| Research Article | ||
Open Vet. J.. 2025; 15(7): 3285-3289 Open Veterinary Journal, (2025), Vol. 15(7): 3285-3289 Research Article Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancyMazin Talib Abbas1, Mayada S. Hassan*, Ali J. AL-Nuaimi2, Marwa Sabah majed2, Ameer Hameed Kadhim2 and Mustafa Ali Noor21Branch of Surgery and Obstetric Veterinary Medicine College, Al-Qasim Green University, Al Qasim, Iraq 2Branch of Physiology, Biochemistry and Pharmacology, University of Kerbala/College of Veterinary Medicine, Kerbala, Iraq *Corresponding Author: Mayada S. Hassan. Branch of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Kerbala, Kerbala, Iraq. Email: mayada.s [at] uokerbala.edu.iq Submitted: 04/02/2025 Revised: 12/06/2025 Accepted: 20/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
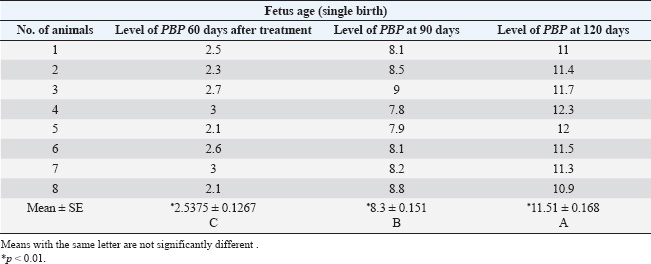
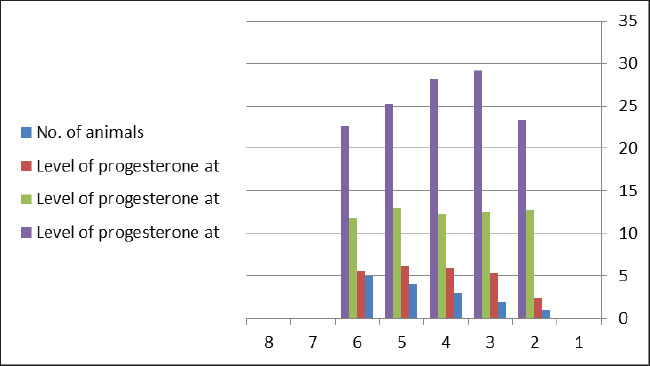
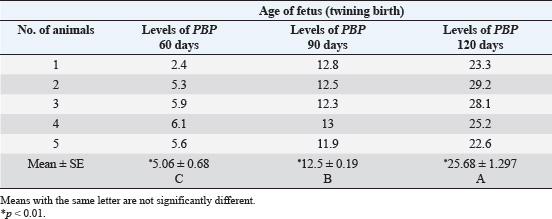
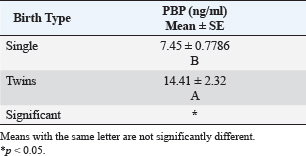
ABSTRACTBackground: The progesterone (PBP) hormone is responsible for maintaining pregnancy. By measuring its level, a single or twin pregnancy in deer can be diagnosed based on its blood PBP concentration. Aim: This study aimed to determine plasma blood PBP levels in Reem gazelles (Gazella subgutturosa marica). Methods: Thirteen pregnant females (G. subgutturosa marica) aged 2–4 years were used. The experiments were conducted from October 2021 to April 2022 in Babylon, Iraq. Jugular venipuncture was used to collect ten milliliters of EDTA-free blood samples. The samples were then centrifuged at 3,000 rpm for 10 minutes to separate the sera, which were then stored at −20°C in sterile test tubes for later analysis. The samples were then transferred to the central laboratory of the Faculty of Veterinary Medicine, Al-Qasim Green University. The electrochemiluminescence immunoassay, or “ECLIA,” was used to measure PBP levels in plasma. It is designed to be used with Elecsys and Cobas immunoassay analyzers. Result: The levels of PBP at 60 days of pregnant was (2.5375 ± 0.1267 ng/ml) less than those at days 90 (8.3 ± 0.151 ng/ml) and 120 days (11.51 ± 0.168 ng/ml) with significantly different (p < 0.01) between stages in the group of single fetuses. PBP concentrations within groups of twin-bearing animals at days (60, 90, and 120) were (5.06 ± 0.68, 12.5 ± 0.19, and 25.68 ± 1.297 ng/ml), respectively, with significantly different (p < 0.01). We found a significant difference (p < 0.05) between the two groups of pregnant females with single and twin fetus, and mean ± SE was (7.45 ± 0.7786, 14.41 ± 2.32 ng/ml), respectively. Conclusions: The current study describes how the content of PBP in the plasma blood of Reem gazelles (G. subgutturosa marica) can be utilized to predict whether a pregnancy is single or twin, as well as during various pregnancy periods. Keywords: Progesterone concentration, Reem gazelles, Single and twin fetus. IntroductionA gazelle is a member of the Cervidae family, which is distributed almost entirely throughout the world (Avent, 2008). Wilson and Reeder (2009) and Mallon (2011) found that the Arabian sand gazelle, Mongolian goitered gazelle, Persian goitered gazelle, and Yarkand gazelle are the four subspecies of goitered gazelle that have been recognized. Gazella subgutturosa marica was categorized as “endangered” by (Mallon, 2011) and as vulnerable by (Temple and Cuttelod, 2009), with a 30% decline in population size during the previous 10 years or three generations. Saudi Arabia accounts for 35% of the global population. Ovarian activity is regulated by sexual steroid hormones (Shahooth et al., 2015). The reproductive health of mammals depends on the levels of progesterone (PBP) (P4) in peripheral blood (Liu et al., 2002). Within a reproductive season, plasma P4 concentrations have been measured in some deer species, such as fallow deer (Asher, 1985; Asher et al., 1986, 1988), white-tail deer (Plotka et al., 1980), Pere Davids deer (Curlewis et al., 1988), Chita deer (Chapple et al., 1993), and red deer (Adam et al., 1985; Kelly et al., 1985; Asher et al., 1997, 2000, 2011; Garcia et al., 2002, 2003). The polyestrous nature of Rusa timorensis deer was demonstrated by the cyclical tendency of its P4 levels. In the mountain gazelle (Gazella gazella), fecal reproductive progestagen monitoring offers a non-invasive technique to monitor reproductive cycling, determining sexual maturity age, and identifying pregnancy (Mohammed et al., 2011). Fecal steroid methods offer several benefits, including the capacity to analyze lengthy datasets. Sexual behavior fluctuates in response to variations in the levels of reproductive hormones in the blood, reflecting variations in each animal’s reproductive physiology (Pickard et al., 2003). Regarding plasma PBP in (G. S. marica), there is no information available. Materials and MethodsThirteen pregnant females (G. subgutturosa marica) aged 2–4 years were used). The experiments were conducted from October 2021 to April 2022 in Iraq. Blood samples were collected by drawing 10 ml from the jugular vein without using EDTA, and the serum was separated using a centrifuge at 3,000 rpm for 10 minutes. Sera were kept in test tubes at −20°C. The electrochemiluminescence immunoassay, or “ECLIA,” was used to measure PBP levels in plasma. It is designed to be used with Elecsys and Cobas immunoassay analyzers. ▪ First incubation: When a (20 µl) sample is incubated with a biotinylated antibody specific for PBP, immune complexes are produced. The number of these complexes depends on the analytical concentration of the sample. ▪ Second incubation: The remaining sites for the antibiotic antibodies are occupied by creating an antibody-hapten combination by adding microparticles coated with streptavidin and a PBP derivative tagged with a ruthenium molecule streptavidin and biotin interact to immobilize the whole complex in the solid phase. The microparticles are guided toward the electrode surface by magnetic forces when the measurement cell is filled with the reaction mixture. Unbound materials are then eliminated with ProCell/ProCell M. Using a photomultiplier, the chemiluminescent emission produced by supplying a voltage to the electrode is then monitored. ▪ The results are obtained by integrating a master curve provided by the reagent barcode or e-barcode with an instrument-specific calibration curve generated through a 2-point calibration process. Statistical analysisThe Statistical Package for Social Scientists (SAS Version 9.1th ed.) and Microsoft Office Excel 2016 were used to examine the data. Ethical approvalUnder the reference number UOK.VET. PH.2025.120, this research was carried out in the anatomical laboratory of the College of Veterinary Medicine at the University of Kerbala – Iraq. ResultsAll 13 females were pregnant at the same time, approximately ±5 days different between it. Plasma PBP concentration differed from female to anther depending on the stage of pregnancy and number of births, Table 1 and Figure 1 show significantly different (p < 0.01) with means ± SE (2.5375 ± 0.1267, 8.3 ± 0.151, and 11.51 ± 0.168) between eight females at 60, 90, and 120 days, respectively, all these females deliver one kids. Five females in the current study had plasma PBP levels at days 60, 90, and 120 of pregnancy with means ± SE (5.06 ± 0.68, 12.5 ± 0.19, and 25.68 ± 1.297) that were significantly different (p < 0.01), and the females were delivered twins; this information is shown in (Table 2, Fig. 2). Table 3 shows a significant difference (p < 0.05) between the first group (single birth) and second group (twining birth), with means ± SE was (7.45 ± 0.7786) in the single birth group and (14.41 ± 2.32) in the twin birth group. DiscussionPBP levels in the first trimester of pregnancy with single and twining births in the current study were 2.5375 ± 0.1267 and 5.06 ± 0.68 ng/ml, respectively. These results are generally comparable to those of other deer species. The results show that PBP concentrations reached their peak 7–14 days following estrus, differing on species: Red deer (Adam et al., 1985; Kelly et al., 1985; Asher et al., 1997) have 2–7.5 ng/ml, chital deer (Chapple et al., 1993) have 5–8 ng/ml, and fallow deer have 3–8 ng/ml (Asher, 1985; Asher et al., 1986, 1988); and white-tailed deer have 3–8 ng/ml (Plotka et al., 1980). Because the corpus luteum is growing in anticipation of pregnancy or may already be pregnant, the concentration of PBP during the first trimester is comparable to the levels of PBP during the luteal phase. Table 1. Plasma PBP concentration at different stage of pregnancy with single birth.
Fig. 1. PBP levels during days 60, 90, and 120 of pregnancy (single birth). Table 2. Plasma PBP concentrations at different stages of pregnancy with twin births.
The level of PBP hormone in other deer species during the period close to the occurrence of estrus was (0.78 ± 0.20 ng/ml) according to (Mahre et al., 2013). In the middle period of pregnancy, the level of PBP was (8.3 ± 0.151, 12.5 ± 0.19 ng/ml) in the Iraqi Reem gazelle with the single and the twin fetus. This difference between the level of PBP in a single and a twin pregnancy is the result of the existence of one (CL) in a single gestation and the presence of two CL as a result of two ovulations, the result in the current study is comparable to (Mahre et al., 2013), which discovered that PBP levels in the blood rose 6–12 times in different species of deer, when the corpus luteum is completely functioning, from the lowest basal levels during estrous to the maximum peak.
Fig. 2. PBP levels during days 60, 90, and 120 of pregnancy (twining birth). Table 3. Plasma PBP concentrations according to birth type (single and twining), means ± SE.
Around days 120 of pregnancy, the P4 concentrations were (11.51 ± 0.168, 25.68 ± 1.297 ng/ml), in single fetus and twin bearing animals, respectively, in these results agreement with (Mahre et al., 2013). In twin-bearing animals, the PBP concentration increase was greater. ConclusionsThe PBP level in the plasma blood of Reem gazelles (G. subgutturosa marica) is described in the current work. This level can be used to identify the kind of pregnancy, whether twin or single, and during the various stages of pregnancy. AcknowledgmentsI would like to thank the professionals of the Department of Surgery and Obstetrics at the Veterinary Medicine College, Al-Qasim Green University, Iraq and the Department of Physiology, Biochemistry, and Pharmacology in the College of Veterinary Medicine, University of Kerbala. Conflict of interestThe authors declare that they have no competing interests. FundingThis research received no external funding. Authors’ contributionsAll authors contributed to this work equally. Data availabilityAll data were provided in the manuscript. ReferencesAdam, C.L., Moir, C.E. and Atkinson, T. 1985. Plasma concentrations of proges-terone in female red deer (Cervus elaphus) during the breeding season, pregnancy and anoestrus. J. Reprod. Fertil. 74, 631–636. Asher, G.W. 1985. Estrous cycle and breeding season of fallow deer, Dama dama. J. Reprod. Fertil. 75, 521–529. Asher, G.W., Barrell, G.K. and Peterson, A.J. 1986. Hormonal changes around estrus of farmed fallow deer, Dama dama. J. Reprod. Fertil. 78, 487–496. Asher, G.W., Peterson, A.J. and Watkins, W.B. 1988. Hormonal changes during luteal regression in farmed fallow deer, Dama dama. J. Reprod. Fertil. 84, 379–386. Asher, G.W., Scott, I.C., Archer, J.A., Ward, J.F. and Littlejohn, R.P. 2011. Seasonal luteal cyclicity of pubertal and adult red deer. J. Anim. Reprod. Sci. 125, 138–147. Asher, G.W., Scott, I.C., O’neill, K.T., Mockett, B.G. and Fisher, M.W. 2000. Genetic influences on the reproduction of female red deer. J. Anim. Reprod. Sci. 59, 43–59. Asher, G.W., Scott, I.C., O’neill, K.T., Smith, J.F., Inskeep, E.K. and Townsend, E.C. 1997. Ultrasonographic monitoring of antral follicle development red deer (Cervus elaphus). J. Reprod. Fertil. 111, 91–99. Avent, T. 2008. Dominance in a mixed-species deer exhibit at ZSL Whipsnade zoo. A study into supplementary feeding methods to create greater equality of access. M.Sc. Thesis, Imperial College, London, UK. Chapple, R.S., English, A.W. and Mulley, R.C., 1993. Characteristics of theolatrous cycle and duration of gestation in chital hinds., Axis axis. J. Reprod. Fertil. 98, 23–26. Curlewis, J.D., Loudon, A.S.I. and Coleman, A.P.M. 1988. Estrous cycles and the breeding season of the Pere David’s deer hind, Elaphurus davidi. J. Reprod. Fertil. 82, 119–126. Garcıa, A.J., Landete-Castillejos, T., Garde, J.J. and Gallego, L., 2002. Reproductive seasonality in the female Iberian red deer. J. Theriogenol. 58, 1553–1562. Garcia, A.J., Landete-Castillejos, T., Gomez-Brunet, A., Garde, J.J. and Gallego, L. 2003. Characteristics of the estrous cycle of Iberianred deer assessed by progesterone profiles. J. Exp. Zool. 298A, 143–149. Kelly, R.W., McNatty, K.P. and Moore, G.H. 1985. Hormonal changes aboutoestrus in female red deer. In Biology of Deer Production, Bulletin 22. Eds. Fennessy, P.F. and Drew, K.R.. Wellington, New Zealand: The Royal Society of New Zealand, pp: 181–184. Liu, B.T., Cheng, S.P., Huang, M.C. and Yu, J.Y.L. 2002. Serum progesterone changes in luteal cyclicity and duration of estrous cycle in Formosan Sika Deer. Zoolog. Sci. 19(9), 1033–1037. Mahre, M.B., Wahid, H., Rosnina, Y., Jesse, F.F.A., Azlan, C.A. and Yap, K.C. 2013. Plasma progesterone changes and length of estrous cycle in Rusa Deer (Rusa timorensis). J. Anim. Reprod. Sci. 141, 148–153. Mallon, D.P. 2011. Global hotspots in the Arabian Peninsula. Zool. Middle East 54, 20. Mohammed, O.B., Green. D.I. and Holt, W.V. 2011. Fecal progesterone metabolites and ovarian activity in cycling and pregnant mountain gazelles (Gazella gazella). Theriogenology 75(3), 542–548. Pickard, A.R., Holt, W.V., Green, D.I., Cano, M. and Abáigar, T. 2003. Endocrine correlates of sexual behavior in the Mohor gazelle (Gazella dama mhorr). Horm. Behav. 44(4), 303–310. Plotka, E.D., Seal, U.S., Verme, L.J. and Ozoga, J.J. 1980. Reproductive steroids deer III Luteinizing hormone, estradiol, and progesterone aroundestrus. Biol. Reprod. 22, 576–581. Shahooth, M.A., Zalzala, S.J. and Hatif, S.A. 2015. Study of reproduction in the local reem gazelle (Gazella subgutturosa marica). World J. Pharm. Sci. 3(5), 787–1024. Temple, H.J. and Cuttelod, A. 2009. The status and distribution of mediterranean ,ammals. The IUCN Red List of Threatened Species, 32. Gland, Switzerland; and Cambridge, UK: IUCN, pp: 8. Wilson, D.E. and Reeder, D.M. 2009. Mammal species of the world: a taxonomic and geographic reference. Baltimore, MD: JHU Press. | ||
| How to Cite this Article |
| Pubmed Style Abbas MT, Hassan MS, Al-nuaimi AJ, Majed MS, Kadhim AH, Noor MA. Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. Open Vet. J.. 2025; 15(7): 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 Web Style Abbas MT, Hassan MS, Al-nuaimi AJ, Majed MS, Kadhim AH, Noor MA. Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. https://www.openveterinaryjournal.com/?mno=240991 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.39 AMA (American Medical Association) Style Abbas MT, Hassan MS, Al-nuaimi AJ, Majed MS, Kadhim AH, Noor MA. Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. Open Vet. J.. 2025; 15(7): 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 Vancouver/ICMJE Style Abbas MT, Hassan MS, Al-nuaimi AJ, Majed MS, Kadhim AH, Noor MA. Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 Harvard Style Abbas, M. T., Hassan, . M. S., Al-nuaimi, . A. J., Majed, . M. S., Kadhim, . A. H. & Noor, . M. A. (2025) Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. Open Vet. J., 15 (7), 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 Turabian Style Abbas, Mazin Talib, Mayada S. Hassan, Ali J. Al-nuaimi, Marwa Sabah Majed, Ameer Hameed Kadhim, and Mustafa Ali Noor. 2025. Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. Open Veterinary Journal, 15 (7), 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 Chicago Style Abbas, Mazin Talib, Mayada S. Hassan, Ali J. Al-nuaimi, Marwa Sabah Majed, Ameer Hameed Kadhim, and Mustafa Ali Noor. "Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy." Open Veterinary Journal 15 (2025), 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 MLA (The Modern Language Association) Style Abbas, Mazin Talib, Mayada S. Hassan, Ali J. Al-nuaimi, Marwa Sabah Majed, Ameer Hameed Kadhim, and Mustafa Ali Noor. "Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy." Open Veterinary Journal 15.7 (2025), 3285-3289. Print. doi:10.5455/OVJ.2025.v15.i7.39 APA (American Psychological Association) Style Abbas, M. T., Hassan, . M. S., Al-nuaimi, . A. J., Majed, . M. S., Kadhim, . A. H. & Noor, . M. A. (2025) Effect of twin or singleton pregnancy on progesterone levels in the blood of Reem gazelles (Gazelle subgutturosa marica) at different stages of pregnancy. Open Veterinary Journal, 15 (7), 3285-3289. doi:10.5455/OVJ.2025.v15.i7.39 |