| Research Article | ||
Open Vet. J.. 2025; 15(7): 3063-3071 Open Veterinary Journal, (2025), Vol. 15(7): 3063-3071 Research Article Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens)Piyapan Manklinniam1 Saranya Phunpruch1,2 and Worakrit Worananthakij1,2*1Department of Biology, School of Science, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand 2Bioenergy Research Unit, School of Science, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand *Corresponding Author: Worakrit Worananthakij. Department of Biology, School of Science, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand. Email: worakrit.wo [at] kmitl.ac.th Submitted: 01/02/2025 Revised: 18/05/2025 Accepted: 05/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
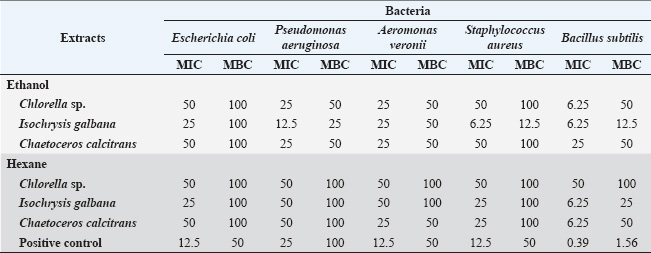
ABSTRACTBackground: Marine microalgae extracts are a promising alternative to commercial drugs because of their antimicrobial properties. Aim: This study aimed to investigate the antibacterial properties of crude extracts containing bioactive compounds derived from Chlorella sp., Isochrysis galbana, and Chaetoceros calcitrans. Methods: Crude extracts from microalgae were obtained via maceration using ethanol and hexane as solvents. Antibacterial activities were initially screened using the agar well diffusion method, whereas antimycobacterial activity was assessed using the microplate 7H11 agar proportion method. Subsequently, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the crude extracts were determined. Gas chromatography-mass spectrometry (GC-MS) was used for phytochemical composition analysis to identify significant antimicrobial compounds in the extracts. Results: The ethanolic extract of I. galbana demonstrated detectable antibacterial activity, with an inhibition zone of 12.40 ± 0.06 mm against Bacillus subtilis, 12.22 ± 0.28 mm against Staphylococcus aureus, 11.10 ± 0.11 mm against Pseudomonas aeruginosa, and 10.99 ± 0.33 mm against Aeromonas veronii at 100 mg/ml. Extracts from I. galbana and C. calcitrans inhibited Mycobacterium marinum, suggesting potential benefits for betta fish health. MIC values were 25 mg/ml for A. veronii in the ethanolic extracts of I. galbana, C. calcitrans, Chlorella sp., and C. calcitrans hexane extract. The GC-MS chromatogram of the ethanolic extract of I. galbana revealed the presence of several bioactive compounds. The major components identified include Phthalate, Tetradecanoic acid, Hexadecanoic acid, and Phytol. Conclusion: This study demonstrated the promising antimicrobial potential of crude extracts from marine microalgae, particularly the ethanolic extract of I. galbana, which showed strong activity against both terrestrial and aquatic pathogens. The findings suggest that bioactive compounds in these extracts, especially those from I. galbana and C. calcitrans, can be effective in managing bacterial infections such as A. veronii and M. marinum in betta fish. These results highlight the potential of marine microalgae as natural, sustainable alternatives for disease control in aquaculture. Keywords: Marine algae, Mycobacterium marinum, Antimycobacteriosis, Antibacterial activity, Siamese fighting fish. IntroductionThe Siamese fighting fish, renowned for its vibrant colors and ease of care, holds a special place in Thailand, where its economic significance is boosted by high export value. However, the primary health problems affecting Siamese fighting fish are infections caused by parasites, fungi, and bacteria. Bacterial infections can be caused by Pseudomonas alcaligenes, Vibrio fluvialis, Aeromonas caviae (Dong et al., 2018), Aeromonas veronii (Manklinniam et al., 2025), and Mycobacterium species (Pleeging and Moons, 2017). Among these infections, tuberculosis infections caused by Mycobacterium have the most significant impact on aquatic health and the fish export industry (Pleeging and Moons, 2017; Dong et al., 2018; Lichak et al., 2022; Dinh-Hung et al., 2023). Mycobacterium spreads through contaminated food, water, and breeding, and is difficult to treat due to antibiotic resistance. The disease can also infect humans, causing skin lesions (Wu et al., 2012). Antibiotics such as amikacin and kanamycin have long been used to treat bacterial and mycobacterial infections in fish (Rhodes and Kator, 1994). However, rising concerns regarding antibiotic resistance and environmental impact have prompted the search for alternative treatments. Natural extracts are emerging as promising alternatives to synthetic antimicrobials in aquaculture, with demonstrated efficacy against bacterial and mycobacterial infections in fish (Sathishkumar and Manoharan, 2014; Sriwatcharakul et al., 2023). Crude extracts from Murraya paniculata L. (flowers, leaves, and bark) and Thymus vulgaris L. have shown notable antibacterial and antimycobacterial activities against fish pathogens (Sriwatcharakul et al., 2023; Guz et al., 2025). However, studies specifically targeting the antimycobacterial effects of fish pathogens are limited. Most research has focused on human-associated mycobacteria, such as Mycobacterium tuberculosis, using bioactive compounds from marine sources, including sponges, algae, seaweeds, and fungi associated with soil sediments and mangroves (Hou et al., 2019). Marine natural extracts have garnered increasing attention as promising alternatives to antibiotics for the prevention and treatment of fish diseases. Several algal species, such as Nannochloropsis oceanica, Chaetoceros gracilis, Isochrysis sp., and Thalassiosira weissflogii, have shown antimicrobial activity, effectively inhibiting Vibrio harveyi infections in barramundi (Lates calcarifer) (Jusidin et al., 2022). Marine algae are rich in bioactive compounds, including phlorotannins, bromophenols, ascorbic acid, and carotenoids, which possess antimicrobial and antioxidant properties (Fujimoto, 1990; Ito et al., 2018). Phlorotannins and bromophenols have been demonstrated to disrupt bacterial membranes and inhibit pathogen growth, highlighting their potential in aquacultural disease management. However, the efficacy of marine algal extracts against Mycobacterium spp. remains underexplored, especially in the Thai aquaculture context. Additionally, few studies have thoroughly characterized the phytochemicals responsible for these bioactivities. This study aimed to evaluate the antimicrobial activity of ethanolic and hexane crude extracts from three marine microalgae species, Chlorella sp., I. galbana, and Chaetoceros calcitrans, against pathogenic bacteria affecting betta fish, including Mycobacterium marinum and A. veronii, as well as four additional strains: Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa. In addition, this study aimed to identify and characterize the phytochemical constituents responsible for the observed antimicrobial effects. This research represents the first investigation in Thailand into the use of natural microalgal extracts for controlling tuberculosis-causing bacteria in aquaculture, with potential implications for improving fish health and promoting sustainable aquaculture practices. Materials and methodsMarine microalgal strains and cultivationIn this study, three marine microalgae (Chlorella sp., I. galbana, and C. calcitrans) were obtained from the Institute of Marine Science, Burapha University, Chonburi province, Thailand. We cultivated them in 6 l plastic aquarium tanks containing 4 l of Guillard’s F/2 medium (Guillard and Ryther, 1962) at room temperature, with an 18:6 hours cycle of fluorescent light and darkness. Air was supplied at a rate of 1.5 l/minute, and the light intensity was set to 2,400 lux. Preparation of marine microalgal crude extractAfter 10 days of cultivation, marine microalgal cells were harvested via centrifugation (Hermle Z513K) at 2,000 × g at 4°C for 15 minutes. The cells were then dried in a hot air oven at 45°C for 5 hours. The dry cells were ground with a mortar and pestle. Five grams of microalgal powder were mixed with 50 ml of 95% ethanol (Qchemical Co., Ltd.) and 100% hexane (Qchemical Co., Ltd). The mixtures were left to macerate at room temperature for 24 hours. The extracts were filtered through Whatman No.1 filter paper (Cytiva, China), and the solvents were evaporated using a rotary evaporator at 60°C. The crude extracts were stored at 4°C under darkness until use. Bacterial strains and cultivationBacillus subtilis Thailand Institute of Scientific and Technological Research (TISTR) 1248, Escherichia coli TISTR 074, P. aeruginosa TISTR 2370, and Staphylococcus aureus TISTR 746 were obtained from the TISTR culture collection, Bangkok, Thailand. Aeromonas veronii WWKMITL-02 and M. marinum WWKMITL-03 were provided by the Laboratory of Fish Diseases, Department of Biology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand. Both isolates were originally obtained from clinically infected Siamese fighting fish (Manklinniam et al., 2025). All bacterial strains, except M. marinum, were cultivated in 250 ml Erlenmeyer flasks containing 100 ml of tryptic soy broth (TSB) and incubated at 37 °C with shaking at 100 rpm for 18 hours (López-Prieto et al., 2019). Mycobacterium marinum was cultured on Ogawa solid medium in 50 ml glass bottles at 30°C for 3 weeks (Dissanayake et al., 2017). Determination of antibacterial activityThe antibacterial activity of the algal extracts was evaluated using the agar well diffusion method. Five indicator bacterial strains, including S. aureus, B. subtilis (Gram-positive), E. coli, P. aeruginosa, and A. veronii (Gram-negative), were cultivated as previously described. Bacterial cells were harvested by centrifugation (Hermle Z513K, Germany) at 10,000 × g for 10 minutes at 4°C, washed, and resuspended in 0.85% NaCl to a final concentration of 1.5×108 CFU/ml. Bacterial suspensions were spread onto Tryptic Soy Agar (TSA) plates using sterile cotton swabs. Wells of 6 mm in diameter were then punched into the agar. Subsequently, 40 μl of algal extracts dissolved in 1% dimethyl sulfoxide (DMSO) (Fisher, England) were added to each well. Streptomycin at concentrations of 2 mg/ml and 1% DMSO served as positive and negative controls, respectively. Plates were incubated at 37°C for 24 hours, and inhibition zones were measured. The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of the crude algal extracts against bacteria were determined using the broth microdilution method in a sterile 96-well microplate. All media and solutions were sterilized prior to use, and the algal extracts were filtered through a 0.22 µm membrane filter. Subsequently, 50 µL of the algal extracts at various final concentrations (0.19, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50, and 100 mg/ml) were added to the wells. An equal volume (50 µl) of bacterial suspension, adjusted to a final concentration of 5×106 CFU/mL, was then added to each well. Streptomycin was used as the positive control, whereas 1% DMSO served as the negative control. The microplates were incubated at 37°C for 24 hours. Bacterial growth was assessed visually by observing turbidity. The MIC was defined as the lowest concentration of the extract that completely inhibited visible bacterial growth. To determine the MBCs, aliquots from wells with no visible growth were plated onto TSA and incubated at 37°C for 24 hours. The MBC was defined as the lowest concentration at which no bacterial colonies were observed on the TSA plates, corresponding to a 99.9% reduction in the initial bacterial population. Determination of antimycobacterial activityAntimycobacterial activity was assessed using the microplate 7H11 agar proportion method (Wahyuningrum et al., 2017). Mycobacterium marinum was cultured in Middlebrook 7H9 broth supplemented with 0.2% glycerol and 10% Oleic Albumin Dextrose Catalase (OADC) (Yam et al., 2020). The mycobacterial suspension was adjusted to McFarland’s standard No. 1 (approximately 3 × 108 CFU/ml). A volume of 250 µl of the M. marinum suspension was added to each of the 12 flat-bottom wells, followed by 250 µl of the test sample, 250 µl of crude extract (final concentration 10 mg/ml), and 1,500 µl of Middlebrook 7H11 medium supplemented with OADC. The plates were incubated at 30°C and were monitored for bacterial growth on days 5, 7, 11, and 21. Phytochemical composition analysisThe crude algal extracts were dissolved in 5% (w/v) ethanol [gas chromatography–mass spectrometry (GC-MS) grade; Merck, Germany] and analyzed using a GC-MS system (Agilent Technologies 5973 Inert). Helium (He) was used as the carrier gas, and the sample was introduced in the split injection mode. The GC-MS interface temperature was maintained at 280°C, while the electron ionization source temperature was 250°C. The electron impact energy was 70 eV, and the quadrupole ion analyzer was set at 150°C. The compounds were analyzed over a mass range of 50–550 atomic mass units (amu), and the resulting spectra were recorded. Statistical analysisAll experiments were conducted in triplicate, and data are presented as the mean ± standard deviation (SD). The differences between groups were compared using one-way analysis of variance with SPSS version 22.0. A significance level of p < 0.05 was considered statistically significant. Ethical approvalNot needed for this study. ResultsAntimicrobial activity of crude extracts from marine microalgaeThe antimicrobial activities of crude extracts derived from Chlorella sp., I. galbana, and C. calcitrans were evaluated against five bacterial strains: E. coli, P. aeruginosa, A. veronii, S. aureus, and B. subtilis (Table 1). The inhibition zones ranged from 6.24 to 10.90 mm for Chlorella sp., 6.74–12.40 mm for I. galbana, and 6.99–9.96 mm for C. calcitrans. The results showed that all algae extracts had antibacterial effects and inhibited the growth of both Gram-positive and Gram-negative strains as follows: the ethanolic extract of I. galbana demonstrated the highest antibacterial effect, with inhibition zones of 12.40 ± 0.66, 12.22 ± 0.28, 11.10 ± 0.11, and 10.99 ± 0.33 mm against B. subtilis, S. aureus, P. aeruginosa, and A. veronii, respectively. The hexane extract of I. galbana also showed notable activity against E. coli, with an inhibition zone of 7.82 ± 0.43 mm. Additionally, the ethanolic extract of Chlorella sp. displayed considerable antibacterial activity, with no statistically significant differences (p > 0.05) among inhibition zones of 10.90 ± 0.06, 10.11 ± 0.17, and 9.34 ± 0.24 mm against A. veronii, B. subtilis, and P. aeruginosa, respectively. The case of ethanolic extract of C. calcitrans had the highest antibacterial activity with 9.96 ± 1.08 mm against A. veronii. The hexane extract of Chlorella sp. was also notable with inhibition zones of 10.39 ± 0.83, 10.02 ± 0.53, 9.75 ± 0.21, and 9.67 ± 0.49 mm mm against P. aeruginosa, B. subtilis, S. aureus, and A. veronii, respectively. The hexane extract of I. galbana exhibited high antibacterial activity with inhibition zones of 11.31 ± 0.17, 10.19 ± 1.00, and 9.40 ± 0.75 mm against B. subtilis, A. veronii, and S. aureus, respectively. C. calcitrans had high antibacterial activity with inhibition zones of 9.69 ± 1.00 mm against A. veronii. The ethanolic extract of I. galbana exhibited effective antibacterial activity against S. aureus, B. subtilis, P. aeruginosa, E. coli, and A. veronii, with MIC values of 6.25, 6.25, 12.5, 25, and 25 mg/ml, respectively (Table 2). This extract had the lowest MIC values among the tested samples, indicating its strong inhibitory potential. In addition, the hexane extracts of I. galbana and C. calcitrans also exhibited antibacterial activity, albeit to a slightly lesser extent. Regarding the MBC, the ethanolic extract of I. galbana exhibited effective bactericidal activity against S. aureus and B. subtilis at a concentration of 12.50 mg/ml (Table 2). For E. coli, all extracts exhibited MBC values of 100 mg/ml (Table 2). Table 1. Antibacterial activity of microalgae extracts.
Table 2. Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of marine microalgae extracts on bacteria (µg/ml).
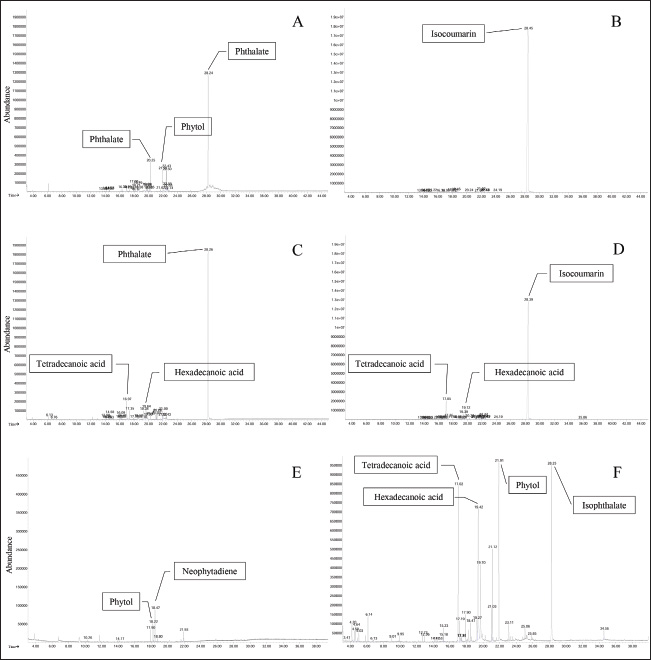
In the antimycobacterial assay against M. marinum, inhibition was assessed using the 7H11 agar proportion method. Both the ethanolic and hexane extracts of I. galbana and C. calcitrans at a concentration of 10 mg/ml demonstrated visible inhibition of M. marinum growth throughout the 21-day experimental period, as shown in Table 3. The inhibitory results were qualitatively recorded as either positive or negative, where positive and negative indicated the presence and absence of antimycobacterial activity, respectively. In contrast, the ethanolic and hexane extracts of Chlorella sp. showed no observable inhibitory effect against M. marinum (Table 3). Phytochemical composition of the microalgal crude extractPhytochemical analysis of the crude algal extracts conducted using GC-MS revealed the presence of several bioactive compounds across different species. In the ethanolic extract of Chlorella sp., the predominant compound was Bis (2-ethylhexyl) phthalate with a retention time (RT) of 28.245 minutes (Fig. 1A). Similarly, the hexane extract of Chlorella sp. contained 3-(2-indolyl) isocoumarin, an isocoumarin, at an RT of 28.450 minutes (Fig. 1B). The ethanol extract of I. galbana predominantly contained Bis (2-ethylhexyl) phthalate, with an RT of 28.255 minutes (Fig. 1C). The ethanolic extract of C. calcitrans contained neophytadiene with an RT of 18.468 minutes (Fig. 1E). In contrast, the hexane extract of C. calcitrans was characterized by the presence of isophthalate at an RT of 19.423 minutes (Fig. 1F). These identified phytochemicals are known for their potential biological activities, particularly antibacterial properties. Table 3. Antimycobacterial activities of the microalgae extracts.
DiscussionThis study investigated the antibacterial and antimycobacterial activities of crude extracts of three marine microalgae (I. galbana, C. calcitrans, and Chlorella sp.) using ethanol and hexane as solvents. The motivation for this work stems from growing concerns over antibiotic resistance, particularly in aquaculture systems where sustainable alternatives are urgently needed. Therefore, our aim was to assess the inhibitory potential of these microalgae extracts against selected bacterial pathogens and M. marinum, a significant pathogen in betta fish farming. The result showed variation in inhibition zones among the different extracts reflects both the type of algal species and the susceptibility of each bacterial strain. The ethanolic extract of I. galbana showed the most promising antibacterial activity, especially against E. coli, S. aureus, and B. subtilis. MIC data confirmed its potency, with the lowest values (6.25–25 mg/ml) across both Gram-positive and Gram-negative bacteria. MBC results further supported its efficacy, showing bactericidal effects against S. aureus and B. subtilis at 12.5 mg/ml. Ethanol has been widely reported as an effective solvent for extracting antibacterial compounds from microalgae. For instance, Pradhan et al. (2012) demonstrated superior antibacterial activity in ethanolic extracts of Spirulina platensis, whereas Jusidin et al. (2022) found similar results with N. oceanica, C. gracilis, Isochrysis sp., and T. weissflogii. According to these results, antibacterial compounds in microalgae may be predominantly hydrophobic, which favors extraction by organic solvents. Our findings are consistent with previous reports showing strong antibacterial activity in I. galbana extracts (Srinivasakumar and Rajashekhar, 2009). However, unlike earlier studies (Seraspe et al., 2012; Maadane et al., 2017), the extracts from Chlorella sp. and C. calcitrans in our study showed no activity against E. coli, P. aeruginosa, and S. aureus. These discrepancies may result from differences in compound profiles, solvent efficiency, environmental conditions, or geographic origin, as noted by Kaufman et al. (1999); Praiboon et al. (2018); and Benhniya et al. (2022). Interestingly, our antimycobacterial tests revealed that the crude extracts of I. galbana and C. calcitrans effectively inhibited M. marinum at 10 mg/ml, with sustained activity over 21 days. In contrast, Chlorella sp. extract showed no notable inhibition. These results highlight species-specific differences in antimicrobial potential, likely due to variations in bioactive compound composition. While most prior studies have focused on human pathogens such as M. tuberculosis (Kim and Ta, 2011; Ruiz-Güereca et al., 2019; Mukherjee et al., 2021), the application of algal extracts against aquaculture pathogens remains underexplored. Phytochemical analysis of the crude extracts identified compounds such as phthalates in I. galbana and Chlorella sp., and neophytadiene and hexadecenoic acid in C. calcitrans. Although phthalates are traditionally considered environmental pollutants derived from synthetic plasticizers, they may also occur naturally; certain freshwater algae and cyanobacteria can produce phthalates under stress conditions (Babu and Wu, 2010). These compounds are known to exhibit various biological activities, including antimicrobial (Ali et al., 2023), antioxidant, antitumor, allelopathic, and phytotoxic effects (Pace et al., 2024). Isocoumarins, also detected, are lactonic natural products primarily produced by fungi and bacteria, with limited presence in higher plants and marine organisms (Shabir et al., 2021). Notably, one of the isocoumarin derivatives identified was previously isolated from an endophytic fungus associated with the red alga Asparagopsis taxiformis, underscoring the potential of marine algae as a source of bioactive isocoumarins with therapeutic applications (Medina et al., 2014). Although natural isocoumarins have been evaluated for antibacterial activity against various human pathogens, the factors influencing their bioactivity remain insufficiently explored (Devienne et al., 2005). Neophytadiene, a bioactive compound found in algae (Plaza et al., 2010), has been reported as a key contributor to the antibacterial activity of the seaweed Ulva lactuca (Anjali et al., 2019). Variations in the chemical composition of natural bioactive compounds contribute to differences in their antibacterial activities. Phthalates have been reported to disrupt bacterial membranes by increasing their permeability, resulting in the leakage of essential intracellular components (Roy et al., 2006). Isocoumarins inhibit key bacterial enzymes such as DNA gyrase and topoisomerase, which are essential for DNA replication and cell survival (Cho et al., 2006). Moreover, neophytadiene, a diterpene with anti-inflammatory properties, has been reported to disrupt membrane integrity and potentially interfere with bacterial enzymes or metabolic proteins (El-Sayed et al., 2019). These mechanisms underscore the potential of natural compounds as promising sources of novel antimicrobial agents. Another important factor is the presence of sulfated polysaccharides in I. galbana, which are known for their antimicrobial and antiviral properties (Ullah, 2021) and may contribute to the sustained antimycobacterial effect observed. Additionally, environmental stressors such as nutrient limitation can enhance the synthesis of bioactive metabolites in microalgae, including lipids, phenolics, and terpenoids, all of which are widely associated with antimicrobial activity (Ördög et al., 2004; Gao et al., 2013; Little et al., 2021).
Fig. 1. GC-MS spectra of different microalgal extracts. Chromatograms of the ethanol extract of Chlorella sp. (A), the hexane extract of Chlorella sp. (B), the ethanol extract of I. galbana (C), the hexane extract of I. galbana (D), the ethanol extract of C. calcitrans (E), and the hexane extract of C. calcitrans (F). The variability in bacterial responses, particularly those of M. marinum, may be attributed to differences in cell wall architecture. Mycobacteria possess a complex, lipid-rich cell envelope that affects their susceptibility to antimicrobial agents (Brennan and Nikaido, 1995). The observed inhibition of M. marinum by I. galbana and C. calcitrans extracts suggests that certain extract components interact with or disrupt these unique structural features. This study is among the first to report the antibacterial and antimycobacterial activities of marine microalgal extracts against bacterial pathogens affecting betta fish in Thailand. The findings underscore the potential of marine microalgae as ecofriendly alternatives to conventional antibiotics in aquaculture, addressing the urgent issue of antibiotic resistance (FAO, 2020). ConclusionThis study investigated the antimicrobial activity of crude extracts of various microalgae strains against multiple pathogenic bacteria. The ethanolic extract of I. galbana exhibited strong inhibitory activity against E. coli, S. aureus, B. subtilis, and A. veronii, with an MIC of 25 mg/ml. In contrast, extracts from C. calcitrans and Chlorella sp. demonstrated limited antibacterial activity, likely due to differences in the bioactive compound profiles and extraction efficiency. Furthermore, extracts of I. galbana and C. calcitrans effectively suppressed M. marinum, a key pathogen in betta fish, indicating their potential utility in aquaculture health management. Phytochemical analysis revealed diverse bioactive constituents, including phthalates, isocoumarins, and diterpenes, which may contribute to the observed antimicrobial activity. Overall, the results highlight the promise of I. galbana and C. calcitrans as sources of natural antimicrobial agents. Further research should explore their application in aquaculture systems, including in vivo efficacy, safety, and formulation development. AcknowledgmentsWe gratefully acknowledge the financial support provided by King Mongkut’s Institute of Technology Ladkrabang (KMITL) under Grant No. RA/TA-2563-D-002, which supported the Ph. D. study of PM, and by the School of Science, KMITL under Grant No. 2565-02-05-011. Conflicts of interestWe declare no conflict of interest. FundingThis work was financially supported by King Mongkut’s Institute of Technology Ladkrabang (KMITL) under Grant No. RA/TA-2563-D-002, which supported the Ph. D. study of PM, and Grant No. 2565-02-05-011 by the School of Science, KMITL. Author contributionsWW conceptualization, formal analysis, project administration, resources, and writing-review and editing. SP bacterial analysis, interpretation of data analysis and writing-review. PM data curation, formal analysis, investigation, methodology, visualization, and writing-original draft. All authors have read and agreed to the publication of the finale version of the manuscript. Data availabilityThe data supporting the findings of this study are available from the corresponding author upon reasonable request. ReferencesAli, M.A., Darweesh, A.O. and Matar, M.M. 2023. Isolation and characterization of an antibacterial compound Bis-(2-ethylhexyl) phthalate from the fungi Penicillium digitatum. Biomedicine 43(5), 1607–1612. Anjali, K.P., Sangeetha, B.M., Devi, G., Raghunathan, R. and Dutta, S. 2019. Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): the compound characterization and functional applications in medicine-a comparative study. J. Phytochem. Phatobiol. 200, 111622 Babu, B. and Wu, J.T. 2010. Production of phthalate esters by nuisance freshwater algae and cyanobacteria. Sci. Total Environ. 408, 4969–4975. Benhniya, B., Lakhdar, F., Noreddine, R. and Etahiri, S. 2022. GC/MS analysis and antibacterial potential of macroalgae extracts harvested on Moroccan Atlantic coast. Egypt. J. Chem. 65, 171–179. Brennan, P.J. and Nikaido, H. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63. Cho, J.Y., Kwon, H.C., Williams, P.G., Kauffman, C.A., Jensen, P.R. and Fenical, W. 2006. Actinofuranones A-D, new polyketides from a marine-derived bacterium related to the Genus Streptomyces. J. Nat. Prod. 69(3), 425–428. Devienne, K.F., Raddi, M.S.G., Coelho, R.G. and Vilegas, W. 2005. Structure-antimicrobial activity of some natural isocoumarins and their analogues. Phytomedicine 12(5), 378–381. Dinh-Hung, N., Dong, H.T., Senapin, S., Pimsannil, K., Thompson, K.D., Shinn, A.P., Soontara, C., Sirimanapong, W., Chatchaiphan, S. and Rodkhum, C. 2023. Insight into characteristics and pathogenicity of five rapidly growing non-tuberculous Mycobacterium species isolated from the Siamese fighting fish, Betta splendens. Aquaculture 575, 739822. Dissanayake, A., Perera, V., Jagoda, S.S.D.S., Roshani Edirisinghe, E.A. and Arulkanthan, A. 2017. Characterisation of nontuberculous mycobacteria isolated from apparently healthy and diseased fresh water ornamental fish in Sri Lanka. Asian Fish. Sci. 30(2), 118–129. Dong, H.T., Senapin, S., Phiwsaiya, K., Techatanakitarnan, C. and Dokladda, K. 2018. Histopathology and culturable bacteria associated with “big belly” and “skin nodule” syndromes in ornamental Siamese fighting fish, Betta splendens. Microb. Pathog. 122, 26–52. El-Sayed, M.M., Ahmed, H.O. and Morsy, N.M. 2019. Antimicrobial diterpenes: sources, biosynthesis, and bioactivity. Fitoterapia 136, 104173. Hou, X.M., Wang, C.Y., Gerwick, W.H. and Shao, C.L. 2019. Marine natural products as potential anti-tubercular agents. Eur. J. Med. Chem. 165, 273–292. Food and Agriculture Organization of the United Nations. 2020. Report of the FAO Expert Working Group Meeting “Scoping exercise to increase the understanding of risks of antimicrobial resistance (AMR) in aquaculture”, Palermo, Italy, 26-29 November 2018. FAO Fisheries and Aquaculture Report No. 1268. Rome, Italy: FAO,. Fujimoto, K. 1990. Antioxidant activity of phlorotannins isolated from the brown alga Eisenia bicyclis. Fish. Sci. 62(6), 923–926. Gao, Y., Yang, M. and Wang, C. 2013. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour. Technol. 147, 484–491. Guillard, R.R.L. and Ryther, J.H. 1962. Studies on marine planktonic diatoms I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239. Guz, L., Puk, K. and Pastuszka, A. 2025. In vitro activity of garden thyme (Thymus vulgaris L.) chloroform extract against non-tuberculous mycobacteria. Med. Weter. 81(2), 93–95. Ito, M., Koba, K., Hikihara, R., Ishimaru, M., Shibata, T., Hatate, H. and Tanaka, R. 2018. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 255, 147–156. Jusidin, M.R., Othman, R., Shaleh, S.R.M., Ching, F.F., Senoo, S. and Oslan, S.N.H. 2022. In Vitro antibacterial activity of marine microalgae extract against Vibrio harveyi. Appl. Sci. 12, 1148. Kaufman, P.B., Cseke, L.J., Warber, S., Duke, J.A. and Brielmann, H.L. 1999. Natural products from plants. Boca Raton, FL: CRC Press; p: 343. Kim, S.K. and Ta, Q.V. 2011. Potential beneficial effects of marine algae sterols on humen health. Adv. Food Nutr. Res. 64, 191–198. Lichak, M.R., Barber, J.R., Kwon, Y.M., Francis, K.X. and Bendesky, A. 2022. Care and use of siamese fighting fish (Betta Splendens) for research. Comp Med. 72(3), 169–180. Little, S.M, Senhorinho, G.N.A., Saleh, M., Basiliko, N. and Scott, J. 2021. Antibacterial compounds in green microalgae from extreme environments: a review. Algae 36(1), 61–72. López-Prieto, A., Martínez-Padrón, H., Rodríguez-López, L, Moldes, A.B. and Cruz, J.M. 2019a. Isolation and characterization of a microorganism that produces biosurfactants in corn steep water. CYTA-J. Food. 17(1), 509–516. Maadane, A., Merghoub, N., Mernissi, N.E., Ainane, T., Amzazi, S., Wahby, I. and Bakri, Y. 2017. Antimicrobial activity of marine microalgae isolated from Moroccan coastlines. J Microbiol Biotechnol. Food Sci. 6(6), 1257–1260. Manklinniam, P., Phunpruch, S. and Worananthakij, W. 2025. Occurrence of parasitic and bacterial pathogen in ornamental and wild populations of Siamese fighting fish (Betta splendens) in a region of Thailand. ASEAN J. Sci. Tech. Report. 28(3), e257870. Medina, R., Biasetto, C., Somensi, A., Yokoya, N., Lopes, M., Araújo, A. and Silva, D. 2014. 5-Hydroxymethylmellein: an isocoumarin derivative from an endophytic fungus from red alga Asparagopsis taxiformis. Planta Med. 80(16), 1395–1396. Mukherjee, G., Mukhopadhyay, B. and Sil, A.K. 2021. Edible marine algae: a new source for anti-mycobacterial agents. Folia Microbiol. 66, 99–105. Ördög, V., Stirk, W.A., Lenobel, R., Bancířová, M., Strnad, M., Van, Staden, J., Szigeti, J. and Németh, L. 2004. Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J. Appl. Phycol. 16, 309–314. Pace, A., Vaglica, A., Maccotta, A. and Savoca, D. 2024. The origin of Phthalates in algae: biosynthesis and environmental bioaccumulation. J. Environ. 11, 78. Plaza, M., Santoyo, S., Jaime, L., García-Blairsy, R.G., Herrero, M., Señoráns, F.J. and Ibáñez, E. 2010. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 51(2), 450–455. Pleeging, C.C.F. and Moons, C.P.H. 2017. Potential welfare issues of the Siamese fighting fish (Betta splendens) at the retailer and the hobbyist aquarium. Vlaams. Diergen. Tijds. 86, 213–223. Pradhan, J., Das, B.K., Sahu, S., Marhual, N.P., Swain, A.K., Mishra, B.K. and Eknath, A.E. 2012. Traditional antibacterial activity of fresh water microalga Spirulina platensis to aquatic pathogens. Aquac. Res. 43, 1287–1295. Praiboon, J., Palaka, S., Noiraksa, T. and Miyashita, K. 2018. Seasonal variation in nutritional composition and anti-proliferative activity of brown seaweed, Sargassum oligocystum. J. Appl. Phycol. 30, 101–111. Rhodes, M.W. and Kator, H. 1994. Mycobacteriosis in fish: a review. J. Fish Dis. 17(3), 197–212. Roy, R.N., Laskar, S. and Sen, S.K. 2006. Dibutyl phthalate, the bioactive compound produced by Streptomyces albidoflavus 321.2. Microbiol. Res. 161(2), 121–126. Ruiz-Güereca, D.A., Licea-Navarro, A.F. and Sánchez-Saavedra, M.P. 2019. Evaluation of antimycobacterial activity from marine and freshwater microalgae. Rev. Biol. Mar. Oceanogr. 54(1), 82–90. Sathishkumar, M. and Manoharan, G. 2014. Evaluation of antibacterial activity of medicinal plants against fish pathogens. Asian Pac. J. Trop. Biomed. 4(1), S316–S320. Seraspe, E.B., Ticar, B.F., Formacion, M.J., Pahila, I.G., Pena, M.R. and Amar, E.C. 2012. Antibacterial properties of the microalgae Chaetoceros calcitrans. Asian Fish. Sci. 25, 343–356. Shabir, G., Saeed, A. and El-Seedi, U.R. 2021. Natural isocoumarins: structural styles and biological activities, the revelations carry on. Phytochemistry 181, 112568. Sriwatcharakul, S., Angsusing, S., Boonyoprakan, N., Worananthakij, W. and Taveekijakarn, P. 2023. Antimicrobial activities and phytochemicals of Murraya paniculata L. flowers, leaves and bark crude extracts. Malays. J. Microbiol. 19(1), 83–86. Srinivasakumar, K.P. and Rajashekhar, M. 2009. In vitro studies on bactericidal activity and sensitivity pattern of isolated marine microalgae against selective human bacterial pathogens. Ind. J. Sci. Technol. 2(8), 16–23. Ullah, I. 2021. Antimicrobial agents from microalgae as potential source for treatment of Mycobacterium tuberculosis: a short review. J. Antimicrob. Agents 7(7), 4. Wahyuningrum, R., Ritmaleni, R., Irianti, T., Wahyuono, S., Kaneko, T. and Nuryastuti, T. 2017. Antituberculosis activity of Brotowali (Tinospora crispa) extract and fractions against Mycobacterium tuberculosis using microplate Alamar blue assay method. J. Tradit. Med. 22(2), 124–130. Wu, T.S., Chiu, C.H., Yang, C.H., Leu, H.S., Huang, C.T., Chen, Y.C., Wu, T.L. Chang, P.Y., Su, L.H., Kuo, A.J., Chia, J.H., Lu, C.C. and Lai, H.C. 2012. Fish tank granuloma caused by Mycobacterium marinum. PLoS One 7(7), e41296. Yam, Y.K., Alvarez, N., Go, M.L. and Dick, T. 2020. Extreme drug tolerance of Mycobacterium abscessus “Persisters”. Front. Microbiol. 11, 359. | ||
| How to Cite this Article |
| Pubmed Style Manklinniam P, Phunpruch S, Worananthakij W. Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). Open Vet. J.. 2025; 15(7): 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 Web Style Manklinniam P, Phunpruch S, Worananthakij W. Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). https://www.openveterinaryjournal.com/?mno=240545 [Access: January 11, 2026]. doi:10.5455/OVJ.2025.v15.i7.17 AMA (American Medical Association) Style Manklinniam P, Phunpruch S, Worananthakij W. Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). Open Vet. J.. 2025; 15(7): 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 Vancouver/ICMJE Style Manklinniam P, Phunpruch S, Worananthakij W. Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). Open Vet. J.. (2025), [cited January 11, 2026]; 15(7): 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 Harvard Style Manklinniam, P., Phunpruch, . S. & Worananthakij, . W. (2025) Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). Open Vet. J., 15 (7), 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 Turabian Style Manklinniam, Piyapan, Saranya Phunpruch, and Worakrit Worananthakij. 2025. Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). Open Veterinary Journal, 15 (7), 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 Chicago Style Manklinniam, Piyapan, Saranya Phunpruch, and Worakrit Worananthakij. "Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens)." Open Veterinary Journal 15 (2025), 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 MLA (The Modern Language Association) Style Manklinniam, Piyapan, Saranya Phunpruch, and Worakrit Worananthakij. "Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens)." Open Veterinary Journal 15.7 (2025), 3063-3071. Print. doi:10.5455/OVJ.2025.v15.i7.17 APA (American Psychological Association) Style Manklinniam, P., Phunpruch, . S. & Worananthakij, . W. (2025) Antibacterial activity of marine microalgae extracts against pathogen of Siamese fighting fish (Betta splendens). Open Veterinary Journal, 15 (7), 3063-3071. doi:10.5455/OVJ.2025.v15.i7.17 |