| Research Article | ||
Open Vet. J.. 2025; 15(6): 2416-2426 Open Veterinary Journal, (2025), Vol. 15(6): 2416-2426 Research Article Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation mediaAthhar Manabi Diansyah1, Muhammad Yusuf1*, Rahmat Rahmat2 and Andi Muhammad Alfian11Department of Animal Production, Faculty of Animal Science, Hasanuddin University, Makassar, Indonesia 2Department of Animals Science, Faculty of Agriculture, Lambung Mangkurat University, Banjarmasin Indonesia *Corresponding Author: Hasanain A. J. Gharban. Department of Internal and Preventive Veterinary Medicine, College of Veterinary Medicine, University of Wasit, Wasit, Iraq. Email: hghirban [at] uowasit.edu.iq Submitted: 21/01/2025 Revised: 26/04/2025 Accepted: 19/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
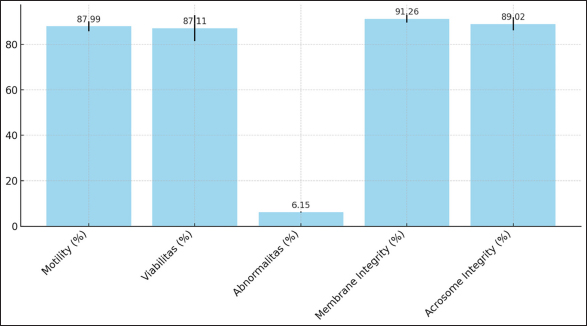
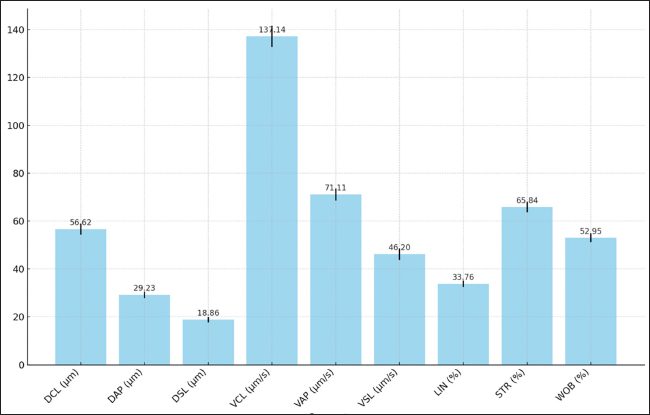
AbstractBackground: Sperm sexing is a crucial technique in livestock breeding, allowing for the selection of offspring of the desired sex. Traditional methods present challenges, including high costs, time consumption, and potential damage to sperm quality. This study explored an alternative approach using freeze-dried albumin as a medium for sperm separation in Bali bulls, a breed of significant economic importance in Indonesia. Aim: This study aimed to compare the advantages of freeze-dried albumin and fresh albumin for the separation of Bali bull sperm based on the existing albumin gradient separation technique. The evaluation focused on the impact of sperm separation, quality preservation, and kinematic properties. In addition, this study seeks to understand the broader applicability of this method beyond Bali bulls, considering its potential integration into various livestock reproductive programs. Methods: Fresh semen from Bali bulls was processed using two different sperm separation media: fresh albumin and freeze-dried albumin. Sperm motility, viability, membrane integrity, acrosome integrity, and kinematic parameters (e.g., velocity and linearity) were measured and compared between the two mediums. The proportions of X- and Y-chromosome-bearing sperm in the separated fractions were also assessed. Results: The study found no significant differences in sperm quality parameters (motility, viability, abnormalities, membrane integrity, and acrosome integrity.) between the two mediums. In contrast, freeze-dried albumin resulted in higher separation efficiency, with a greater proportion of X-bearing sperm in the upper gradient layer, compared with fresh albumin. Kinematic analysis showed a slight increase of about 2%–4% inmotility and stability in sperm treated with freeze-dried albumin. Conclusion: Freeze-dried albumin is a viable alternative to fresh albumin for sperm sexing because it improves separation efficiency while maintaining sperm quality. The advantages of stability, ease of storage, and cost- effectiveness make it an ideal choice for resource-limited settings, potentially advancing reproductive biotechnology in livestock breeding. Keywords: Albumin freeze-dried, Bali bull, Kinematic, Medium, Sexing, Sperm. IntroductionBali cattle (Bos javanicus), a native Indonesian breed, hold significant economic and cultural value because of their adaptability, disease resistance, and contribution to sustainable livestock farming. As demand for high-quality livestock continues to grow, enhancing reproductive efficiency has become a priority. Sperm sexing, a technique that allows the selective production of offspring of a desired sex, holds significant promise for optimizing breeding strategies, improving productivity, and meeting market-specific demands. For example, for fattening, male cattle are needed, while female cattle are needed for breeding. However, the success of this technique relies heavily on both the efficiency of sperm separation and the preservation of sperm quality throughout the process. Flow cytometry is currently the most widely used method for sperm sexing because of its high accuracy, which often exceeds 90% in separating X- and Y-chromosome-bearing sperm based on DNA content. Despite its precision, this method has several limitations, including high operational costs, the need for specialized equipment and expertise, and potential adverse effects on sperm quality, particularly motility, viability, membrane integrity, and kinematic properties (Maulana and Said, 2019). These challenges are further compounded in regions with limited resources, such as rural cattle-breeding programs, highlighting the need for alternative, cost-effective, and accessible methods. Albumin gradient separation has emerged as an alternative to flow cytometry, offering simplicity, affordability, and minimal impact on sperm quality (Susilawati, 2014). This technique capitalizes on the natural biophysical differences between X- and Y-bearing sperm, separating them based on their density and sedimentation behavior within a gradient medium. Recent advancements in this approach, particularly the use of freeze-dried albumin, have opened new possibilities for improving the efficiency and practicality of sperm sexing (Rahman and Pang, 2020). Freeze-dried albumin has distinct advantages over fresh albumin, including enhanced stability, standardized composition, and ease of storage and transportation. These attributes are particularly beneficial in resource- constrained settings, where consistent preparation and storage conditions are challenging (Amaliah et al., 2023). In addition to its logistical benefits, freeze-dried albumin is hypothesized to improve the efficiency of sperm separation by providing a more uniform medium that facilitates selective spermatozoa sedimentation. This may lead to higher proportions of X- or Y-bearing sperm in the desired gradient layers compared with fresh albumin (Madyawati et al., 2021). Furthermore, it is expected to better preserve critical sperm quality parameters, including motility, viability, membrane integrity, and acrosome integrity, which are essential for maintaining fertility potential. The theoretical advantages extend to sperm kinematics, such as curvilinear velocity (VCL), straight-line velocity (VSL), and average path velocity (VAP), which are key indicators of sperm functionality and their ability to fertilize oocytes. The preservation of these kinematic properties during the separation process is vital for ensuring the success of sexed semen in reproductive applications (Raafi et al., 2021; Maulana et al., 2022). The efficiency of a sperm sexing technique is defined not only by its ability to achieve high separation accuracy but also by its impact on sperm quality and its adaptability to different operational conditions (Bhalakiya et al., 2018; Joshi et al., 2021). Freeze-dried albumin is hypothesized to strike a favorable balance between these factors, making it a promising alternative to existing methods such as flow cytometry and fresh albumin- based separation. This study evaluated the efficiency of freeze-dried albumin in sperm sexing in Bali bulls by examining its impact on sperm separation, quality preservation, and kinematic properties. By addressing these aspects, the research aims to establish freeze- dried albumin as an accessible, efficient, and sustainable solution for reproductive biotechnology, contributing to the advancement of Bali cattle breeding programs and broader livestock production systems. Material and MethodsPreparation of the diluent and sexing mediumPreparation of tris-egg yolk (TEY) diluent The TEY diluent was prepared using a modified protocol based on the study of Arif et al. (2020). In this process, 3.63 g of Tris-hydroxymethyl aminomethane, 1.78 g of citric acid, and 1.25 g of fructose were accurately weighed and dissolved in a volumetric flask. Distilled water (aquabidest) was added to adjust the volume to 100 ml, and the solution was homogenized for 15 minutes. Subsequently, 80 ml of the prepared solution was transferred into a separate volumetric flask, and 20 ml of egg yolk was added to achieve a total volume of 100 ml. The mixture was further homogenized for 10–20 minutes to ensure a consistent emulsion. Freeze-dried albumin preparation Freeze-dried albumin was prepared from albumin derived from chicken eggs. Albumin was separated from the yolk and stored under controlled environmental conditions of 30°C and 75% relative humidity. The albumin was subjected to a freeze-drying process involving initial freezing at –10°C for 3 hours, followed by drying at 45°C under a vacuum pressure of 25 Pa for 12 hours. The entire freeze-drying process spanned 15 hours. Upon completion, the albumin was pulverized into a fine powder and stored in sterile containers. The processed albumin was subsequently incorporated into the experimental solutions according to the requirements outlined by Ishwarya (2022). Semen evaluation The semen sample from each bull was collected twice a week using an artificial vagina. The parameters of semen quality tested in this study were sperm motility, kinematics, viability, abnormality, membrane integrity, and acrosome integrity, (Diansyah et al., 2023b). Sperm motility and kinematic analysis were performed using computer-assisted semen analysis with the Sperm- Vision Program (Minitüb, Tiefenbach, Germany), which was linked to Carl Zeiss Microimaging GmbH (Gottingen, Germany) and equipped with a warm stage at 38°C. The parameters analyzed were motility, VCL, VSL, VAP, linearity (LIN), straightness (STR), and wobble (WOB) (Raafi et al., 2021; Diansyah et al., 2022). The mean values were calculated for each parameter based on approximately 1,000 spermatozoa (Maulana et al., 2021). Sperm viability (%) and abnormality (%) were assessed according to the modified method of Madyawati et al. (2021). Sperm morphological defects are classified into three groups: head defects, neck and midpiece defects, and tail defects (Surahman et al., 2021; Diansyah et al., 2023a). Analysis of the membrane integrity was assessed using the hypo-osmotic swelling test. Sperm cells with intact plasma membranes were indicated by curled or swollen tails, whereas straight tails indicated defective or dead sperm (Arifiantini et al., 2014). Sperm acrosome integrity was assessed using the fluorescein- conjugated lectin Pisum sativum agglutinin staining protocol (Gholami et al., 2023). Sexing spermsSperm separation and sexing protocol Preparation of freeze-dry albumin using chicken eggs; egg yolks and egg white (albumin) are separated in a cup; albumin and yolk are separated on a plate. Albumin was then frozen in the freezer before freezing and drying. Furthermore, albumin that had been frozen was put into a freeze dryer at a heating temperature of 45°, freezing temperature of –10°, and pressure of 25 Pa for 48 hours. The separation of X- and Y-bearing spermatozoa was conducted using a gradient medium prepared with freeze-dried albumin by dissolving 1.5 g of freeze-dried albumin per 1 ml of distilled water. The gradient medium comprised upper and lower fractions, created by mixing freeze-dried albumin diluted with TEY diluent in a single tube. The experimental setup included six treatments with varying gradient concentrations: T1 (fresh albumin) and T2 (freeze- dried albumin). Each gradient was constructed by layering 2 ml of the respective fractions. The sexing sperms following the protocol Susilawati (2014) were pre-diluted with TEY diluent in a 1:1 ratio, and 1 ml of this diluted semen was introduced into each tube containing the prepared gradient medium. The samples were incubated at room temperature for 30 minutes to facilitate separation. Following incubation, the upper and lower fractions were carefully isolated and subjected to centrifugation at 1,500 rpm for 5 minutes. The supernatant was discarded, and the pellet (sediment) was collected for subsequent analyses. The parameters evaluated included sperm motility, concentration, viability, morphological abnormalities, membrane integrity, acrosome integrity, and the proportion of sexed spermatozoa. Determination of the X and Y spermatozoa proportions Differentiation between X- and Y-chromosome-bearing spermatozoa was conducted based on a quantitative assessment of spermatozoa head dimensions, following the protocol established by Amaliah et al. (2023). Spermatozoa head size was assessed using smear preparations and a trinocular microscope (AxioCam Erc 5s, ZEISS, Germany) under 100 × 10 magnification. The mean head size of spermatozoa in fresh Bali bull semen was used as the reference value. Sperms were classified as follows: X-bearing spermatozoa: Head size ≤ Mean head size + standard error (SE). Y-bearing spermatozoa: Head size ≤ Mean head size SE. Uncategorized spermatozoa: Head size within the range of Mean head size ± SE. Statistical analysisThe data collected during the study were organized and tabulated using Microsoft Excel. Subsequently, statistical analysis was performed using Student’s T-test with SPSS software (version 25) (IBM Corp., Chicago, IL). Descriptive analyses were used to evaluate the proportions of X- and Y-chromosome-bearing spermatozoa. AnimalsThe study was conducted at the Laboratory of Animal Reproduction, Faculty of Animal Science, Hasanuddin University, Makassar, Indonesia. A total of 5 Bali bulls aged 4 years. The bull was fed 10% of its body weight in elephant grass and concentrated. The stages of this study included five biological repeats. Ethical approvalThe Animal Ethics Commission of the National Research and Innovation Agency approved this study’s animal models and experimental designs (certificate number: 050/KE.02/SK/03/2023. The procedure for producing frozen semen complies with the Indonesian National Standard SNI 4869-2: 2021. ResultsCharacteristics and kinematics of fresh semen in Bali bullsThe characteristics and kinematics of fresh semen in Bali bulls are presented in Figures 1 and 2. The results presented in Figure 1 provide valuable insights into the quality parameters necessary for standardizing the continuous sperm sexing process. The motility percentage of 87.99% indicates that the majority of sperm cells exhibit active movement, which is critical for successful separation during the sexing process. Similarly, the viability rate of 87.11% reflects a high proportion of living sperm cells with intact membranes, ensuring their structural and functional integrity is a vital aspect of maintaining sperm quality during the procedure. The low abnormality rate (6.15%) demonstrates minimal morphological defects, further supporting the reliability of the sample for sexing processes. Additionally, the measured membrane integrity (91.26%) highlights the robustness of the cells to withstand the physical and chemical stresses involved in the sexing process. The acrosome integrity rate of 89.02% further reinforces the functional capability of sperm cells, as this region is essential for successful fertilization. The data presented in Figure 1 emphasize the importance of maintaining high-quality benchmarks for sperm preparation. Achieving and maintaining these parameters of motility and viability above 85%, abnormalities below 10%, and membrane and acrosome integrity above 90% are critical for establishing a standardized, reliable, and continuous sperm sexing process. These benchmarks enhance not only the efficiency but also the reproducibility of sexing procedures, ensuring optimal outcomes in reproductive technologies. The results illustrated in Figure 2 provide an in-depth analysis of sperm motion parameters, which are essential for assessing quality and efficiency in applications such as sperm sexing. The DCL was measured at 56.62 µm, reflecting the overall path traveled by the sperm. The DAP and DSL were recorded at 29.23 and 18.86 µm, respectively, representing the intermediate and shortest paths traversed by the sperm cells. The velocity parameters provide insight into the sperm’s speed and trajectory. The VCL was the highest among the motion metrics at 131.14 µm/s, indicating rapid movement along the actual sperm trajectory. The VAP and VSL were recorded at 71.11 and 46.20 µm/s, respectively, demonstrating the efficiency of sperm in traveling along defined paths. Metrics assessing motion precision and directionality were also analyzed. The LIN was recorded at 33.76%, indicating the proportion of straight-line motion relative to the curvilinear path.
Fig. 1. Characteristic of fresh semen in Bali bulls.
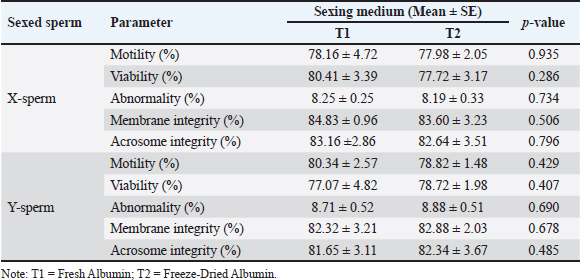
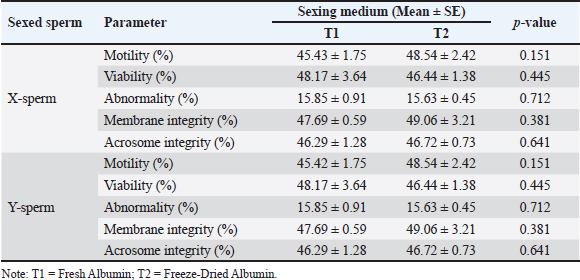
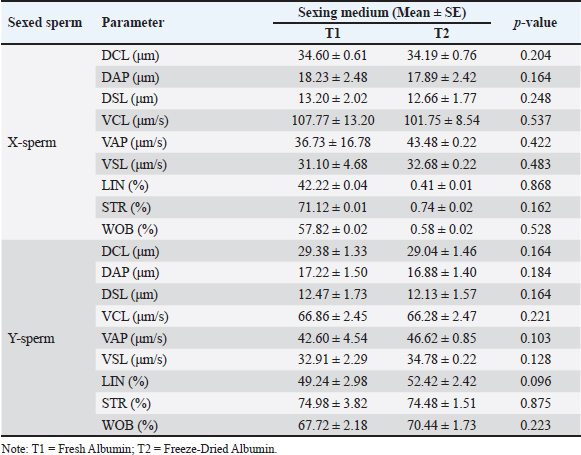
Fig. 2. Kinematic of fresh semen in Bali bulls. STR, which reflects the degree of direct sperm motion toward the target, was measured at 65.84%, indicating moderately high directionality. Finally, the WOB was 52.95%, reflecting the balance between VCL and VAP. Collectively, these motion parameters provide a comprehensive understanding of sperm motility, directionality, and trajectory efficiency. Such insights are critical for optimizing processes such as sperm sexing, in which precision and motility play vital roles in achieving successful outcomes. Comparison of sperm sexing efficiency in Bali bulls at different mediumThe comparison of sperm sexing efficiency of Bali bulls in different mediums is presented in Tables 1–4. The results in Table 1 demonstrate that the use of T1 and T2 as medium for sexed sperm did not result in significant differences across all measured parameters for both X-sperm and Y-sperm. Motility, viability, abnormality, membrane integrity, and acrosome integrity were comparable between the two treatments, with no statistically significant variations (p > 0.05) observed. Table 1. Characteristic of sexed fresh semen of Bali bulls at different medium.
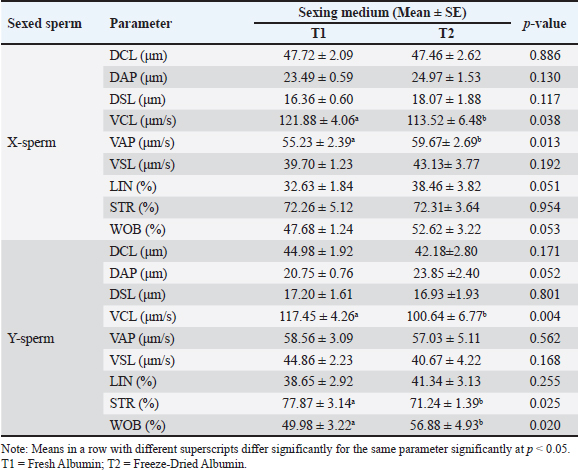
Table 2. Kinematics of fresh sexed Bali bull semen in different mediums.
Table 3. Characteristics of sexed frozen semen of Bali bulls at different medium.
Table 4. Kinematic analysis of sexed frozen semen of Bali bulls at different medium.
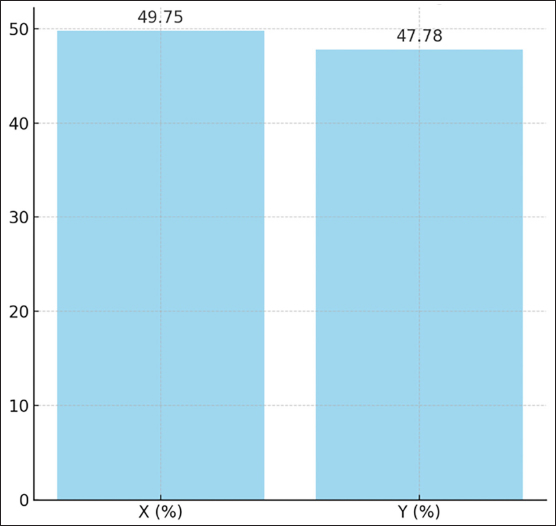
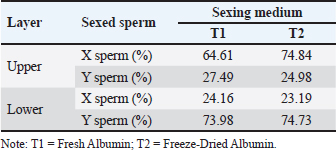
This indicates that the medium is equally effective in preserving the quality of sexed sperm. For X-sperm, T1 consistently showed slightly higher values across most parameters compared with T2, although the differences were negligible. Similarly, for Y-sperm, T2 exhibited slightly better viability and membrane integrity, but these differences were not significant. The low abnormality rates and consistent integrity metrics across both mediums suggest that T1 and T2 can maintain the structural and functional characteristics of sperm. The kinematic evaluation of sexed fresh semen from Bali bulls demonstrated statistically significant differences in motility parameters when incubated in two different mediums: T1 and T2. For X-chromosome-bearing spermatozoa (X-sperm), the VCL was significantly higher in T1 compared with T2 (p = 0.038), indicating that T1 provides more favorable conditions for enhancing the overall velocity of nonlinear trajectories. Conversely, the VAP was significantly greater in T2 (p = 0.013), suggesting improved efficiency of progressive motility in this medium. Moreover, the LIN and WOB parameters in T2 were higher than those in T1, with results approaching significance (p = 0.051 and p = 0.053, respectively), indicating a trend toward more linear and stable movement in T2. For Y-chromosome-bearing spermatozoa (Y-sperm), T1 resulted in significantly higher VCL compared to T2 (p = 0.004), suggesting enhanced motility in curved trajectories under T1 conditions. Additionally, STR was significantly elevated at T1 (p = 0.025), indicating a better efficiency of straight-line movement in this medium. In contrast, the WOB was significantly higher in T2 (p = 0.020), indicating that T2 cells may promote more stable and consistent movement patterns in Y-sperm. The data indicate that T1 provides optimal conditions for enhancing the velocity and efficiency of both X- and Y-sperm, particularly in curved and progressive movements, while T2 offers advantages in stabilizing movement and increasing VAP. The findings in Table 3 reveal that the use of Fresh Albumin (T1) and Freeze-Dried Albumin (T2) as the medium for sexed frozen semen of Bali bulls resulted in no statistically significant differences (p > 0.05) across all parameters for both X and Y sperm. Parameters such as motility, viability, abnormality, membrane integrity, and acrosome integrity were consistent between the two mediums, indicating similar efficacy in preserving the quality of sexed frozen sperm. For X-sperm, T2 exhibited slightly higher motility and membrane integrity, suggesting a marginal advantage in preserving these functional aspects. However, viability and acrosome integrity were slightly higher in T1 than in T2, although these differences were not significant. Similar trends were observed for Y-sperm, with T2 showing a slight improvement in motility and membrane integrity, while T1 exhibited slightly better viability and acrosome integrity. Overall, the comparable performance of T1 and T2 in maintaining sperm quality after freezing indicates that Freeze-Dried Albumin (T2) could serve as an effective alternative to Fresh Albumin (T1) for sexed frozen semen processing. This provides flexibility and practicality in semen handling, particularly in contexts requiring long-term storage or transportation. Kinematic analysis of sexed frozen semen from Bali bulls revealed no significant differences in motility parameters between the T1 and T2 groups. For X-sperm, measurements of DCL, DAP, and DSL were consistent across both mediums, with no statistically significant variation. The velocity parameters, including VCL, VAP, and VSL, were also similar, indicating that the medium had comparable effects on the motility profile of frozen X-sperm. Linear indicators, such as LIN, STR, and WOB, demonstrated similar values between T1 and T2, further supporting the equivalent performance of both mediums in preserving the structural and functional integrity of frozen X-sperm. For Y-sperm, the findings mirrored those of X-sperm, with no significant differences observed in kinematic parameters between the two mediums. The DCL, DAP, and DSL measurements were comparable, as were velocity parameters such as VCL, VAP, and VSL. Although there were slight numerical differences, such as marginally higher values for VAP, VSL, and WOB in T2, these variations did not reach statistical significance, suggesting that the performance of T1 and T2 is equivalent in maintaining Y-sperm motility. Additionally, the LIN, STR, and WOB parameters remained consistent between the two mediums, indicating that neither medium exerts a superior influence on the directional movement or trajectory stability of frozen Y-sperm. Proportions of sexed semen in Bali bulls at different mediumThe proportions of sexed semen in Bali bulls in different mediums are presented in Figure 3 and Table 5. The results indicate a nearly balanced distribution of X and Y sperm in fresh semen, with a slight predominance of X sperm at 49.75% and 47.78% for Y sperm. This minimal difference reflects the natural equilibrium in sperm populations, suggesting that fresh semen maintains a consistent distribution without significant bias toward either sperm type. The close proportions observed also highlight the stability of the sample and suggest minimal external influences affecting the natural sperm balance. The proposed method serves as a useful baseline for assessing the effects of various processing techniques or separation methods in future studies, particularly when deviations from this natural distribution are observed. The findings reinforce the reliability of fresh semen for applications in which a natural proportion of sperm types is desired. Table 5 presents the distribution of X and Y sperm proportions in Bali bulls across the upper and lower layers when using two albumin medium types. The results demonstrate a clear partitioning pattern between the two sperm types, with the upper layer favoring X sperm and the lower layer favoring Y sperm. This distribution highlights the efficacy of the albumin gradient in sperm separation, a critical factor in semen sexing techniques. The observed difference in sperm proportions between the two albumin preparations suggests that the freeze-dried medium (T2) may provide enhanced separation efficiency. The increased concentration of X sperm in the upper layer in T2 compared with T1 indicates a potential improvement in the selectivity and consistency of the process. This finding is particularly relevant for applications requiring higher X sperm yields, such as those targeting female offspring production. Conversely, the similar distribution patterns of Y sperm in the lower layer across both mediums highlight the robustness of the separation mechanism, regardless of the albumin type. These results emphasize the importance of optimizing medium preparation to maximize the efficiency and reliability of sexed semen production, offering valuable implications for improving reproductive biotechnology in Bali bulls.
Fig. 3. Proportions of X and Y in fresh semen from Bali bulls. Table 5. Proportions of sexed semen in Bali bulls at different medium.
DiscussionThis study investigated the potential of freeze-dried albumin as an innovative medium for sperm sexing in Bali bulls, addressing the hypothesis that it surpasses fresh albumin in both separation efficiency and sperm quality preservation. The results indicate that freeze- dried albumin achieves superior partitioning of X- and Y-bearing sperm while maintaining essential parameters such as motility, viability, and membrane integrity. These findings emphasize the dual benefits of enhanced functionality and practical scalability, highlighting freeze-dried albumin as a promising alternative to traditional methods in reproductive biotechnology (Amaliah et al., 2023). The study confirmed that freeze-dried albumin achieves a higher proportion of X-bearing sperm in the upper gradient layer than fresh albumin, indicating improved separation efficiency. Both media effectively maintained sperm quality, with parameters such as motility, viability, and acrosome integrity remaining consistent across treatments. Notably, freeze-dried albumin slightly enhanced the progressive motility and stability of trajectory parameters such as LIN and VAP. These findings highlight freeze-dried albumin’s ability to balance separation efficiency with the preservation of sperm functionality, which is essential for fertilization success. The improved separation efficiency of freeze- dried albumin can be attributed to its molecular stability and uniformity. The freeze-drying process removes water and stabilizes albumin molecules, reducing the variability in gradient composition. This ensures a more predictable and consistent medium that facilitates the sedimentation of spermatozoa based on biophysical differences, such as density and DNA content (Kang et al., 2020). In contrast, fresh albumin is more prone to inconsistencies due to preparation and storage variability, which may compromise separation efficiency. The ability of freeze-dried albumin to preserve sperm quality stems from its stable osmotic environment and reduced potential for oxidative stress. A uniform gradient minimizes osmotic fluctuations and mechanical stress during centrifugation, protecting sperm membranes and cellular structures (Rahman and Pang, 2020). This stability also helps maintain energy reserves within spermatozoa, enabling higher motility and improved trajectory stability post separation. The enhanced progressive motility and directional stability observed in this study further highlight the role of freeze-dried albumin in supporting sperm kinematics. These properties are likely influenced by the medium’s biochemical stability, which prevents disturbances to the sperm’s motility apparatus and intracellular signaling pathways critical for movement (Ratnawati et al., 2019; Fernandez et al., 2021). Such preservation of kinematic parameters ensures that sperm remains functional for reproductive applications, including artificial insemination and in vitro fertilization. This is in accordance with the results by Rahmat et al. (2024) and Amaliah et al. (2023) that the use of freeze-dry albumin as a sexing medium can reduce the decrease in semen quality after sexing because the viscosity of freeze-dry albumin can be more evenly uniformity than fresh albumin. Additionally, previous studies on fresh albumin have shown variability in separation efficiency and quality preservation due to its preparation- dependent nature. This study is consistent with findings that standardizing albumin gradients, such as freeze- drying, enhances reliability and reduces variability. The superior performance of freeze-dried albumin observed here adds to the growing body of evidence supporting its use in advanced reproductive technologies. This study recommends the adoption of freeze-dried albumin for sperm sexing, particularly in regions where access to advanced techniques like flow cytometry, is limited. The ease of preparation, storage stability, and cost-effectiveness of this method make it a scalable option for decentralized breeding programs. For large- scale applications, freeze-dried albumin provides a practical solution for sexed semen production while maintaining high sperm quality (Kaiin et al., 2017). Future research should focus on optimizing gradient composition and centrifugation protocols to further enhance separation efficiency. Comparative studies across different livestock species and under varied environmental conditions would provide valuable insights into the generalizability of these findings. Additionally, the integration of freeze-dried albumin into commercial artificial insemination programs could validate the scalability and impact of this agent on reproductive outcomes (Amaliah et al., 2023). Although the study demonstrated the effectiveness of freeze-dried albumin, several limitations should be noted. First, this study did not include a direct comparison with flow cytometry, which is the gold standard for sperm sexing. This comparison provides valuable insights into the relative efficiency and quality preservation capabilities of freeze-dried albumin compared with a well-established method. Second, this study did not explore the molecular mechanisms underlying the interactions between spermatozoa and albumin gradients. Molecular analyses, such as proteomic or transcriptomic profiling, can be used to elucidate the specific pathways or factors influencing sperm behavior and quality during the separation process. We also discussed the challenges related to scalability, including the need for consistent sourcing and quality control of freeze-dried albumin, as well as the adaptation of the method to commercial breeding programs. These challenges may involve optimizing the process to handle larger semen volumes and ensuring cost-effectiveness for large-scale applications. Addressing these gaps in future research will enhance our understanding of the mechanisms driving the observed benefits of freeze-dried albumin and support the optimization of sperm sexing techniques for broader applications. These findings highlight the promise of freeze-dried albumin as an efficient, reliable, and accessible medium for sperm sexing. By addressing its limitations and further exploring its scalability, freeze-dried albumin could play a transformative role in advancing livestock reproduction and genetic improvement programs. Addressing these gaps in future research will enhance our understanding of the mechanisms driving the observed benefits of freeze- dried albumin and support the optimization of sperm sexing techniques for broader applications. These findings highlight the promise of freeze-dried albumin as an efficient, reliable, and accessible medium for sperm sexing. By addressing its limitations and further exploring its scalability, freeze-dried albumin could play a transformative role in advancing livestock reproduction and genetic improvement programs. ConclusionIn conclusion, this study demonstrated that freeze- dried albumin achieves superior separation efficiency compared with fresh albumin, with higher proportions of X-bearing sperm in the upper gradient layer and effective preservation of critical quality parameters, such as motility, viability, membrane integrity, and kinematics. The stability and uniformity of freeze-dried albumin create an optimized gradient environment, enhancing sperm partitioning and minimizing stress on sperm cells. The practical advantages of this approach, including ease of storage, preparation, and cost- effectiveness, make it a promising alternative to flow cytometry, particularly in resource-limited settings. These findings support the potential of freeze-dried albumin to advance sustainable livestock reproduction and genetic improvement programs. AcknowledgmentsThe authors were supported by the Research Assistance for Research and Innovation Talents (BARISTA) scholarship from the National Research and Innovation Agency. We thank all members of the Laboratory of Animal Reproduction, Semen Processing Unit, Faculty of Animal Science, Hasanuddin University. Conflict of interestThe authors declare no conflict of interest. FundingThe authors did not receive support from any organization for the submitted work. Authors contributionA.M.D., M.Y., and R.R. contributed to supervised the experiment and conception and design the study; A.M.D. and M.Y. contributed to conception and design the study and improvement of the manuscript; R.R. collected data and drafted the manuscript; A.M.F. collected data, data analysis, and drafted the manuscript. Data availabilityAll data were provided in the manuscript. ReferencesAmaliah, R., Yusuf, M. and Toleng, A.L. 2023. The quality of Bali bull sexed semen using freeze-dried albumin. In AIP Conference Proceedings, Melville, NY: AIP Publishing, Vol. 2628(1). Arif, A.A., Maulana, T., Kaiin, E.M., Purwantara, B., Arifiantini, R.I. and Memili, E. 2020. Comparative analysis of various step-dilution techniques on the quality of frozen Limousin bull semen. Vet. World 13(11), 2422. Arifiantini, R.I., Yuliani, E., Pardede, B.P., Said, S. and Purwantara, B. 2024. Semen characteristics, freezability, and application of motility-based protein markers (proAKAP4) in assessing the suitability of superior Bali bulls (Bos sondaicus) at the Regional AI Center. Reprod. Breed. 4(4), 279–286. Bhalakiya, N., Haque, N., Patel, D., Chaudhari, A., Patel, G., Madhavatar, M., Patel, P., Hossain, S. and Kumar, R. 2018. Sperm sexing and its application in livestock sector. Int. J. Curr. Microbiol. App. Sci. 2018(Special 7), 259–272. Diansyah, A.M., Yusuf, M., Toleng, A.L. and Dagong, M.I.A. 2022. Characteristic and kinematics of Bali-polled bull sperms. Adv. Anim. Vet. Sci. 10(8), 1787–1796. Diansyah, A.M., Yusuf, M., Toleng, A.L. and Dagong, M.I.A. 2023a. The effect of thawing duration on the sperms quality of Bali polled bull. In AIP Conference Proceedings, Melville, NY: AIP Publishing, vol. 2628(1). Diansyah, A.M., Yusuf, M., Toleng, A.L., Dagong, M.I.A., Maulana, T. and Hasrin, B.A. 2023b. The sperm post-thawing quality and proteomic seminal plasma on fertility performance of Bali-polled bull. Adv. Anim. Vet. Sci. 11(4), 517–525. Fernandez-Novo, A., Santos-Lopez, S., Barrajon- Masa, C., Mozas, P., de Mercado, E., Caceres, E. and Perez-Garnelo, S.S. 2021. Effect of extender, storage time and temperature on kinetic parameters (CASA) on bull semen samples. Biology 10(8), 806. Gholami, D., Sharafi, M., Esmaeili, V., Nadri, T., Alaei, L., Riazi, G. and Shahverdi, A. 2023. Beneficial effects of trehalose and gentiobiose on human sperm cryopreservation. PLoS One 18(4), e0271210. Ishwarya, S.P. 2022. Spray-freeze-drying of foods and bioproducts: theory, applications and perspectives. Boca Raton, FL: CRC Press. Joshi, H., Mathur, M., Mohanty, A., Kumar, S., Kaushik, J., Mohanty, T., Kumar, D., Singh, S.K.., Bhushan, V., Parashar, A., Bhardwaj, P. and Yata, V.K. 2021. Semen sexing in bovine: current status and the need to develop alternative techniques. Anim. Reprod. Update 1(1), 17–31. Kaiin, E.M., Gunawan, M. and Maulana, T. 2017. Morphometry and abnormality evaluation of sex- sorted sperm of spotted buffalo (Tedong bonga). Nus. Biosci. 9(2), 175–180. Kang, S.S., Kim, U.H., Lee, M.S., Lee, S.D. and Cho, S.R. 2020. Spermatozoa motility, viability, acrosome integrity, mitochondrial membrane potential and plasma membrane integrity in 0.25 mL and 0.5 mL straw after frozen-thawing in Hanwoo bull. J. Anim. Reprod. Biotechnol. 35(4), 307–314. Madyawati, S.P., Toleng, A.L., Yusuf, M., Ako, A. and Amrullah, M.F. 2021. The quality of Bali bull sexed sperms at different incubation time using egg white sedimentation method. IOP Conf. Earth Environ. Sci. 788(1), 012142. Maulana, T., Afiati, F., Gunawan, M. and Kaiin, E.M. 2021. Kinematics motility of friesian-holstein sperm sexing in l-ascorbic acid treatments. IOP Conf. Earth Environ. Sci. 762(1), 012081. Maulana, T., Agung, P.P., Gunawan, M. and Said, S. 2022. Computer aided semen analysis (CASA) to determine the quality and fertility of frozen thawed sumba ongole sperm supplemented with amino acids. Livest. Anim. Res. 20(2), 194–201. Maulana, T. and Said, S. 2019. Kinematics motility of frozen-thawed X and Y sperm of Sumba Ongole bull. IOP Conf. Earth Environ. Sci. 387(1), 012030. Raafi, M., Yusuf, M., Toleng, A.L. and Diansyah, A.M. 2021. Movement patterns of sperms at different bull breeds using computer-assisted sperm analysis (CASA). IOP Conf. Earth Environ. Sci. 788(1), 012137. Rahman, M.S. and Pang, M.G. 2020. New biological insights on X and Y chromosome-bearing spermatozoa. Front. Cell Dev Biol. 7, 388. Rahmat, R., Yusuf, M., Toleng, A.L., Herdis, H., Diansyah, A.M. and Hasrin. H. 2024. The quality of Bali bull sexed sperms using freeze dry albumin at different concentrations of sexing medium. Adv. Anim. Vet. Sci. 12(2):249–258. Ratnawati, D., Isnaini, N. and Susilawati, T. 2019. Factors affecting spermatozoa motility analysis using CASA. Indonesian Bull. Anim. Vet. Sci. 29, 145–152. Surahman, S., Yusuf, M.Y., Garantjang, S.G. and Toleng, A.L. 2021. Sperms motility, viability, and abnormality of the frozen semen at different bull breeds. IOP Conf. Earth Environ. Sci. 788(1), 012140. Susilawati, T. 2014. Sexing spermatozoa. Malang, Indonesia: UB Press. | ||
| How to Cite this Article |
| Pubmed Style Diansyah AM, Yusuf M, Rahmat R, Alfian AM. Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. Open Vet. J.. 2025; 15(6): 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 Web Style Diansyah AM, Yusuf M, Rahmat R, Alfian AM. Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. https://www.openveterinaryjournal.com/?mno=238997 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.14 AMA (American Medical Association) Style Diansyah AM, Yusuf M, Rahmat R, Alfian AM. Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. Open Vet. J.. 2025; 15(6): 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 Vancouver/ICMJE Style Diansyah AM, Yusuf M, Rahmat R, Alfian AM. Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 Harvard Style Diansyah, A. M., Yusuf, . M., Rahmat, . R. & Alfian, . A. M. (2025) Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. Open Vet. J., 15 (6), 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 Turabian Style Diansyah, Athhar Manabi, Muhammad Yusuf, Rahmat Rahmat, and Andi Muhammad Alfian. 2025. Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. Open Veterinary Journal, 15 (6), 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 Chicago Style Diansyah, Athhar Manabi, Muhammad Yusuf, Rahmat Rahmat, and Andi Muhammad Alfian. "Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media." Open Veterinary Journal 15 (2025), 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 MLA (The Modern Language Association) Style Diansyah, Athhar Manabi, Muhammad Yusuf, Rahmat Rahmat, and Andi Muhammad Alfian. "Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media." Open Veterinary Journal 15.6 (2025), 2416-2426. Print. doi:10.5455/OVJ.2025.v15.i6.14 APA (American Psychological Association) Style Diansyah, A. M., Yusuf, . M., Rahmat, . R. & Alfian, . A. M. (2025) Enhanced sperm sexing efficiency and quality preservation in Bali bulls using freeze-dried albumin separation media. Open Veterinary Journal, 15 (6), 2416-2426. doi:10.5455/OVJ.2025.v15.i6.14 |