| Research Article | ||
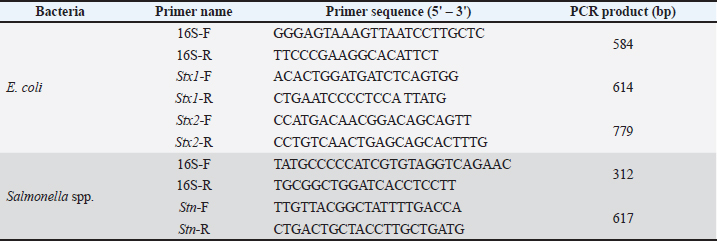
Open Vet. J.. 2025; 15(6): 2355-2364 Open Veterinary Journal, (2025), Vol. 15(6): 2355-2364 Research Article Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxin-carrying bacteria: In vitro and pathogenicity assessment in chickensPhan Vu Hai1, Nguyen Xuan Hoa1 and Hoang Thi Anh Phuong2*1Faculty of Animal Husbandry and Veterinary Medicine, College of Agriculture and Forestry, Hue University, Hue, Vietnam 2Faculty of Animal Husbandry and Veterinary Medicine, Tay Nguyen University, Buon Ma Thuot, Vietnam *Corresponding Author: Hoang Thi Anh Phuong. Faculty of Animal Husbandry and Veterinary Medicine, Tay Nguyen University, Buon Ma Thuot, Vietnam. Email: htaphuong [at] ttn.edu.vn Submitted: 08/03/2025 Revised: 07/05/2025 Accepted: 17/05/2025 Published: 30/06/2025 This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License ( AbstractBackground: Antibiotic resistance in Escherichia coli and Salmonella spp. poses a significant threat to poultry health and productivity. Overuse of antibiotics in poultry farming has led to the emergence of multidrug-resistant (MDR) pathogens, which have contributed to food safety concerns and limited treatment options. Natural antimicrobial alternatives, particularly herbal agricultural products such as purple onions and chives, have gained interest as sustainable solutions. Furthermore, fermentation with probiotic bacteria enhances the antimicrobial properties of botanical extracts, improving their efficacy against pathogenic bacteria. Aim: This study aimed to evaluate the antibacterial efficacy of fermented purple onion (FPO) and fermented chive (FC) extracts against E. coli and Salmonella spp., both causative agents of diarrhea, in broiler chickens. The research also compared their effectiveness with conventional antibiotics used in poultry farming. Methods: We fermented fresh purple onion and chive extracts using Lactobacillus plantarum and Bacillus subtilis for 72 hours under anaerobic conditions. Pathogenic E. coli (22) and Salmonella spp. (9) were isolated from 30 diarrheal chicken fecal samples and identified through biochemical tests, in vivo virulence testing in chicks, polymerase chain reaction detection of toxin genes (Stx1, Stx2, and Stn), and 16S rRNA sequencing. The antibacterial activity of the fermented extracts was evaluated using disk diffusion assays, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) tests. The bioactive compound content, including phenolics, flavonoids, and sulfur compounds, was quantified using an aluminum chloride colorimetric assay to assess their contribution to antimicrobial efficacy. Results: In vivo virulence assessment of chickens revealed that 4 E. coli strains (18.2%) and 5 Salmonella strains (26.3%) induced diarrhea. Of these, 2 E. coli strains (50%) harbored the Stx1 toxin gene, and all 5 Salmonella strains (100%) carried the Stn gene. 16S rRNA gene sequencing identified 2 E. coli strains and 4 Salmonella strains. All bacterial isolates exhibited MDR, particularly beta-lactam and macrolide antibiotics. Both fermented extracts exhibited significant antibacterial activity against MDR E. coli and Salmonella spp., with FC exhibiting superior efficacy compared to FPO. The inhibition zones of FC (12.5–17.6 mm) were significantly larger than those of FPO (10.1–16.8 mm), particularly against E. coli FG31-1 and Salmonella pullorum NCTC10705. The MIC and MBC values indicated that FC had a stronger bactericidal effect, requiring lower concentrations to inhibit and kill bacterial growth. FC contained higher levels of flavonoids and allicin, which were correlated with its enhanced antibacterial properties. Conclusion: FPO and FC, particularly FPO, show strong antibacterial potential as natural alternatives to antibiotics in poultry farming. Their ability to inhibit MDR pathogens suggests their role in reducing antibiotic dependency and improving poultry health. Further, in vivo studies and commercial formulation strategies are required to validate the practical application of these formulations in the poultry industry. Keywords: Antibiotic alternative, Chive, E. coli, Fermentation, Purple onion, Salmonella spp.. IntroductionGastrointestinal diseases pose a significant challenge to the poultry industry, leading to losing weight gain, increasing feed conversion ratio, and mortality rates as well (Timbermont et al., 2011). Escherichia coli and Salmonella are major enteric pathogens, and their control is becoming increasingly difficult (Azam et al., 2019). While antibiotics have been used to combat these pathogens, their indiscriminate use has resulted in antibiotic residues in meat products and the appearance of antimicrobial pathogens, which have caused an extreme threat to animal and human health (Shivaramaiah et al., 2011). Therefore, there is an urgent need for alternative strategies to control pathogenic microorganisms and promote growth performance in poultry. Herbal medicines are a promising source of novel antimicrobial agents and can serve as alternatives to conventional antibiotics (Al-Mariri and Safi, 2014). Among Allium species, onions (purple onion and chive) are readily available agricultural products in the central region of Vietnam, offering a cost-effective solution. According to Ankri and Mirelman (1999), the primary bioactive compounds in onions are allicin and quercetin, which exert antibacterial effects by disrupting energy metabolism, compromising cytoplasmic membrane function, and inhibiting nucleic acid biosynthesis. In vivo studies have demonstrated that onion extract enhances the immune response and promotes growth in broiler chickens (Hai and Hoa, 2020). However, the pungent and spicy nature of raw onions probably irritates the digestive tract and affects their palatability to livestock. Fermented onions have been used traditionally in order to enhance flavor and extend shelf life. During the fermentation process, various antimicrobial compounds, such as bacteriocins, reuterin, diacetyl, carbon dioxide, ethanol, propionic acid, formic acid, acetic acid, and lactic acid, are produced and considered antimicrobial compounds (Gaggia et al., 2011). There are a variety of lactic acid bacteria (LAB) for fermenting onions, including Lactobacillus casei, Lactobacillus brevis, Lactobacillus malefermentans, Lactobacillus zymae, Lactobacillus paraplantarum, and Lactobacillus plantarum (Gardner et al., 2001; Kimoto-Nira et al., 2020). LAB not only inhibits the growth of harmful intestinal microorganisms but also enhances gut health by regulating the mucosal barrier, reducing inflammation, enhancing resistance to infections, and stimulating IgA secretion (Wan et al., 2016). The probiotic bacterium Bacillus subtilis is also gaining popularity in animal husbandry (Mingmongkolchai and Panbangred, 2018). Spore formation assists the probiotic bacterium with unfavorable conditions in the animal gastrointestinal tract. Our recent study successfully isolated L. plantarum 1582 (Hai et al., 2024a) and B. subtiis BSn5 (Hai et al., 2024b) from free-range native chicken feces, demonstrating bacteria inhibition, purple onion fermentation capability, and survivability in the chicken digestive tract. While fermented garlic has been widely studied, this research focuses on evaluating fermented purple onion (FPO) and fermented chive (FC) extracts, these particular probiotic strains, against well-characterized toxin-producing poultry pathogens (E. coli and Salmonella spp.), with the efficacy linked to the quantification of bioactive compounds—an area with limited prior exploration. Materials and MethodsMaterialsChives (Allium schoenoprasum: NCBI GenBank ID: NC_057575.1) and purple onions (Allium cepa L. var. aggregatum: NCBI GenBank ID: OR605568.1) were cultivated organically according to the VietGAP standard TCVN 11892-1:2017 in Dien Mon, Phong Dien district, Thua Thien Hue province, Vietnam. They were harvested after 4–5 months of growth. The bulbs were selected for quality, washed, and surface-sterilized by soaking in a 5% NaCl solution for 120 minutes, followed by UV irradiation at 30 mW/cm² for 15 minutes. The sterilized bulbs were then homogenized in a blender. They were then filtered through two layers of gauze to remove debris followed by centrifugation at 5,000 rpm in 15 minutes to remove insoluble particles, and sterile extracts were obtained for experiments. Lactobacillus plantarum 1582 (NCBI GenBank ID: MT597487.1) and B. subtilis BSn5 (NCBI GenBank ID: CP026662.1), each at a concentration of 109 CFU/g, were used as starter cultures for onion fermentation. These strains were isolated from the digestive tracts of native free-range chickens, as described by Hai et al. (2024a, b). Bacterial strains were maintained at −80°C in the freezer of the Microbiology Laboratory, Hue University, Vietnam. The fermentation process was adapted based on the reference of (Hai et al., 2024b) with some minor changes. Briefly, each probiotic strain was inoculated into 50 ml of 10% (w/v) skim milk, which was dissolved in sterile distilled water with 10 g of skim milk for 100 ml of sterile water, then incubated at 37°C within 48 hours. The probiotic cultures were then combined with sterilized purple onion or chive extract at a ratio of 1:20 (w/v), mixed thoroughly, and transferred to a Kuvings KGC-712CB fermentation vessel. Fermentation was performed anaerobically for 72 hours. The FPO extract and FC extract were stored at 4°C. MethodsIsolation and identification of Salmonella spp. and E. coliFresh fecal samples were collected from the rectums of diarrheic chickens, which showed clinical signs of diarrhea (watery feces, lethargy), using sterile cotton swabs, kept refrigerated, and transferred to the laboratory for processing on the same day. Briefly, for E. coli isolation, 200 μl of diluted fecal samples were evenly spread on MacConkey agar and incubated at 37°C for 24 hours. Subsequently, 3–5 lactose-positive colonies were inoculated onto Eosin Methylene Blue (EMB) agar. Presumptive E. coli colonies displaying a green metallic sheen on EMB agar were sub-cultured in Tryptic Soy Broth (TSB) for biochemical identification using E. coli ATCC 25922 as the reference strain (da Silva et al., 2018). For Salmonella isolation, 200 μl of diluted fecal samples were cultured in Rappaport-Vassiliadis Soya broth and incubated at 41.5°C within 24 hours. An amount of 10 μl of bacterium culture was then streaked on xylose lysine deoxycholate (XLD) agar and incubated at 37°C within 24 hours; presumptive Salmonella colonies from XLD agar plates were sub-cultured in TSB for biochemical identification (motility, sugar metabolism, indole production, H2S production, oxidase activity, catalase activity, and carbohydrate fermentation); Salmonella ATCC 25957 was used as the reference strain (TCVN 8400-12:2011). Identification of toxin genes and pathogenicity assessmentVirulence testing in chickens: Three-day-old Viet chicks were orally inoculated with an amount of 1 ml of a bacterial suspension at a concentration of 108 CFU. Each strain was tested in triplicate, using a total of 90 chickens. Control chickens (n = 30) received an amount of 1 ml of sterile distilled water and were housed in separate houses to prevent cross-contamination. We monitored chicks for 7 days post-infection for clinical signs, including diarrhea, weight loss, and other indicators of disease. Pathogenic strains were screened for the performance of Stx1 and Stx2 genes of E. coli and the Stn gene of Salmonella spp. using a DNA extraction kit (ABT Vietnam). The DNA was then used as a template for polymerase chain reaction (PCR) screening to detect the specified genes, as detailed in the PCR section (Table 1). PCR amplification and gene sequencingThe total volume of PCR reactions was 25 μl which consisted of 15.25 μl of nuclease-free water, 2 μl of template DNA, 0.5 μl of reverse primer (10 pmol/μl), 0.5 μl of forward primer (10 pmol/μl) and 6.25 μl of 2× GoTaq Green Master Mix (Promega). PCR amplification was conducted in a thermal cycler followed by the manufacturer’s instructions. PCR products were analyzed by electrophoresis on a 1% agarose gel in 1× TAE buffer at 100 V for 35 minutes. Gels were visualized and imaged using the Gel Doc system (Bio-Rad). For gene sequencing, target genes from diarrheagenic E. coli and Salmonella spp. (carrying toxin genes) were amplified using the oligonucleotide primer pairs listed (Table 1). The total volume of PCR reactions was 25 μl, including primers at a final concentration of 0.2 μM, 24 μl of a pre-made mixture containing Platinum Blue PCR SuperMix (Invitrogen, Carlsbad, CA), and 1 μl of crude DNA. The conditions of PCR cycling consist of 94°C in 2 minutes for initial denaturation, 92°C in 30 seconds for denaturation (40 cycles), 59°C in 30 seconds for annealing, 72°C in 30 seconds for extension, and 72°C in 5 minutes for final extension. The PCR products were then purified for sequencing. Sequencing was performed using the Sanger method at DNA Sequencing Ltd. Co. (Vietnam). Quantification of bioactive compounds of FPO and FC extractsThe Folin–Ciocalteu method quantifies total phenolics based on their ability to reduce the reagent under alkaline conditions, with measurements taken spectrophotometrically at 765 nm, using gallic acid as the standard reference (Georgé et al., 2005). Similarly, the aluminum chloride colorimetric assay measures flavonoids by forming a colored complex with aluminum chloride, with absorbance measured at 430 nm (Edeoga et al., 2005). Both assays were performed in triplicate for the final FPO and FC extracts to ensure reliable data for quantifying total phenolic (TPC) and flavonoid content (TFC) quantification. Antibiotic and extract susceptibility testingA disk diffusion method was used to determine antibiotic susceptibility of Salmonella spp. and E. coli isolates, following the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2024). Briefly, Salmonella spp. and E. coli were adjusted at 0.5 McFarland (108 CFU/ml), dipping a sterile cotton swab in suspension, then streaking over Mueller–Hinton (MH) agar plates, following the guidelines of CLSI, and incubating at 37°C within 16–20 hours. Table 1. Target genes, toxin genes of E. coli, and Salmonella spp. for PCR amplification.
Table 2. PCR detection of toxin-encoding genes of E. coli and Salmonella spp.
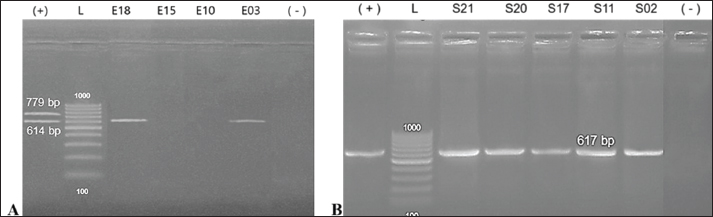
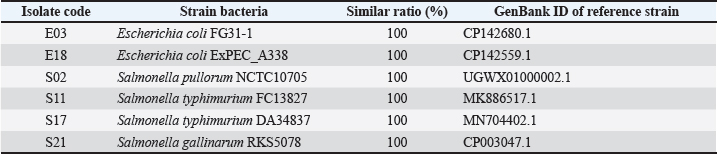
For herbal extract susceptibility testing, wells with diameters of 6 mm were created into MH agar plates inoculated with the bacterial cultures. Each extract (50 μl) was transferred into a well and incubated at 37°C within 24 hours. Measuring inhibition zones was conducted and compared with established standards for antibiotic and herbal extract sensitivity (Kumar et al., 2024). Experimented antibiotics included amoxicillin (30 μg), cefotaxime (15 μg), doxycycline (30 μg), amikacin (10 μg), ciprofloxacin (30 μg), ceftriaxone (10 μg), ofloxacin (10 μg), nitrofurantoin (15 μg), and spiramycin (30 μg) (CLSI, 2024). Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) valuesThe broth microdilution method was used to determine the MIC and MBC of onion and chive extracts. Each extract at an initial concentration of 500 mg/ml was serially two-fold diluted to achieve a concentration range of 250–0.988 mg/ml. Nine sterile test tubes were used for each extract. One tube served as a negative control (extract only), and another tube containing tetracycline served as a positive control. A volume of 1 ml of the bacterial suspension was transferred to each tube, and incubated at 37°C for 24 hours. The MIC value was identified at the lowest concentration of each extract that prevented visible bacterial growth compared with the control group. For MBC values, all observed tubes without visible growth of bacteria were sub-cultured on Tryptic soy agar and incubated at 37°C within 24 hours. The MBC value of each extract was identified at the lowest concentration without forming bacterial colonies (CLSI, 1999). Statistical analysesAll data from the findings were analyzed with SPSS software (version 21.0) and were presented as mean values with standard deviation. Each assay was performed in triplicate, and bacterial counts were transformed into log10 CFU/ml (or CFU/g) for analysis. Pairwise t-tests were used to analyze significant differences between groups (FC or FPO) in biochemical content and pathogenic bacteria, with a statistical confidence interval of 0.05. Ethical approvalAll procedures involving chickens were conducted in accordance with the ethical standards and guidelines approved by the Animal Ethics Committee of Hue University, Vietnam (Approval No.: HUVNO39). ResultsIsolation of Salmonella spp. and E. coli from chicken fecesTwenty-four Salmonella spp. and 28 E. coli isolates were obtained from diarrheic chicken fecal samples based on characteristic colony morphology. Biochemical testing confirmed the identity of 22 out of 28 isolates (78.6%) as E. coli. These isolates were positive for lactose fermentation, methyl red, catalase, indole production, and nitrate reduction and negative for urease, H2S production, oxidase, citrate utilization, and Voges–Proskauer. Similarly, 19 out of 24 isolates (79.2%) were confirmed to be Salmonella. These isolates were negative for urease, phenylalanine deaminase, H2S production, gelatinase, nitrate reduction, motility, methyl red, Voges–Proskauer, indole production, citrate utilization, lactose fermentation, and oxidase activity (data not shown). In vivo virulence testing of Salmonella spp. and E. coliTo assess the virulence of the isolates, 3-day-old chicks were orally inoculated with 1 ml of bacterial suspension (108 CFU/chicken). This in vivo assay identified five virulent Salmonella spp. strains and four virulent E. coli strains capable of causing diarrhea in chickens (data not shown). Detection of toxin genes in E. coli and Salmonella spp.To further confirm the pathogenicity of the isolates, PCR was performed to detect the genes encoding toxins (Table 2, Fig. 1). Virulence genes (Stx1 and Stx2) in isolated E. coli were detected using multiplex PCR. The results showed that of the 4 E. coli isolates, strains E03 and E18 harbored the Stx1 gene, producing a characteristic band at 614 bp. In addition, all 5 Salmonella isolates were positive for Stn, which generated a specific amplicon at 617 bp. The virulent isolates were further identified using 16S rRNA gene sequencing. The sequencing results (Table 3) were compared with those from the NCBI GenBank database. The 4 Salmonella strains (S02, S11, S17, and S21) were identified as Salmonella pullorum strain NCTC10705, Salmonella typhimurium strain FC13827, S. typhimurium strain DA34837, and S. gallinarum strain RKS5078, with the corresponding GenBank IDs UGWX01000002.1, MK886517.1, CP029568.1, and CP003047.1, respectively. The Salmonella strain (S20) could not be definitively identified. The 2 E. coli strains (E03 and E18) were identified as E. coli FG31-1 and E. coli ExPEC_A338 with corresponding GenBank IDs CP142680.1 and CP142559.1, respectively, showing 100% sequence similarity.
Fig. 1. Electrophoresis results of multiplex PCR products for virulence genes. (A) E. coli: positive for the Stx1 gene (614 bp) in lanes E03 and E18. (B) Salmonella: positive for the Stn gene (617 bp) in lanes S02, S11, S17, S20, and S21. *Statistically significant differences (P < 0.05). Table 3. Identification of toxigenic bacterial strains using 16S rRNA gene sequencing.
Table 4. Antibiotic resistance profiles of pathogenic bacterial strains.
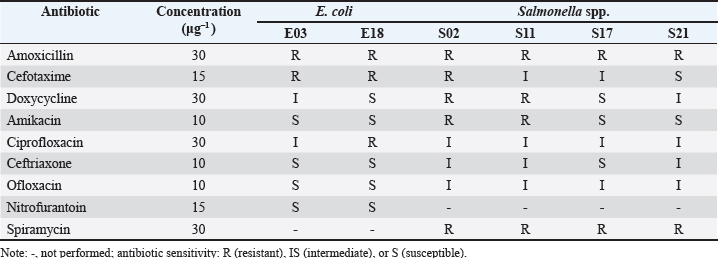
The antibiotic resistance status of pathogenic bacteriaThe E. coli and Salmonella isolates exhibited multidrug resistance (MDR), particularly for beta-lactam antibiotics (amoxicillin and cefotaxime) and macrolide antibiotics (spiramycin). However, various antibiotic resistance strains were recorded for both species. Escherichia coli isolates generally showed greater sensitivity to amikacin and nitrofurantoin than Salmonella spp. (Table 4).
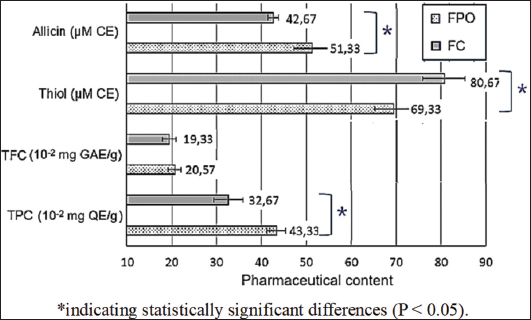
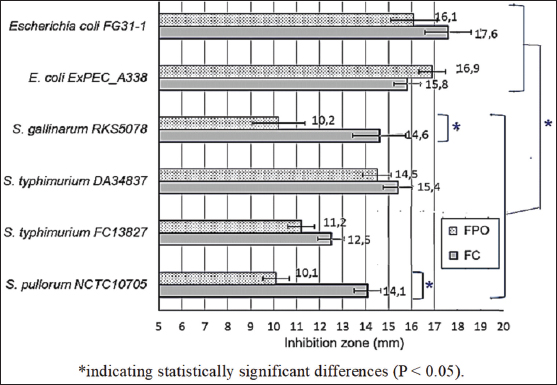
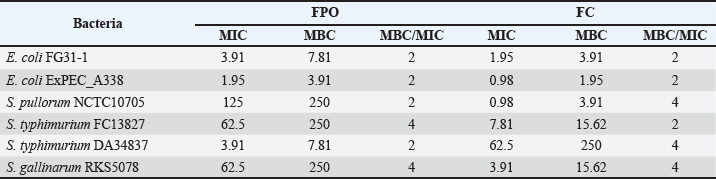
Fig. 2. Allicin, thiol, TFC, and TFC of FPO, and FC. Note: CE, chive extract; GAE, gallic acid equivalents; QE, quercetin equivalent. *Statistically significant differences (P < 0.05). Bioactive compound content and antibacterial activity of fermented onionsThe phenolic and flavonoid contents of the extracts from FPO and FC are shown in Figure 2. As shown in Figure 2, allicin and TPC are key components significantly affecting the antibacterial and antioxidant activities of onion and chive extracts. FC extract contained significantly higher levels of allicin (51.33 μM) and TPC (0.43 mg gallic acid equivalents/g) compared with FPO extract (42.67 μM allicin and 0.32 mg GAE/g) (p < 0.05). Furthermore, FC extract had a significantly higher thiol content (80.67 μM) than FPO extract (69.33 μM) (p < 0.05). The results are presented in Figure 3. Both extracts exhibited inhibitory effects against the tested pathogens (inhibition zone diameter, D > 10 mm), suggesting their potential as natural antibacterial agents in poultry production. However, FC exhibited superior efficacy compared to FPO against most bacterial strains. This was evidenced by the significantly larger (p = 0.036) inhibition zones produced by FC (12.5–17.6 mm) compared with FPO (10.1–16.8 mm) for most strains. Both FPO and FC showed good inhibitory activity against E. coli, with significantly (p < 0.05) larger inhibition zones (15.8–17.6 mm) than those observed for Salmonella spp. (10.1–15.4 mm). For Salmonella spp., the antibacterial activity varied significantly between strains and between the two fermented onion extracts. FC exhibited significantly stronger inhibition (14.1 mm; p = 0.008) than FPO (10.1 mm) against S. gallinarum RKS5078 and S. pullorum NCTC10705. MICs and MBCs of FPO and FC extractsTable 5 presents the MIC and MBC values of the fermented onion and chive extracts against pathogenic microorganisms. The concentrations of the extracts ranged from 0.98 to 250 mg/ml FC extract exhibited stronger antibacterial activity against most of the tested strains, as evidenced by its lower MIC and MBC values compared to FPO extract. Notably, FC had a significantly lower MIC (0.98–1.95 mg/ml vs. 1.95–62.5 mg/ml) against both E. coli strains and S. pullorum, highlighting its potent inhibitory effect against these bacteria. DiscussionThe findings of antibiotic resistance from pathogenic bacterial strains illustrated that Salmonella and E. coli are resistant to multi-antibiotics. These findings align with a recent study in Greece, which reported high resistance rates among E. coli isolates from broiler farms to streptomycin (70.8%), nalidixic acid (73.6%), and sulfamethoxazole (81.1%) (Xexaki et al., 2023). In contrast, the Salmonella isolates in our study exhibited varied resistance patterns. Salmonella strain S21, in particular, exhibited resistance to multiple drug classes, reflecting the global trend toward MDR in Salmonella, as highlighted by Alenazy (2022). A study in Vietnam reported an antibiotic resistance rate of up to 37.89% among Salmonella spp. isolated from chicken meat, with some strains resistant to as many as 11 different antibiotics (Yen et al., 2019). The relatively high rate of antimicrobial resistance in E. coli and Salmonella is likely due to the under-controlled use of various categories of antibiotics in poultry production, including those used for treatment, prevention, and growth promotion. The lack of legal restrictions on the use of antibiotics further exacerbates this issue.
Fig. 3. Inhibitory effects of FPO and FC on pathogenic bacteria. Table 5. MIC (mg/ml) and MBC (mg/ml) of FPO and FC against pathogenic bacteria.
Flavonoids and phenolic compounds exert antibacterial efficacy through disrupted bacterial cell membranes, resulting in the leakage of intracellular constituents, altering membrane permeability, and interfering with protein and nucleic acid synthesis (Cushnie and Lamb, 2005; Xie et al., 2015). The findings of this study are consistent with prior research demonstrating the antibacterial activity of fermented garlic extract (Bhatwalkar et al., 2021) and other fermented herbs (Ricci et al., 2021) against various pathogens such as E. coli and Salmonella. Ebrahimi Pure and Pure (2016) reported that kombucha-fermented garlic extract exhibited a larger inhibition zone (21.7 mm) against S. typhimurium PTCC 1596 and E. coli PTCC 1395 compared to garlic extracts fermented from vinegar (17.9 mm). Rouf et al. (2020) demonstrated the antibacterial efficacy of fermented garlic mainly derived from its sulfur compounds, particularly allicin. The enhanced antibacterial activity of FC observed in this study may be attributed to its relatively high TFC and allicin. Previous studies have established a strong correlation between plant-derived phenolic compounds and their antibacterial properties (Velioglu et al., 1998; Ranjan et al., 2012). The superior antibacterial activity of FC compared to FPO can likely be explained by changes in the sulfur compound profile, particularly allicin, during the fermentation process. This process likely enhances bioactive compounds, especially sulfur-based compounds such as allicin and thiols, which are known for their antimicrobial properties. Similar to garlic, water-soluble organic sulfur compounds such as S-allyl cysteine, S-methyl-L-cysteine, and S-allylmercapto-L-cysteine are produced during fermentation, as observed in the preparation of aged garlic extract (Rais et al., 2023). Additionally, fermentation conditions, such as the type of microorganism used and duration of fermentation, can influence the outcomes. An interesting detail is that fermentation not only enhances sulfur compounds but also produces organic acids, such as lactic acid, which creates a low pH environment that inhibits bacterial growth. However, as mentioned earlier, the primary effect of sulfur compounds, as they exert antibacterial effects through various mechanisms, including disruption of cell membranes, inhibition of protein or DNA synthesis, and interference with bacterial metabolism. Furthermore, the probiotic strains (B. subtilis and L. plantarum) used for botanical fermentation significantly contribute to the antibacterial efficacy of fermented plant extracts. Bacillus spp. producing bacteriocins, antimicrobial peptides may inhibit pathogenic growth through disrupted cell membranes and protein or DNA synthesis (Abriouel et al., 2011). Some Bacillus strains produce polymyxin and gramicidin, which are effective against various bacteria such as E. coli and Salmonella (Stein, 2005). Lactobacillus spp. exert antimicrobial efficacy through multiple mechanisms, including lactic acid being produced from carbohydrate fermentation, which had a lower pH level in comparison with the gastrointestinal environment, resulting in inhibiting bacterial growth (O’Shea et al., 2012). Additionally, Lactobacillus spp. could produce other antimicrobial constituents consisting of bacteriocins, hydrogen peroxide (Alvarez-Sieiro et al., 2016), and organic acids, such as propionic acid and acetic acid (Makras and De Vuyst, 2006). The MBC/MIC ratio is a valuable parameter for evaluating the efficacy of novel antimicrobial agents during drug development. This ratio allowed researchers to compare the bactericidal activity of different agents and select the most promising candidates (Balouiri et al., 2016). In this study, both fermented onion extracts effectively inhibited and killed E. coli strains, with an MBC/MIC ratio of 2, indicating a strong bactericidal effect at concentrations twice the MIC. For the remaining bacterial strains, the MBC/MIC ratios were ≤ 4, except for S. pullorum with FC extract (MBC/MIC=4). According to French (2006), an MBC/MIC ratio ≤4 is indicative of bactericidal activity, indicating that the agent not only inhibited bacterial growth but also effectively killed the bacteria. ConclusionThis study demonstrated the potential of FPO and FC extracts as viable antibiotic alternatives for combating Salmonella spp. and E. coli in broiler chicken. The research highlights that FC exhibits superior antibacterial efficacy compared with FPO, which is attributed to its higher flavonoid and allicin content. Both extracts demonstrated inhibitory effects against MDR bacterial strains, suggesting their utility in reducing antibiotic dependence in poultry farming. AcknowledgmentsThis project was supported by the Ministry of Education and Training, Vietnam (code number B2023-DHH-24. Conflict of interestThe authors declare no conflicts of interest. FundingThis study was supported by the Ministry of Education and Training, Vietnam, and the Russian Science Foundation (no. 22-16-00070). Authors’ contributionsExperiment design: P.V.H.., N.X.H., H.T.A.P.; Data analysis: P.V.H., N.X.H., H.T.A.P.; Administrative and technical support: P.V.H., N.X.H.; Drafting the manuscript: P.V.H., H.T.A.P. Data availabilityThe findings are provided upon request from the corresponding author. ReferencesAbriouel, H., Franz, C.M., Omar, N.B. and Gálvez, A. 2011. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35(1), 201–232. Alenazy, R. 2022. Antibiotic resistance in Salmonella: targeting multidrug resistance by understanding efflux pumps, regulators and the inhibitors. J. King Saud Univ. Sci. 34(7), 102275. Al-Mariri, A. and Safi, M. 2014. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran J. Med. Sci. 39(1), 36–43. Alvarez-Sieiro, P., Montalbán-López, M., Mu, D. and Kuipers, O.P. 2016. Bacteriocins of lactic acid bacteria: extending the family. Appl. Microbiol. Biotechnol. 100, 2939–2951. Ankri, S. and Mirelman, D. 1999. Antimicrobial properties of allicin from garlic. Microbes Infect. 1(2), 125–129. Azam, M., Mohsin, M. and Saleemi, M.K. 2019. Virulence-associated genes and antimicrobial resistance among avian pathogenic Escherichia coli from colibacillosis affected broilers in Pakistan. Trop. Anim. Health. Prod. 51, 1259–1265. Balouiri, M., Sadiki, M. and Ibnsouda, S.K. 2016. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 6(2), 71–79. Bhatwalkar, S.B., Mondal, R., Krishna, S.B.N., Adam, J.K., Govender, P. and Anupam, R. 2021. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 12, 613077. CLSI. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. Wayne, PA: Clinical and Laboratory Standards Institute. CLSI. 2024. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. 5th ed. CLSI standard VET01. Wayne, PA: Clinical and Laboratory Standards Institute. Cushnie, T.P. and Lamb, A.J. 2005. Antimicrobial activity of flavonoids. Inter. J. Antimicrob. Agents. 26(5), 343–356. da Silva, N., Taniwaki, M.H., Junqueira, V.C., Silveira, N., Okazaki, M.M. and Gomes, R.A.R. 2018. Microbiological examination methods of food and water: a laboratory manual. Boca Raton, FL: CRC Press. Edeoga, H.O., Okwu, D.E. and Mbaebie, B.O. 2005. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 4(7), 685–688. French, G.L. 2006. Bactericidal agents in the treatment of MRSA infections—the potential role of daptomycin. J. Antimicrob. Chemother. 58(6), 1107–1117. Gaggia, F., Di Gioia, D., Baffoni, L. and Biavati, B. 2011. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food. Sci. Technol. 22, S58–S66. Gardner, N.J., Savard, T., Obermeier, P., Caldwell, G. and Champagne, C.P. 2001. Selection and characterization of mixed starter cultures for lactic acid fermentation of carrot, cabbage, beet and onion vegetable mixtures. Int. J. Food. Microbiol. 64(3), 261–275. Georgé, S., Brat, P., Alter, P. and Amiot, M.J. 2005. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food. Chem. 53(5), 1370–1373. Hai, P.V. and Hoa, N.X. 2020. Effect of Allium schoenoprasum extract on immune status against Newcastle virus and growth performance of broiler chicken. Hue Univ. J. Sci. Nat. Sci. 4, 2058–2064. Hai, P.V., Khuong, N.D.T., Lai, N.H., Liem, T.N. and Hoa, N.X. 2024b. Optimizing fermentation conditions for purple onion (Allium cepa L.) using Bacillus subtilis BSn5 to produce products rich in carboxymethyl cellulase for poultry farming. TNU J. Sci. Technol. 230(2), 474–482. Hai, P.V., Phuong, H.T.A., Hung, P.H.S., Na, T.T., Lai, N.H., Khuong, N.D.T., Liem, T.N. and Hoa, N.X. 2024a. Selection of Lactobacillus strains from native chicken feces for the fermentation of purple onion (Allium cepa L.) as an antibiotic alternative against Salmonella spp. in chickens. Open Vet. J. 14(12), 3525–3538. Kimoto-Nira, H., Ohashi, Y., Amamiya, M., Moriya, N., Ohmori, H. and Sekiyama, Y. 2020. Fermentation of onion (Allium cepa L.) peel by lactic acid bacteria for production of functional food. Food. Meas. Charact. 14, 142–149. Kumar, A., Singh, B.R., SN, J.P., Kumar, S., Ahuja, D. and Singh, P. 2024. Study of antimicrobial efficacy of garlic oil loaded ectosome against clinical microbial isolates of diverse origin. J. Herb. Med. 43, 100824. Makras, L. and De Vuyst, L. 2006. The in vitro inhibition of Gram-negative pathogenic bacteria by bifidobacteria is caused by the production of organic acids. Int. Dairy J. 16(9), 1049–1057. Mingmongkolchai, S. and Panbangred, W. 2018. Bacillus probiotics: an alternative to antibiotics for livestock production. J. Appl. Microb. 124(6), 1334–1346. O’Shea, E.F., Cotter, P.D., Stanton, C., Ross, R.P. and Hill, C. 2012. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int. J. Food Microbiol. 152(3), 189–205. Pure, A.E. and Pure, M.E. 2016. Antioxidant, antibacterial and color analysis of garlic fermented in kombucha and red grape vinegar. Appl. Food Biotechnol. 3(4), 246–252. Rais, N., Ved, A., Ahmad, R., Kumar, M., Deepak Barbhai, M., Radha, Chandran, D., Dey, A., Dhumal, S., Senapathy, M., Deshmukh, V.P., Anitha, T., Balamurugan, V. and Lorenzo, J.M. 2023. S-Allyl-L-Cysteine-garlic bioactive: physicochemical nature, mechanism, pharmacokinetics, and health promoting activities. J. Funct. Foods 107, 105657. Ranjan, S., Dasgupta, N., Saha, P., Rakshit, M. and Ramalingam, C. 2012. Comparative study of antibacterial activity of garlic and cinnamon at different temperature and its application on preservation of fish. Adv. Appl. Sci. Res. 3(1), 495–501. Ricci, A., Bertani, G., Maoloni, A., Bernini, V., Levante, A., Neviani, E. and Lazzi, C. 2021. Antimicrobial activity of fermented vegetable byproduct extracts for food applications. Foods 10(5), 1107–1117. Rouf, R., Uddin, S.J., Sarker, D.K., Islam, M.T., Ali, E.S., Shilpi, J.A., Nahar, L., Tiralongo, E. and Sarker, S.D. 2020. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: a systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 104, 219–234. Shivaramaiah, S., Pumford, N.R., Morgan, M.J., Wolfenden, R.E., Wolfenden, A.D., Torres-Rodríguez, A., Hargis, B.M. and Téllez, G. 2011. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult. Sci. 90(7), 1574–1580. Stein, T. 2005. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 56(4), 845–857. Timbermont, L., Haesebrouck, F., Ducatelle, R. and Van Immerseel, F. 2011. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 40(4), 341–347. Velioglu, Y., Mazza, G., Gao, L. and Oomah, B.D. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food. Chem. 46(10), 4113–4117. Wan, M.L., Turner, P.C., Allen, K.J. and El-Nezami, H. 2016. Lactobacillus rhamnosus GG modulates intestinal mucosal barrier and inflammation in mice following combined dietary exposure to deoxynivalenol and zearalenone. J. Funct. Foods 22, 34–43. Xexaki, A., Papadopoulos, D.K., Alvanou, M.V., Giantsis, I.A., Papageorgiou, K.V., Delis, G.A., Economou, V., Kritas, S.K., Sossidou, E.N. and Petridou, E. 2023. Prevalence of antibiotic-resistant E. coli strains isolated from farmed broilers and hens in Greece, based on phenotypic and molecular analyses. Sustainability 15(12), 9421. Xie, Y., Yang, W., Tang, F., Chen, X. and Ren, L. 2015. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr. Med. Chem. 22(1), 132–149. Yen, H.X, Thuan, N.K. and Khai, L.T.L. 2019. Study on Salmonella spp. in chicken and environment from households at Vinh Long province. Can Tho Univ. J. Sci. 55(6), 1–6. | ||
| How to Cite this Article |
| Pubmed Style Hai PV, Hoa NX, Phuong HTA. Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. Open Vet. J.. 2025; 15(6): 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 Web Style Hai PV, Hoa NX, Phuong HTA. Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. https://www.openveterinaryjournal.com/?mno=241251 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.8 AMA (American Medical Association) Style Hai PV, Hoa NX, Phuong HTA. Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. Open Vet. J.. 2025; 15(6): 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 Vancouver/ICMJE Style Hai PV, Hoa NX, Phuong HTA. Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 Harvard Style Hai, P. V., Hoa, . N. X. & Phuong, . H. T. A. (2025) Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. Open Vet. J., 15 (6), 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 Turabian Style Hai, Phan Vu, Nguyen Xuan Hoa, and Hoang Thi Anh Phuong. 2025. Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. Open Veterinary Journal, 15 (6), 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 Chicago Style Hai, Phan Vu, Nguyen Xuan Hoa, and Hoang Thi Anh Phuong. "Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens." Open Veterinary Journal 15 (2025), 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 MLA (The Modern Language Association) Style Hai, Phan Vu, Nguyen Xuan Hoa, and Hoang Thi Anh Phuong. "Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens." Open Veterinary Journal 15.6 (2025), 2355-2364. Print. doi:10.5455/OVJ.2025.v15.i6.8 APA (American Psychological Association) Style Hai, P. V., Hoa, . N. X. & Phuong, . H. T. A. (2025) Fermented purple onion (Allium cepa L.) and chive (Allium schoenoprasum) bulb extracts as antibiotic alternatives against toxincarrying bacteria: In vitro and pathogenicity assessment in chickens. Open Veterinary Journal, 15 (6), 2355-2364. doi:10.5455/OVJ.2025.v15.i6.8 |