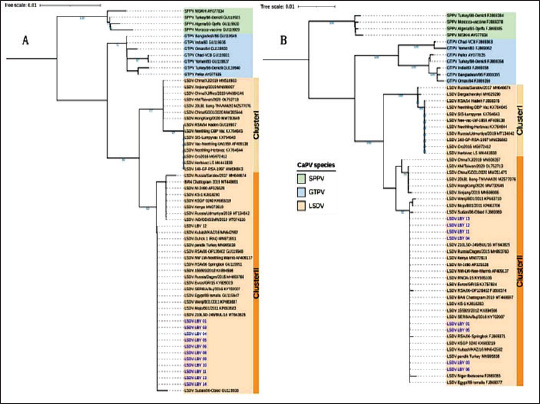
Fig. 1. Map of the outbreak and study areas. Sample preparation for DNA extractionThe tissue samples were cut into small pieces, ground, and homogenized in PBS buffer using a homogenizer, followed by centrifugation at 3,000 rpm at 4°C for 10 minutes. The supernatant was collected in sterile vials. 200 l of the supernatant was used for DNA extraction using QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The DNA was eluted in 50 μL of elution buffer and stored at 20°C for further use (Bowden et al., 2008) Amplification of RPO30, GPCR, EEV glycoprotein, and B22R genesThe tissue samples were finely chopped, ground, and homogenized in PBS buffer using a homogenizer. The homogenate was then centrifuged at 3,000 rpm and 4°C for 10 minutes. The resulting supernatant was collected in sterile vials, and 200 µl of it was used for DNA extraction using a QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s protocol. The extracted DNA was eluted in 50 µl of elution buffer and stored at −20°C for future analysis (Bowden et al., 2008). Sequencing and phylogenetic analysisThe positive PCR products were purified and sent for automated DNA sequencing, utilizing the same primers employed during the amplification process. The nucleotide sequences were subsequently analyzed using DNA Baser V3 software to ensure accurate base calling and sequence assembly. To identify the closest genetic relatives of the obtained sequences, a BLAST search was conducted against the GenBank database (http://www.ncbi.nlm.nih.gov). Sequence similarity was assessed by aligning the nucleotide sequences with entries for Sheep Pox Virus (SPPV), Goat Pox Virus (GTPV), and LSDV in GenBank, selecting those with the highest nucleotide sequence identity. The partial EEV gene sequences obtained from the samples were aligned with representative LSDV sequences retrieved from GenBank for comparative analysis. In order to better understand the evolutionary connections between the viruses we identified, especially the strains of LSDV from Libya, we conducted a detailed phylogenetic analysis. This analysis focused on two important viral gene markers: RPO30 and GPCR. Using the neighbor-joining method available in MEGA X, a phylogenetic tree was constructed. The resulting tree was then visualized using the Interactive Tree of Life platform, which provided an intuitive way to interpret the data. To ensure the accuracy of the evolutionary distances between the sequences, we applied the maximum composite likelihood technique, which is a widely accepted method for estimating genetic differences. To confirm the reliability of the tree structure, 1,000 bootstrap replicates were performed, which is a statistical approach that strengthens confidence in the observed branching patterns. In the final tree, the LSDVs from Libya are highlighted in blue, making it easy to see where they fit within the larger family of Capripoxviruses (Saad et al., 2014; He et al., 2020; Kumar et al., 2022). Ethical approvalThis investigation obtained approval from the Ethics Review Committee of the National Center for Animal Health and was conducted in accordance with its regulations and guidelines. Furthermore, the samples were collected from the LSDV suspect cattle according to standard practices and with the farmer’s consent. ResultsObserved clinical manifestationClinical signs, including fever, appetite loss, nasal and ocular discharge, formation of various-sized skin nodules, and enlargement of superficial lymph nodes, were observed in most LSD-affected cattle. The ill animals also displayed an extreme decrease in milk production along with swollen subscapular and prefemoral lymph nodes. A number of infected cattle also showed severe scab development and ulcerated nodules. Figure 2 shows the nodular skin lesions that cover the cattle’s entire body, including the face, neck, and limbs. Amplification and sequencingThe real-time PCR method was used to examine 14 skin nodule samples for the detection of LSDV. As summarized in Table 2, the virus was detected in 13 of the 14 samples. The whole RPO30 and GPCR genes, as well as the partial EEV glycoprotein and B22R genes, were successfully amplified and sequenced from the positive samples. Appropriately sized sequences for the RPO30, GPCR, EEV, and B22R genes have been submitted to the GenBank database after quality control and sequence modification. Sequence and phylogenetic analysisAs illustrated in Figure 3, multiple sequence alignments of the RPO30 and GPCR genes, along with partial EEV glycoprotein and B22R gene sequences, revealed 100% similarity among the Libyan isolates. Phylogenetic analysis of the complete RPO30 (Fig. 3a) and GPCR (Fig. 3b) genes placed the Libyan isolates within LSDV Cluster II. This cluster includes commonly circulating LSDV field isolates detected across Africa, the Middle East, and Europe. These isolates include LSDV Sudan/06-Obied (GU119938), LSDV Egypt/89-Ismalia (GU119947), and LSDV Serbia/Buj/2016 (KY702007). As shown in Figure 4, amplification and sequencing of the EEV gene revealed 100% genetic identity among all positive Libyan samples. The phylogenetic analysis of the EEV gene detected the presence of a 27-nucleotide insertion (175–201) in all Libyan samples, which distinguishes them from Neethling-derived vaccines and the LSDV recombinants from Southeast Asia and Russia. Furthermore, in addition to the single nucleotide polymorphism (SNP) at position 240 (A→G), which differentiates ancient Kenyan LSDV isolates from common field isolates, Libyan LSDV strains possess an additional SNP at position 111 (C→A).
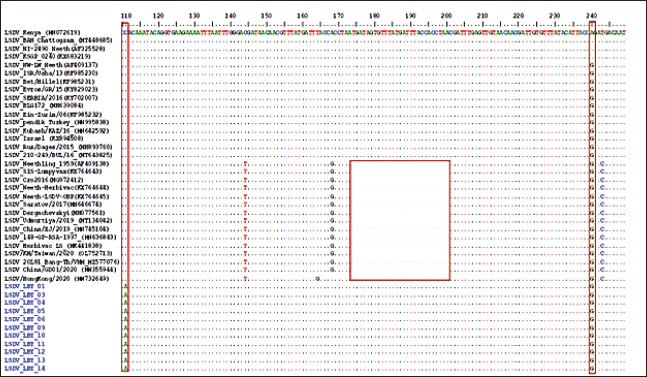
Fig. 2. Lesions observed in animals with LSD infection. (a) A local bovine exhibiting nodular skin lesions across the facial region, neck, and udder. (b) A local cow exhibiting nodular skin lesions distributed across its body and limbs. Multiple sequence alignment of the partial nucleotide sequences of the EEV glycoprotein gene showed that the Libya isolates (highlighted in blue) were aligned with representative LSDV sequences obtained from GenBank. A distinct 27-nucleotide insertion and specific single-nucleotide polymorphisms (SNPs) were identified in the Libyan LSDV isolates marked within the highlighted boxes. The identical nucleotides in the alignment are represented by dots. DiscussionThe diagnosis of LSD was performed by using real-time PCR of samples that showed clinical symptoms during the outbreaks, along with clinical signs. The disease, which is not endemic to Libya, first appeared in the northwest (coastal district) of the country in May 2023, before spreading nationwide. The clinical picture was characterized by fever, loss of appetite, decreased milk production, ulcerated nodules, circumscribed nodules on the skin, enlargement of the superficial lymph nodes, and disseminated skin lesions all over the body. These findings are consistent with results described by Gari et al. (2010), and Rouby et al. (2021). Table 2. Identification of LSD virus (DNA) in skin nodule samples collected from cattle in Al-Zawia, Libya, which were clinically affected.
Fig. 3. Phylogenetic analysis of LSDV isolates: phylogenetic trees based on the RPO30 (A) and GPCR (B) genes reveal that Libyan LSDV isolates cluster within LSDV Cluster II, alongside circulating field strains from Africa, the Middle East, and Europe. Sequence alignments confirmed 100% genetic similarity among Libyan isolates, highlighting their close evolutionary relationship with common LSDV strains.
Fig. 4. Molecular characteristics of EEV glycoprotein gene: multiple sequence alignment of the partial EEV gene shows a unique 27-nucleotide insertion and specific SNPs (C→A at position 111 and A→G at position 240) in Libyan isolates, distinguishing them from vaccine-derived and ancient Kenyan strains. These molecular markers emphasize the genetic distinctiveness of Libyan LSDV isolates. Diagnosis using PCR without the application of VI is a sensitive and precise method characterized by its ability to detect the virus even in small quantities in collected samples. This can assist with early management of positive instances and the quick adoption of control actions (Sharawi and Abd El-Rahim, 2011). However, viral isolation of VI by tissue culture technique can be time-consuming and has a contamination issue(van Rooyen et al., 1969; Fawzi et al., 2022). The virus can be found in a variety of samples, such as nasal, oral, and ocular discharges; however, skin nodules are recognized as the most appropriate samples because of their susceptibility to transportation over long distances at varying temperatures. Furthermore, VI in tissue culture is considered time-consuming and more likely to have bacterial and fungal contamination. Serological tests are used as diagnostic tests since they cannot distinguish between diseased and vaccinated animals, nor can they produce antibodies as a result of LSD infection, or the infection caused by other members of the poxvirus (Abd El-Rahim et al., 2002; Awad et al., 2010). To the best of our knowledge, this is the first study to identify the molecular traits of LSDV that are spreading in Libyan fields. In this scenario, four different genes were amplified and sequenced: RPO30, GPCR, EEV, and B22R. The two genes of RPO30, GPCR, were targeted to classify CaPV into three genotypes: SPPV, GTPV, and LSDV. The sequence of four genes revealed 100% similarity between all Libyan samples. This is due to the enduring nature of the LSDV genome, which is recognized by its highly preserved nature despite its spread across several geographical locations(Sameea Yousefi et al., 2018; Adedeji et al., 2019; El-Khabaz et al., 2020). Three groups of CaPV were identified based on the phylogenetic analysis of GPCR and RPO30, which also grouped Libyan field isolates with LSDV from Africa, the Middle East, and Europe. The sequences were closely related to LSDV isolates, including LSDV Sudan/06-Obied_GU119938, LSDV Egypt/89-Ismalia_GU119947, and LSDV Serbia/Buj/2016_KY702007. These findings are in contrast with those described by (Badhy et al., 2021). These findings suggest that the isolates responsible for the outbreaks may have originated from neighboring countries, especially Sudan, Chad, and Niger, due to open borders and livestock smuggling. This study revealed the insertion of 27 nucleotides in the EEV glycoprotein gene of Libyan samples, which distinguishes them from LSDV Neethling-derived vaccines and LSDV recombinants from Southeast Asia and Russia. This outcome was in line with the findings of Chibssa et al. (2021), Badhy et al. (2021). Furthermore, Libyan samples presented with an SNP at position 111 (C→A), which make them unique among all LSDV isolates. ConclusionOur results found that the phylogenetic analysis of LSDV was closely related to LSDV Sudan/06-Obied_GU119938, LSDV Egypt/89-Ismalia_GU119947, and LSDV Serbia/Buj/2016_KY702007. In recent efforts to mitigate the spread of the disease in the region, a targeted approach involving genetic analysis was implemented. This effort aimed to reduce the risk of disease transmission through vaccination programs and to identify the specific virus strains circulating among cattle herds in the country. This study highlights the importance of conducting an epidemiological analysis of the disease and further genetic analysis of Libyan LSDV, as it is unique among all LSDV isolates due to the additional SNP at position 111 (C→A), which may impact the effectiveness of immunization efforts to control the disease. AcknowledgmentsThe National Center for Animal Health (NCAH) team in Libya is acknowledged by the authors for their technical help. The authors would like to thank the employees of the Joint FAO/IAEA Center of Nuclear Techniques in Food and Agriculture, Animal Production and Health Laboratory, Seibersdorf, Austria. Conflicts of interestThe authors declare that they have no conflicts of interest. FundingThe authors thank the National Center for Animal Health for conducting the sample analyses in its laboratories, in collaboration with its international partners. Author’s contributionAAS, MAM, MMH, TAZ, and NAA prepared the initial draft of the manuscript. AAS, MMH, and NAA revised and edited the manuscripts contributed to the development of the research methodology, data analysis, interpretation, and manuscript writing. EIS interpreted the test results. Sample collection and laboratory analysis were carried out by ONE, EMI, and AJJ. All authors have read and approved the final manuscript. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAbd El-Rahim, I.H., El-Ballal, S. and Hussein, M. 2002. An outbreak of lumpy skin disease among cattle in upper egypt (EL-MENIA GOVERNORATE). Minufyia Vet. J. 2, 185-200. Abutarbush, S.M., Ababneh, M.M., Al Zoubi, I.G., Al Sheyab, O.M., Al Zoubi, M.G., Alekish, M.O. and Al Gharabat, R.J. 2015. Lumpy skin disease in Jordan: disease emergence, clinical signs, complications and preliminary-associated economic losses. Transbound. Emerg. Dis. 62, 549–554. Adedeji, A.J., Möller, J., Meseko, C.A., Adole, J.A., Tekki, I.S., Shamaki, D. and Hoffmann, B. 2019. Molecular characterization of Capripox viruses obtained from field outbreaks in Nigeria between 2000 and 2016. Transbound. Emerg. Dis. 66, 1631–1641. Alkhamis, M.A. and VanderWaal, K. 2016. Spatial and temporal epidemiology of lumpy skin disease in the Middle East, 2012–2015. Front. Vet. Sci. 3:19. Awad, W.S., Ibrahim, A.K., Mahran, K., Fararh, K.M. and Abdel Moniem, M.I. 2010. Evaluation of different diagnostic methods for diagnosis of Lumpy skin disease in cows. Trop. Anim. Health. Prod. 42, 777–783. Babi Babiuk, S., Bowden, T.R., Boyle, D.B., Wallace, D.B. and Kitching, R.P. 2008. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transbound. Emerg. Dis. 55, 263–272. Badhy, S.C., Chowdhury, M.G., Settypalli, T.B., Cattoli, G., Lamien, C.E., Fakir, M.A., Akter, S., Osmani, M.G., Talukdar, F., Begum, N. and Khan, I.A. 2021. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet. Res. 17, 61. Body, M., Singh, K.P., Hussain, M.H., Al-Rawahi, A., Al-Maawali, M., Al-Lamki, K. and Al-Habsy, S. 2012. Clinico-histopathological findings and PCR based diagnosis of lumpy skin disease in the Sultanate of Oman. Pak. Vet. J. 8318, 2074–7764. Bowden, T.R., Babiuk, S.L., Parkyn, G.R., Copps, J.S. and Boyle, D.B. 2008. Capripoxvirus tissue tropism and shedding: a quantitative study in experimentally infected sheep and goats. Virology 371, 380–393. Calistri, P., De Clercq, K., Gubbins, S., Klement, E., Stegeman, A., Cortiñas Abrahantes, J., Marojevic, D., Antoniou, S.E. and Broglia, A. 2020. Lumpy skin disease epidemiological report IV: data collection and analysis. EFSA J. 18, e06010. Chibssa, T.R., Sombo, M., Lichoti, J.K., Adam, T.I., Liu, Y., Elraouf, Y.A., Grabherr, R., Settypalli, T.B., Berguido, F.J., Loitsch, A. and Sahle M. 2021. Molecular analysis of east African lumpy skin disease viruses reveals a mixed isolate with features of both vaccine and field isolates. Microorganisms 9, 1142. Chihota, C.M., Rennie, L.F., Kitching, R.P. and Mellor, P.S. 2003. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med. Vet. Entomol. 17, 294–300. El-Khabaz, K., Shosha, E. and Abdel Raouf, A. 2020. New epizootic of lumpy skin disease in Assiut- Egypt: molecular identification and characterization. Adv. Anim. Vet. Sci. 9, 446–452. Elhaig, M.M., Selim, A. and Mahmoud, M. 2017. Lumpy skin disease in cattle: frequency of occurrence in a dairy farm and a preliminary assessment of its possible impact on Egyptian buffaloes. Onderstepoort J. Vet. Res. 84, e1–e6. FAO. 2013. Emergence of lumpy skin disease in the Eastern Mediterranean Basin countries. EMPRES WATCH, Vol. 29, November 2013. Rome. Fawzi, E.M., Morsi, A.M. and Abd-Elfatah, E.B. 2022. Molecular diagnosis of three outbreaks during three successive years (2018, 2019, and 2020) of Lumpy skin disease virus in cattle in Sharkia Governorate, Egypt. Open Vet. J. 12, 451–462. Gari, G., Bonnet, P., Roger, F. and Waret-Szkuta, A. 2011. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev. Vet. Med. 102, 274–283. Gari, G., Waret-Szkuta, A., Grosbois, V., Jacquiet, P., and Roger, F. 2010. Risk factors associated with observed clinical lumpy skin disease in Ethiopia. Epidemiol. Infect. 138, 1657–1666. Gelaye, E., Lamien, C.E., Silber, R., Tuppurainen, E.S., Grabherr, R. and Diallo A. 2013. Development of a cost-effective method for capripoxvirus genotyping using snapback primer and dsDNA intercalating dye. PLos One 8, e75971. He, C., Tong, J., Zhang, X., Tuohetiniyazi, M., Zhang, Y. and Li, Y. 2020. Comparative analysis of ankyrin (ANK) genes of five capripoxviruses isolate strains from Xinjiang province in China. Virol. J. 17, 133. Kumar, A., Venkatesan, G., Hosamani, M., Bhanuprakash, V., Balamurugan, V., Ramakrishnan, M.A. and Singh, R.K. 2022. The complete genome sequence of Indian sheeppox vaccine virus and comparative analysis with other capripoxviruses. Gene 810, 146085. Lamien, C.E., Le Goff, C., Silber, R., Wallace, D.B., Gulyaz, V., Tuppurainen, E., Madani, H., Caufour, P., Adam, T., El Harrak, M. and Luckins, A.G. 2011a. Use of the capripoxvirus homologue of vaccinia virus 30 kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate goat poxvirus from sheep poxvirus. Vet. Microbiol. 149, 30–39. Lamien, C.E., Lelenta, M., Goger, W., Silber, R., Tuppurainen, E., Matijevic, M., Luckins, A.G. and Diallo, A. 2011b. Real time PCR method for simultaneous detection, quantitation and differentiation of capripoxviruses. J. Virol. Methods 171, 134–140. Le Goff, C., Lamien, C.E., Fakhfakh, E., Chadeyras, A., Aba-Adulugba, E., Libeau, G., Tuppurainen, E., Wallace, D.B., Adam, T., Silber, R. and Gulyaz, V. 2009. Capripoxvirus G-protein-coupled chemokine receptor: a host-range gene suitable for virus animal origin discrimination. J. Gen. Virol. 90, 1967–1977. Menasherow, S., Erster, O., Rubinstein-Giuni, M., Kovtunenko, A., Eyngor, E., Gelman, B., Khinich, E. and Stram, Y. 2016. A high-resolution melting (HRM) assay for the differentiation between Israeli field and Neethling vaccine lumpy skin disease viruses. J. Virol. Methods 232, 12–15. Molla, W., de Jong, M.C., Gari, G. and Frankena, K. 2017. Economic impact of lumpy skin disease and cost effectiveness of vaccination for the control of outbreaks in Ethiopia. Prev. Vet. Med. 147, 100–107. Ochwo, S., VanderWaal, K., Ndekezi, C., Nkamwesiga, J., Munsey, A., Witto, S.G., Nantima, N., Mayanja, F., Okurut, A.R.A., Atuhaire, D.K. and Mwiine, F.N. 2020. Molecular detection and phylogenetic analysis of lumpy skin disease virus from outbreaks in Uganda 2017–2018. BMC Vet. Res. 16, 66. OIE. 2010. Lumpy skin disease. In Manual of diagnostic tests and vaccines for terrestrial animals. Ed., OIE. Paris, France: Office International des Epizooties/World Organization for Animal Health. Rouby, S.R., Safwat, N.M., Hussein, K.H., Abdel-Ra’ouf, A.M., Madkour, B.S., Abdel-Moneim, A.S. and Hosein, H.I. 2021. Lumpy skin disease outbreaks in Egypt during 2017-2018 among sheeppox vaccinated cattle: epidemiological, pathological, and molecular findings. PLoS One 16, e0258755. Saad, I.I., Saha, S.B. and Thomas, G. 2014. The RAS subfamily evolution - tracing evolution for its utmost exploitation. Bioinformation 10, 293–298. Sameea Yousefi, P., Dalir-Naghadeh, B., Mardani, K. and Jalilzadeh-Amin, G. 2018. Phylogenetic analysis of the lumpy skin disease viruses in northwest of Iran. Trop. Anim. Health Prod. 50, 1851–1858. Selim, A., Radwan, A. and Arnaout, F. 2020. Seroprevalence and molecular characterization of West Nile Virus in Egypt. Comp. Immunol. Microbiol. Infect. Dis. 71, 101473. Sharawi, S.S. and Abd El-Rahim, I. H. 2011. The utility of polymerase chain reaction for diagnosis of lumpy skin disease in cattle and water buffaloes in Egypt. Rev. Sci. Tech. 30, 821-830. Sprygin, A., Artyuchova, E., Babin, Y.U., Prutnikov, P., Kostrova, E., Byadovskaya, O. and Kononov, A. 2018. Epidemiological characterization of lumpy skin disease outbreaks in Russia in 2016. Transbound. Emerg. Dis. 65, 1514–1521. Tian, H., Chen, Y., Wu, J., Shang, Y. and Liu, X. 2010. Serodiagnosis of sheeppox and goatpox using an indirect ELISA based on synthetic peptide targeting for the major antigen P32. Virol. J. 7, 245. Tuppurainen, E.S.M., Venter, E.H., Shisler, J.L., Gari, G., Mekonnen, G.A., Juleff, N., Lyons, N.A., De Clercq, K., Upton, C. and Bowden, T.R. 2017. Review: capripoxvirus diseases: current status and opportunities for control. Transbound. Emerg. Dis. 64, 729–745. van Rooyen, P.J., Munz. E.K. and Weiss, K.E. 1969. The optimal conditions for the multiplication of Neethling-type lumpy skin disease virus in embryonated eggs. Onderstepoort J. Vet. Res. 36, 165-174. | ||
| How to Cite this Article |
| Pubmed Style Saad AAA, Murshid MA, Hussein MM, Zahmol TA, Abdulsalam NAI, Saad EIF, Elwaer ON, Ismaeil EM, Jbeel AJ. Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. Open Vet. J.. 2025; 15(7): 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 Web Style Saad AAA, Murshid MA, Hussein MM, Zahmol TA, Abdulsalam NAI, Saad EIF, Elwaer ON, Ismaeil EM, Jbeel AJ. Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. https://www.openveterinaryjournal.com/?mno=235282 [Access: January 11, 2026]. doi:10.5455/OVJ.2025.v15.i7.15 AMA (American Medical Association) Style Saad AAA, Murshid MA, Hussein MM, Zahmol TA, Abdulsalam NAI, Saad EIF, Elwaer ON, Ismaeil EM, Jbeel AJ. Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. Open Vet. J.. 2025; 15(7): 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 Vancouver/ICMJE Style Saad AAA, Murshid MA, Hussein MM, Zahmol TA, Abdulsalam NAI, Saad EIF, Elwaer ON, Ismaeil EM, Jbeel AJ. Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. Open Vet. J.. (2025), [cited January 11, 2026]; 15(7): 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 Harvard Style Saad, A. A. A., Murshid, . M. A., Hussein, . M. M., Zahmol, . T. A., Abdulsalam, . N. A. I., Saad, . E. I. F., Elwaer, . O. N., Ismaeil, . E. M. & Jbeel, . A. J. (2025) Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. Open Vet. J., 15 (7), 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 Turabian Style Saad, Ahmad A. A., Mohammed A. Murshid, Marium M. Hussein, Tarek A. Zahmol, Najwa A. I. Abdulsalam, Esmaiel I. F. Saad, Osama N. Elwaer, Esmahan M. Ismaeil, and Abderrahman. J. Jbeel. 2025. Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. Open Veterinary Journal, 15 (7), 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 Chicago Style Saad, Ahmad A. A., Mohammed A. Murshid, Marium M. Hussein, Tarek A. Zahmol, Najwa A. I. Abdulsalam, Esmaiel I. F. Saad, Osama N. Elwaer, Esmahan M. Ismaeil, and Abderrahman. J. Jbeel. "Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya." Open Veterinary Journal 15 (2025), 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 MLA (The Modern Language Association) Style Saad, Ahmad A. A., Mohammed A. Murshid, Marium M. Hussein, Tarek A. Zahmol, Najwa A. I. Abdulsalam, Esmaiel I. F. Saad, Osama N. Elwaer, Esmahan M. Ismaeil, and Abderrahman. J. Jbeel. "Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya." Open Veterinary Journal 15.7 (2025), 3044-3053. Print. doi:10.5455/OVJ.2025.v15.i7.15 APA (American Psychological Association) Style Saad, A. A. A., Murshid, . M. A., Hussein, . M. M., Zahmol, . T. A., Abdulsalam, . N. A. I., Saad, . E. I. F., Elwaer, . O. N., Ismaeil, . E. M. & Jbeel, . A. J. (2025) Molecular characterization and phylogenetic analysis of the lumpy skin disease virus isolated in Al-Zawiya, Western Libya. Open Veterinary Journal, 15 (7), 3044-3053. doi:10.5455/OVJ.2025.v15.i7.15 |