| Research Article | ||
Open Vet. J.. 2025; 15(6): 2511-2517 Open Veterinary Journal, (2025), Vol. 15(6): 2511-2517 Research Article Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicityFaisal Fikri1,2*, Auliyauna Miftahurrohmah1, Muhammad Sbastian Pratama1, Danis Farid Qosdina1, Gigih Fikrillah Sya’ban1, Salipudin Tasil Maslamama3 and Muhammad Thohawi Elziyad Purnama1,2,41Division of Veterinary Medicine, Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 2Research Group of Animal Biomedical and Conservation, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 3Department of Agricultural Biotechnology, Faculty of Agriculture, Eskişehir Osmangazi Üniversitesi, Eskişehir, Türkiye 4Department of Biology, Graduate School of Natural and Applied Sciences, Eskişehir Osmangazi Üniversitesi, Eskişehir, Türkiye * Correspondence to: Faisal Fikri. Division of Veterinary Medicine, Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia. Email: faisalfikri [at] fkh.unair.ac.id Submitted: 03/02/2025 Revised: 10/05/2025 Accepted: 16/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
AbstractBackground: In therapeutic scenarios, the anti-inflammatory medication dexamethasone is frequently administered. However, the consequences of prolonged use and dose residue may result in dexamethasone toxicity. Astaxanthin is a carotenoid antioxidant that may lessen the harmful effects of dexamethasone. Aim: This study aimed to investigate the efficacy of astaxanthin in a dexamethasone toxicity model in the kidney of albino rats based on hematological profiles and interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) expression. Methods: The study involved the random assignment of 25 albino rats into five treatment groups with five replications: (C–) placebo, (C+) intramuscular injection of 0.75 mg/kg BW of dexamethasone, and (T1, T2, and T3) intramuscular injection of 0.75 mg/kg BW of dexamethasone and oral administration of 2, 6, and 12 mg/kg BW of astaxanthin, respectively. All experimental animals were administered therapy for 10 days. Hematological profiles were evaluated using a blood analyzer, whereas IL-6 and TNF-α expression was evaluated using immunohistochemical staining. Results: Based on leukocyte, erythrocyte, hemoglobin, and hematocrit characteristics, groups T2 and T3 were generally found to be significantly different (p < 0.05) from group C+ regarding the reduction of the effects of dexamethasone. In the meantime, group T3 showed a significant (p < 0.05) decrease in TNF-α and IL-6 expression compared with group C+. Conclusion: The administration of 12 mg/kg BW of astaxanthin may be an antidote to dexamethasone toxicity in albino rats, according to the overall assessment of the hematological profile, IL-6, and TNF-α cytokines in the kidney. Keywords: Astaxanthin, Dexamethasone toxicity, Drug safety, Hematology, Interleukin-6, Tumor necrosis factor-α.. IntroductionCorticosteroids are considered a beneficial medication for treating several illnesses, including inflammation, anaphylaxis, and asthma attacks. The preparation of dexamethasone is one of the new synthesizers of corticosteroids, and it has undergone numerous variations and is still being developed (Stone et al., 2021). Dexamethasone is an anti-inflammatory medication that may have a long half-life. Because dexamethasone is readily available and reasonably priced, it is fairly widespread in the community. Dexamethasone has a lot of negative adverse effects. Dexamethasone use may result in harmful side effects that harm the kidneys, particularly the proximal tubules (Ye et al., 2017). Long-term usage of dexamethasone may result in oxidative stress due to adverse effects such as free radicals (Hidayatik et al., 2021). Furthermore, dexamethasone-induced potassium deficiency may cause salt retention in the proximal tubules of the kidneys (Rossi et al., 2020). The kidney injury process can be recognized when pro-inflammatory cytokines, particularly interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), are present. When IL-6 and TNF-α are present, injured cells can be moved to preserve homeostasis in pathological situations. In addition to being a pleiotropic cytokine that functions as a primary regulator of the inflammatory process, IL-6 is a pro-inflammatory cytokine that has biological activity as a regulator of the immune response, hematopoietic, and acute phase reactions (Alwahaibi et al., 2016). Antioxidants that protect and maintain the integrity of cell membranes can be employed to reduce the negative effects of dexamethasone on the kidneys and prevent cell degeneration (Akmal et al., 2024). According to recent studies, the body’s antioxidant defense can be strengthened by exogenous carotenoids-derived antioxidant supplementation (Bahonar et al., 2017; Hajizadeh-Sharafabad et al., 2022). Astaxanthin is a carotenoid antioxidant that has been extensively investigated lately (Lim et al., 2018). Exogenous antioxidants, such as astaxanthin, are necessary to combat oxidative stress because endogenous antioxidants are insufficient. Astaxanthin has the ability to combat oxidative damage, lipid peroxidation, and free radical digestion. Astaxanthin has an immunomodulatory function that raises the relative lymphocyte cell count and shows signs of possible impacts without causing any pathological alterations (Kuraji et al., 2016). The effectiveness of astaxanthin in this study was essential for investigating hematological profiles and the expression of IL-6 and TNF-α in the kidneys of albino rats using a dexamethasone poisoning model. Materials and MethodsAnimalsFor this investigation, a total of 25 male albino rats that were healthy and free of deformities were recruited. The room where they were reared had a 12-hour light-dark cycle and an average temperature of 27°C ± 2°C. The animals were provided with an ordinary feed and drinking water ad libitum. Rats were given 1 week to adapt to the laboratory setting before receiving a dose. Following the acclimation period, all albino rats were randomly assigned to five treatment groups with five replications: (C–) placebo, (C+) intramuscular injection of 0.75 mg/kg BW of dexamethasone, (T1) intramuscular injection of 0.75 mg/kg BW of dexamethasone and oral administration of 2 mg/kg BW of astaxanthin, (T2) intramuscular injection of 0.75 mg/kg BW of dexamethasone and oral administration of 6 mg/kg BW of astaxanthin, and (T3) intramuscular injection of 0.75 mg/kg BW of dexamethasone and oral administration of 12 mg/kg BW of astaxanthin. An oral dose of astaxanthin (Asthin Force®, PT SOHO Industri Pharmasi, Indonesia) was administered an hour after the intramuscular injection of a lethal dose of 0.75 mg/kg BW of dexamethasone (Dexamethasone®, PT Phapros Tbk, Indonesia) (Akmal et al., 2024). Each experimental animal received treatment at 8 am for 10 days on consecutive days. Euthanasia and Organ RemovalEuthanasia was performed ethically using the cervical decapitation method on the 11th day after the treatment period. After the animal lost consciousness, which was marked by decreased pupil reflex and respiratory rate, 1 ml of blood was withdrawn via intracardial puncture with vacutainer and ethylenediaminetetraacetic acid and then stored at 4°C until hematological evaluation. During abdominal surgery, the kidneys of the rats in each treatment group were removed. The rats were then cleaned and stored in 15% formalin for immunohistochemistry evaluation. Evaluation of the hematological profile and the cytokines IL-6 and TNF-αA hematology analyzer (Hitachi 902®, Roche Diagnostics, USA) was used to assess the hematology profile of blood samples after collection. In the meantime, the intensity of the brown color in the cells was used to quantify the expression of IL-6 and TNF-α (Table 1) (Hamid et al., 2022a). A trinocular microscope (Eclipse E200®, Nikon, Japan) was used to observe IL-6 and TNF-α expressions in five fields of view at 400× magnification. Statistical AnalysisThe mean ± SD format was used to represent all data. To analyze the data distribution, the Kolmogorov–Smirnov normality test was used. A one-way ANOVA was used to analyze continuous variables with parametric distributions. The significance of the findings was assessed using post hoc Duncan’s comparison test. For multiple comparisons of nonparametrically distributed data, the post hoc Mann–Whitney and Kruskal–Wallis tests were used. For each analysis, the statistical software SPSS (IBM, USA) was used. p-values less than 0.05 were considered statistically significant. Ethical approvalThe current research was approved by the Universitas Airlangga Faculty of Veterinary Medicine’s Animal Care and Use Committee under Certificate No. 1.KE.104.08.2021. In order to prevent cruelty to animals and lessen the discomfort that the animals experienced throughout the study, this ethical approval was considered. Table 1. Immunohistochemistry scoring method for IL-6 and TNF-α.
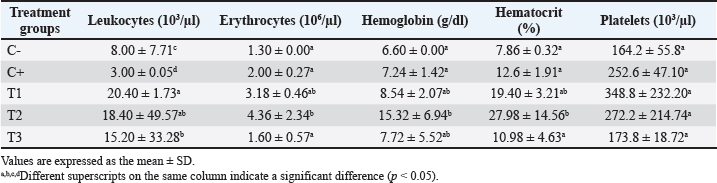
Table 2. Hematological profiles at the end of the study for all treatment groups.
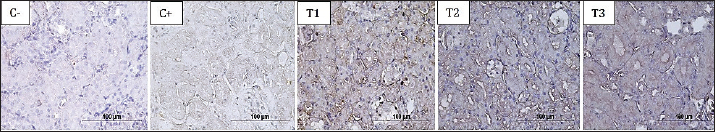
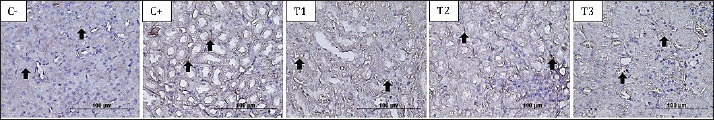
Fig 1. The brownish color gradation represents differences in IL-6 expression in the proximal tubule at 400× magnification in all treatment groups. ResultsEvaluation of the hematological profileBased on the hematology profile evaluation, the lowest number of leukocytes was found in group C–(3.00 ± 0.05d), whereas the highest number was found in group T1 (20.40 ± 1.73a). A gradual decrease in leukocytes was observed with increasing doses of astaxanthin from groups T1 to T3. Thus, it was revealed that group C–was significantly different from C+, T1, T2, and T3. The lowest erythrocyte level was found in group C–(1.30 ± 0.00a), whereas the highest was found in group T2 (4.36 ± 2.34b). The increase in leukocytes in group T2 then decreased with increasing dose of astaxanthin. Thus, group T3 was significantly different from T1 and T2 but not significantly different from C–and C+ (Table 2). In hemoglobin parameters, the lowest level was found in group C–(6.60 ± 0.00a) and the highest in group T2 (15.32 ± 6.94b). The increase in hemoglobin in group T2 decreased with the addition of an astaxanthin dose. Thus, group T2 was significantly different from C–and C+. The lowest hematocrit level was found in group C–(7.86 ± 0.32a), whereas the highest was found in group T2 (27.98 ± 14.56b). In addition, the increase in hemoglobin in T2 cells then decreased with the addition of astaxanthin dose. Thus, group T3 was not significantly different from groups C–, C+, and T1, but was significantly different from group T2. At the platelet level, the highest level was observed in group C–(164.2 ± 55.8a), whereas the highest level was observed in group T1 (348.8 ± 232.20a). However, no significant difference was observed in all treatment groups (Table 2). Evaluation of IL-6 and TNF-α cytokinesThe cytokines IL-6 and TNF-α were reported to be not significantly different between the T3 and T2 groups compared to C–. Meanwhile, groups T1, T2, and T3 were reported to be significantly different compared to C+, with a trend of decreasing IL-6 (Fig. 1) and TNF-α (Fig. 2) expression as the dose of astaxanthin increased. Thus, it can be highlighted that the T3 group decreased IL-6 and TNF-α expression in the dexamethasone toxicity model (Table 3).
Fig 2. The brownish color gradation represents the differences in TNF-α expression in the proximal tubule at 400× magnification in all treatment groups. Table 3. Expression of IL-6 and TNF-α in the proximal tubule in all treatment groups.
DiscussionHematology evaluations are crucial tests for evaluating immunity and physiology, and they can also reveal preclinical shifts in an animal’s internal balance (Purnama et al., 2022). An imbalance in an individual’s blood profile indicates an illness or blood function issue that may cause problems in other organs (Hamid et al., 2022b). In this study, group C + showed decreased leukocyte levels due to the immunosuppressive administration of dexamethasone, whereas administration of astaxanthin improved the condition of the animals with a decrease in leukocytes as the dose increased approaching group C–. Cytochrome P4503A is responsible for the liver’s metabolism of dexamethasone. Consequently, overindulgence may seriously impair liver function. The triggering of cytochrome P4503A can also affect some vital hepatocyte processes, such as glucose metabolism and oxidative xenobiotic metabolism (Jaber and Al-Bakri, 2024). Furthermore, excessive ingestion causes hepatocytes to store significant glycogen, leading to steatosis or glycogenesis and liver enlargement. High dosages of dexamethasone, on the other hand, have been linked to oxidative damage by increasing lipid peroxidation and reactive oxygen species (ROS) generation, which in turn inhibit antioxidant enzymes (Motafeghi et al., 2023). Dexamethasone increases ROS levels because inflammation in cells exposed to hazardous substances can activate nuclear factor-kappaB and macrophages, causing them to express pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1 (Solfaine et al., 2024). Leukocyte counts increase as a normal reaction to the body’s defense against microbial infection (Quaresma, 2019). The positive aspect of increased oxygen is that it makes it easier for nutrients to reach all tissues. All ROS with one unpaired free electron that were neutralized by exogenous antioxidants were the sources of the erythrocyte decline observed in this study. Because of oxygen infiltration, the treatment group’s erythrocyte count increased (Gwozdzinski et al., 2021). Anemia, hemorrhage, hemolysis, and starvation can all result in erythrocyte counts below physiologically normal ranges (Listyorini et al., 2021; Fadila and Kusumarini, 2024). According to the variables influencing this study, mice suffer from anemia as a result of drugs that interfere with the metabolism of carbohydrates, such as corticosteroid administration, vitamin B12 or folic acid insufficiency, and disruption of DNA nucleic acid synthesis (Wardhana et al., 2023). Erythrocyte maturation is affected by the interruption of DNA nucleic acid synthesis caused by corticosteroid use, which can result in vitamin B12 or folic acid deficiencies and cobalt insufficiency. The quantity of erythrocytes will be significantly affected by damage or disturbance to one of the processes involved in erythrocyte production. Clinical issues such as cancer, persistent infectious anemia, kidney illness, and long-term medication use all have an impact on decreased hemoglobin (Majid et al., 2023). Rats’ typical hemoglobin levels fall between 11.6 and 16.1 g/dl, according to earlier research (Kim et al., 2020). Other research, however, has shown that rats’ hemoglobin levels typically range between 11.1 and 18 g/dl (Topkan et al., 2019) and 12.8 and 15.7 g/dl (Houghton et al., 2020). Hemoglobin levels will increase in tandem with an elevated hematocrit. When an animal is under stress or discomfort, its erythrocytes, hemoglobin, and hematocrit will all rise. This is consistent with the study’s findings that the three components work together well (Seibel et al., 2021). The hematocrit value and the blood erythrocyte number were regarded as the same. The volume of blood cells relative to the volume of blood determines changes in hematocrit levels (Kristanto and Septiyani, 2023). Hematocrit values decreased across all treatment groups, according to the data. Anemia, or a shortage of blood, is characterized by a drop in hematocrit readings below normal. This can be caused by erythrocyte destruction, a reduction in the size or quantity of erythrocytes produced, or even a lack of nutrients necessary for blood formation. On the other hand, if the percentage number rises noticeably, blood viscosity will also rise, thereby slowing blood flow to the vessels (Sloop et al., 2020). Platelet counts typically range between 150 and 450 × 103/µl (Franciuc et al., 2022). Out of all the treatment groups, the C+ group had the greatest level of IL-6 cytokine expression. Possible factors that can cause increased levels of IL-6 cytokines include the loss of kidney function and its complications, such as oxidative stress caused by toxic substances, fluid retention, and susceptibility to infection (Hendrawan et al., 2023; Rao et al., 2023). Dexamethasone affects the majority of immune cell types and is crucial for controlling inflammatory and immunological responses. Dexamethasone can control monocyte and macrophage phenotype, survival, and functions; it has anti-apoptotic properties that help anti-inflammatory macrophages survive; it enhances macrophage phagocytic activity; it promotes neutrophil clearance; it suppresses the release of pro-inflammatory mediators such as cytokines, chemokines, and ROS; it controls dendritic cell maturation, survival, and migration to lymph nodes, as well as the function of dendritic cells (Khaleel et al., 2024). Dexamethasone inhibits transcription factors that regulate the production of pro-inflammatory cells and mediators, such as mast cells, dendritic cells, lymphocytes, eosinophils, and macrophages. Another important effect is the inhibition of phospholipase A2, which is responsible for the production of pro-inflammatory mediators. Dexamethasone inhibits the genes responsible for the expression of cyclooxygenase-2, iNOS, and pro-inflammatory cytokines. Concomitantly, glucocorticoids (GCs) produce an increase in lipocortin and annexin A1 and a subsequent reduction in prostaglandin and leukotriene synthesis. Dexamethasone suppresses transcription factors that control the generation of proinflammatory cells and mediators, including macrophages, mast cells, dendritic cells, lymphocytes, and eosinophils. The suppression of the enzyme phospholipase A2, which produces pro-inflammatory mediators, is another significant consequence. Dexamethasone inhibits the genes that produce pro-inflammatory cytokines, cyclooxygenase-2, and iNOS. The synthesis of prostaglandins and leukotrienes is subsequently decreased as GCs concurrently result in a rise in lipocortin and annexin A1 (Guţu et al., 2023). In addition, the degeneration process occurring in the kidneys can be observed in the presence of pro-inflammatory cytokines, namely TNF-α. The degree of inflammation is reflected in TNF-α levels; the higher the TNF-α levels, the more severe the inflammation, and the lower the TNF-α expression, the less inflammation there is in the organ (Hamid et al., 2018). The study findings demonstrated that when astaxanthin was administeredtothe T3 group, their TNF-αexpression was the lowest, followed by the C–group. On the other hand, the highest average TNF-α expression is found in the C+ group. There was a considerable deviation in the average expression of TNF-α along with the increase in the dose of astaxanthin. These findings support the hypothesis that the more astaxanthin is administered, the more effective the treatment of tissue damage will be (Alugoju et al., 2023). Previous research findings have also demonstrated that astaxanthin can reduce ROS by reducing oxidation in lipids, proteins, and DNA, increase the endogenous defense system (Pereira et al., 2021), and act as a chain breaker of initiation, propagation, and termination in the formation of ROS, thereby protecting against kidney disorders (Gu et al., 2022). In our perspective, a more comprehensive evaluation is needed to evaluate the efficacy of astaxanthin on liver function, antioxidant activity, and serum liver enzyme levels, which are other possible outcomes that can be improved after dexamethasone toxicity. However, the evaluation of hematology, IL-6, and TNF-α expression in this study may reveal the early efficacy of astaxanthin that can protect renal physiology in case of dexamethasone toxicity. ConclusionBased on the overall evaluation of the hematological profile, IL-6, and TNF-α cytokines in kidney, administration of 12 mg/kg BW astaxanthin can be an antidote to dexamethasone toxicity in albino rats. During the study period, astaxanthin ameliorated the leukocyte, erythrocyte, hemoglobin, and hematocrit levels. In addition, the expression of IL-6 and TNF-α cytokines in kidneys observed by immunohistochemistry, decreased gradually associated with the increase in astaxanthin dose. AcknowledgmentsThe authors thank the Rector of Universitas Airlangga for funding this study under the Novice Research Program grant number 212/UN3/2021. The authors also thank Universitas Airlangga’s deans of the faculties of veterinary medicine and health, medicine, and life sciences for providing facilities for the study. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThe authors acknowledge the Rector of Universitas Airlangga for providing fund support for this study through the Novice Research Program grant number 212/UN3/2021. Authors’ ContributionFF contributed to conceptualization, methodology, funding acquisition, and supervision. AM, MSP, DFQ, GFS, and MTEP contributed to the investigation, prepared medical treatment, and treated and euthanized animals. AM, GFS, and MSP evaluated the renal cytokine parameters. DFQ and FF evaluated the hematological profiles. STM, FF, and MTEP contributed to project administration, visualization, validation, data curation, writing, original drafts, and editing. All authors have read and approved the final manuscript. Data AvailabilityAll data were provided in the manuscript. ReferencesAkmal, T., Dina, C., Yudhana, A., Fikri, F., Purnomo, A., Maslamama, S. and Purnama, M.T.E. 2024. Protective effect of bajakah tampala (Spatholobus littoralis Hassk.) on dexamethasone-induced renal toxicity in albino rats. Pak. Vet. J. 44(2), 458–464. Alugoju, P., Krishna Swamy, V.K.D., Anthikapalli, N.V.A. and Tencomnao, T. 2023. Health benefits of astaxanthin against age-related diseases of multiple organs: a comprehensive review. Crit. Rev. Food Sci. Nutr. 63(31), 10709–10774. Alwahaibi, N.Y., Alissaei, H.K., Alshihi, S.A., Alabri, N., Albalushi, S.S. and Albalooshi, M. 2016. Serum levels of TNF-α, IL-6 and IL-10 in haemodialysis and renal transplant patients and in healthy subjects. Port J. Nephrol. Hypert. 30(3), 194–198. Bahonar, A., Saadatnia, M., Khorvash, F., Maracy, M. and Khosravi, A. 2017. Carotenoids as potential antioxidant agents in stroke prevention: a systematic review. Int. J. Prev. Med. 8(1), 70. Fadila, K.A. and Kusumarini, S. 2024. Diagnosis, hematologic profile, and treatment of cystoisosporiasis in domestic dog. J. Med. Vet. 7(1), 205–211. Franciuc, I., Matei, E., Aschie, M., Mitroi, A., Chisoi, A., Poinareanu, I. and Cozaru, G.C. 2022. Changes in platelet function in preterm newborns with prematurity related morbidities. Children 9(6), 791. Gu, J., Wang, X., Chen, Y., Xu, K., Yu, D. and Wu, H. 2022. An enhanced antioxidant strategy of astaxanthin encapsulated in ROS-responsive nanoparticles for combating cisplatin-induced ototoxicity. J. Nanobiotechnol. 20(1), 268. Guţu, I., Bachinsky, N.G. and Gudumak, V. 2023. The impact of imuheptin and imupurin on cytokine profile and antioxidant status in rat model of inflammation. Mold. J. Health Sci. 10(3), 18–24. Gwozdzinski, K., Pieniazek, A. and Gwozdzinski, L. 2021. Reactive oxygen species and their involvement in red blood cell damage in chronic kidney disease. Oxidative Med. Cell. Longevity. 2021(1), 6639199. Hajizadeh-Sharafabad, F., Zahabi, E.S., Malekahmadi, M., Zarrin, R. and Alizadeh, M. 2022. Carotenoids supplementation and inflammation: a systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 62(29), 8161–8177. Hamid, I.S., Aksono, E.B., Sukmanadi, M. and Purnama, M.T.E. 2018. Antiangiogenesis activity test of tin leaf (Ficus carica L.) on the number of blood vessels and VEGF expression of chorioallantoic membrane of embryonated chicken eggs. Eur. J. Oncol. Pharm. 1(4), e00007. Hamid, I.S., Ekowati, J., Solfaine, R., Chhetri, S. and Purnama, M.T.E. 2022a. Efficacy of probiotic on duodenal TNF-α expression and the histological findings in the liver and lung in animal model canine coronavirus. Pharmacogn. J. 14(3), 591–597. Hamid, I.S., Fikri, F., Purnama, M.T.E., Solfaine, R. and Chhetri, S. 2022b. Effects of Tithonia diversifolia on blood glucose levels, renal and pancreatic histopathology of Wistar rats: a model of diabetic nephropathy. Indian Vet. J. 99(11), 37–39. Hendrawan, V.F., Oktanella, Y., Firmawati, A. and Agustina, G.C. 2023. The Effect of black cumin (Nigella sativa) on histopathology of liver and kidney in albino rats with organophosphate exposure. J. Med. Vet. 6(1), 35–42. Hidayatik, N., Purnomo, A., Fikri, F. and Purnama, M.T.E. 2021. Amelioration on oxidative stress, testosterone, and cortisol levels after administration of Vitamins C and E in albino rats with chronic variable stress. Vet. World. 14(1), 137. Houghton, D.E., Koh, I., Ellis, A., Key, N.S., Douce, D.R., Howard, G. and Zakai, N.A. 2020. Hemoglobin levels and coronary heart disease risk by age, race, and sex in the reasons for geographic and racial differences in stroke study (REGARDS). Am. J. Hematol. 95(3), 258–266. Jaber, N.R. and Al-Bakri, N.A. 2024. The morphological and histopathological liver abnormalities caused by carbamazepine-induced injury in female albino mice. J. Biotechnol. Res. Center. 18(1), 105–118. Khaleel, Z.I., Abdulwahhab, I.G. and Genan, A. 2024. The effect of infection with Entamoeba histolytica on the level of some biological variables and histological changes in the liver. J. Biotechnol. Res. Center. 18(2), 94–102. Kim, I.S., Lee, B.K., Yang, P.S., Joung, B. and Kim, J.Y. 2020. Sex-based approach for the clinical impact of the increased hemoglobin on incident AF in the general population. Korean Circ. J. 50(12), 1095–1110. Kristanto, D. and Septiyani. 2023. Comparison of hematological levels of simmental-ongole crossbreed (SimPO) and ongole crossbreed (PO) cattle reared semi-intensively. J. Med. Vet. 6(2), 237–243. Kuraji, M., Matsuno, T. and Satoh, T. 2016. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J. Clin. Biochem. Nutr. 59(2), 79–85. Lim, K.C., Yusoff, F.M., Shariff, M. and Kamarudin, M.S. 2018. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 10(3), 738–773. Listyorini, L., Mustofa, I., Hernawati, T., Rimayanti, R. and Suprayogi, T.W. 2021. Honey can increase the length of the small intestinal villi in malnourished albino rats. J. Med. Vet. 4(2), 175–179. Majid, R.A., Gradia, R., Rosdianto, A.M. and Hidayatik, N. 2023. Hematological profile in dairy cattle with foot and mouth diseases in lembang, West Bandung. J. Med. Vet. 6(3), 381–389. Motafeghi, F., Mortazavi, P., Ghassemi-Barghi, N., Zahedi, M. and Shokrzadeh, M. 2023. Dexamethasone as an anti-cancer or hepatotoxic. Toxicol. Mech. Methods. 33(2), 161–171. Pereira, C.P.M., Souza, A.C.R., Vasconcelos, A.R. and Prado, P.S. 2021. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med. 47(1), 37–48. Purnama, M.T.E., Hendrawan, D., Wicaksono, A.P., Fikri, F., Purnomo, A. and Chhetri, S. 2022. Risk factors, hematological and biochemical profile associated with colic in Delman horses in Gresik, Indonesia. F1000Res. 10, 950. Quaresma, J.A.S. 2019. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin. Microbiol. Rev. 32(4), 10–1128. Rao, S.B.S., Widjiati, Lokapirnasari, W.P., Triakoso, N., Safitri, E., Kuncorojakti, S. and Proboningrat, A. 2023. Protective effects of Apis dorsata honey supplementation on kidney histopathology in mice with monosodium glutamate exposure. J. Med. Vet. 6(3), 374–380. Rossi, G.M., Regolisti, G., Peyronel, F. and Fiaccadori, E. 2020. Recent insights into sodium and potassium handling by the aldosterone-sensitive distal nephron: implications on pathophysiology and drug discovery. J. Nephrol. 33(3), 447–466. Seibel, H., Baßmann, B. and Rebl, A. 2021. Blood will tell: what hematological analyses can reveal about fish welfare. Front. Vet. Sci. 8, 616955. Sloop, G.D., De Mast, Q., Pop, G., Weidman, J.J. and St Cyr, J.A. 2020. The role of blood viscosity in infectious diseases. Cureus 12(2), e7090. Solfaine, R., Fikri, F., Maslamama, S.T., Purnama, M.T.E. and Hamid, I. 2024. Exploring the protective efficacy of Lactobacillus acidophilus against interleukin-1beta (IL-1β) expression in mice induced with canine coronavirus vaccine. Adv. Anim. Vet. Sci. 12(4), 673–678. Stone, S., Malanga, G.A. and Capella, T. 2021. Corticosteroids: review of the history, the effectiveness, and adverse effects in the treatment of joint pain. Pain Physic. 24(S1), S233. Topkan, E., Ekici, N.Y., Ozdemir, Y., Besen, A.A., Yildirim, B.A., Mertsoylu, H. and Selek, U. 2019. Baseline hemoglobin< 11.0 g/dL has stronger prognostic value than anemia status in nasopharynx cancers treated with chemoradiotherapy. Int. J. Biol. Markers. 34(2), 139–147. Wardhana, A.W., Jihan, P. and Suwardani, G.W. 2023. Comparison of corticomedullary ratio and medullary rim sign in feline lower urinary tract disease recurrence in Persian breed. J. Med. Vet. 6(3), 447–450. Ye, Q., He, X.O. and D’Urzo, A. 2017. A review on the safety and efficacy of inhaled corticosteroids in the management of asthma. Pulmonary Ther. 3, 1–18. | ||
| How to Cite this Article |
| Pubmed Style Fikri F, Miftahurrohmah A, Pratama MS, Qosdina DF, Sya'ban GF, Maslamama ST, Purnama MTE. Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. Open Vet. J.. 2025; 15(6): 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 Web Style Fikri F, Miftahurrohmah A, Pratama MS, Qosdina DF, Sya'ban GF, Maslamama ST, Purnama MTE. Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. https://www.openveterinaryjournal.com/?mno=240862 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.23 AMA (American Medical Association) Style Fikri F, Miftahurrohmah A, Pratama MS, Qosdina DF, Sya'ban GF, Maslamama ST, Purnama MTE. Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. Open Vet. J.. 2025; 15(6): 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 Vancouver/ICMJE Style Fikri F, Miftahurrohmah A, Pratama MS, Qosdina DF, Sya'ban GF, Maslamama ST, Purnama MTE. Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 Harvard Style Fikri, F., Miftahurrohmah, . A., Pratama, . M. S., Qosdina, . D. F., Sya'ban, . G. F., Maslamama, . S. T. & Purnama, . M. T. E. (2025) Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. Open Vet. J., 15 (6), 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 Turabian Style Fikri, Faisal, Auliyauna Miftahurrohmah, Muhammad Sbastian Pratama, Danis Farid Qosdina, Gigih Fikrillah Sya'ban, Salipudin Tasil Maslamama, and Muhammad Thohawi Elziyad Purnama. 2025. Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. Open Veterinary Journal, 15 (6), 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 Chicago Style Fikri, Faisal, Auliyauna Miftahurrohmah, Muhammad Sbastian Pratama, Danis Farid Qosdina, Gigih Fikrillah Sya'ban, Salipudin Tasil Maslamama, and Muhammad Thohawi Elziyad Purnama. "Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity." Open Veterinary Journal 15 (2025), 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 MLA (The Modern Language Association) Style Fikri, Faisal, Auliyauna Miftahurrohmah, Muhammad Sbastian Pratama, Danis Farid Qosdina, Gigih Fikrillah Sya'ban, Salipudin Tasil Maslamama, and Muhammad Thohawi Elziyad Purnama. "Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity." Open Veterinary Journal 15.6 (2025), 2511-2517. Print. doi:10.5455/OVJ.2025.v15.i6.23 APA (American Psychological Association) Style Fikri, F., Miftahurrohmah, . A., Pratama, . M. S., Qosdina, . D. F., Sya'ban, . G. F., Maslamama, . S. T. & Purnama, . M. T. E. (2025) Astaxanthin improves hematology, interleukin-6, and tumor necrosis factor-α in the albino rats’ kidney model of dexamethasone toxicity. Open Veterinary Journal, 15 (6), 2511-2517. doi:10.5455/OVJ.2025.v15.i6.23 |