| Research Article | ||
Open Vet. J.. 2025; 15(6): 2750-2761 Open Veterinary Journal, (2025), Vol. 15(6): 2750-2761 Short Communication The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicinNabeel Mahdi Abed1, Hakeem Jawad Kadhim2*, Fadil Mohsen Hamed31Department of Physiology, College of Veterinary Medicine and Surgery, University of Shatrah, Alshatrah, Iraq 2Department of Microbiology, College of Veterinary Medicine and Surgery, University of Shatrah, Alshatrah, Iraq 3University Presidency, University of Shatrah, Al-Shatrah, Iraq * Correspondence to: Hakeem Jawad Kadhim. Department of Microbiology, College of Veterinary Medicine and Surgery, University of Shatrah, Alshatrah, Iraq. Email: hakim.jawad [at] shu.edu.iq Submitted: 06/02/2025 Revised: 12/05/2025 Accepted: 17/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
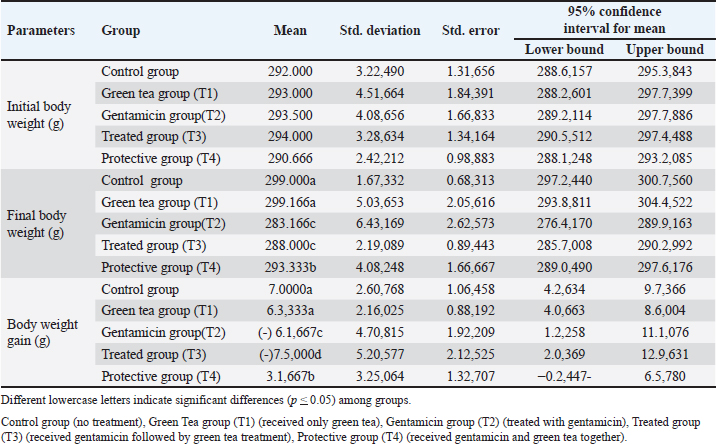
AbstractBackground: Green tea (GT), which is rich in polyphenolic compounds, has an extensive array of proactive preventive and therapeutic properties for the mitigation of various diseases, including acute kidney damage. This study hypothesized that GT attenuates gentamicin-induced renal damage by decreasing the levels of inflammatory biomarkers and exerting antioxidant properties. Aims: This experiment was designed to investigate the effects of gentamicin on kidney functions and to determine whether GT extract prevents or minimizes the adverse effects of gentamicin in male Sprague–Dawley rats. Materials and Methods: Five groups were formed: a control (untreated) group and four experimental groups. For 2 weeks, the control group received distilled water. In the first experimental group (T1), rats orally received GT extract (20 µg/g body weight) for 2 weeks. In the second group (T2), gentamicin (100 mg/kg body weight) was injected intraperitoneally for 1 week. In the third group (T3), rats were intraperitoneally injected with gentamicin for a week; then, an oral administration of GT extract. In the last group (T4), an intraperitoneal injection of gentamicin was administered, followed by an oral administration of GT extract for 2 weeks. Blood and kidney tissues were collected at the end of the treatment for biochemical and histological examinations. Results: The administration of gentamicin induced a decline in body weight and increases in the levels of inflammatory markers (kidney injury molecule I, tumor necrosis factor-alpha, and interleukin-6), urea, and creatinine, which are indicative of renal damage. Histopathological examination revealed extensive renal tissue damage. However, concurrent administration of gentamicin and GT diminished these parameters by decreasing the malondialdehyde concentration and improving the activity of antioxidant enzymes (glutathione peroxidase and superoxide dismutase). Furthermore, gentamicin injections caused nephrotoxicity, as evidenced by a reduction in body weight and higher levels of urea, creatinine, and inflammatory markers. Conclusion: GT mitigated the effects of gentamicin by improving the activity of antioxidant enzymes, resulting in a reduction in oxidative stress and minimization of inflammation. These findings suggest a potential complementary role for GT extract in the minimization of gentamicin-induced nephrotoxicity. Keywords: Green tea, Inflammatory markers, Renal function. IntroductionGentamicin is a broad-spectrum aminoglycoside antibiotic that is effective in treating various diseases caused by Gram-negative bacteria. However, it is limited by its nephrotoxic side effects, inflammation, oxidative stress, and cellular apoptosis of the kidney, causing damage to the kidney tissue (Mahi-Birjand et al., 2020). This accounts for approximately 60% of all toxicities (Campos et al., 2018), and 3.2% of the side effects are associated with renal tissues and cells (Pierson-Marchandise et al., 2017). It has been shown that approximately 29% of damage to the renal cells were caused by intravenous antibiotics, including vancomycin and gentamicin (Pierson-Marchandise et al., 2017; Slater et al., 2017). The nephrotoxicity parameters of gentamicin include increased plasma levels of creatinine and urea as well as significant necrosis in the renal tubules, leading to renal failure (Al-Timimi et al., 2019). Furthermore, studies have found that gentamicin increases the levels of reactive oxygen species (ROS) and mitigates antioxidant enzymes in renal tissue (Schmidlin et al., 2020). Notably, studies have shown that nephrotoxicity is mainly attributable to oxidative stress (Gonsalez et al., 2019). Green tea (GT) is well-known worldwide for its potent antioxidant properties and various health advantages, including improved heart health and reduced potential cancer risk. Its global popularity is bolstered by historical significance and widespread cultural integration. GT contains polyphenols, which comprise approximately 30% of the dry weight of GT. Polyphenols refer to three chemical substances: phenolic acids, flavonoids, and flavanols (Elzoghby et al., 2014). The active ingredient of GT, polyphenols, has anti-inflammatory and antioxidant properties. Specifically, the polyphenolic active substances in GT are epicatechin, epicatechin gallate, epigagallocatechin, and epigagallocatechin gallate (Jang et al., 2015). Extensive studies have highlighted the potential health advantages of GT, demonstrating its positive impact on many diseases, including heart disease, diabetes, and cancer (Basu and Lucas, 2008). Interestingly, several studies have explored the antioxidant function of GT, but the mechanism by which GT reduces oxidative stress remains unclear. Thus, the present study aimed to assess the impact of green tea extracts on the reduction of adverse effects of gentamicin in renal tissue. Specifically, inflammatory markers, oxidative stress, and renal tissue alterations will be evaluated in rats. Materials and MethodsAnimals and HousingThis research was conducted at the University of Shatrah/College of Veterinary Medicine and Surgery, during the period from 15/10/2023 to 15/12/2023. Thirty adult male rats weighing 285–295 g were used in this research. Rats were raised in an animal house at a temperature of 25°C ± 2°C and 12 hours of light/ dark for 15 days for adaptation before the experiments were initiated. The experimental rats were fed standard pellets and tap water and divided into five groups of six rats each. Specifically, one group was the control group, and the other four groups were the experimental groups. Note: The dose of green tea was 20 µg per gram of body weight (BW), and it was given orally by gavage and prepared in 1 ml of distilled water (DW). Gentamicin was injected intraperitoneally at a dose of 100 mg per gram BW. Furthermore, the experiment lasted for 2 weeks. The details of the groups as follows: Control group: It refers to untreated rats that received only DW. In this group, rats received 1 ml of DW orally and another ml of DW intraperitoneally (IP) for 2 weeks. Green tea group (T1): In this group, the animals were given green tea extract for 14 days. Gentamicin group (T2): Rats in this group were IP injected with gentamicin for 1 week. Treatment group (T3): Rats were administered both gentamicin and GT extract. Specifically, the gentamicin was IP injected for one week. GT extracts were administered for another week. Protective group (T4): The rats were treated with gentamicin and GT extract for 2 weeks. Collection of SamplesThe samples were collected at the end of treatment, which was continued for 2 weeks. In detail, rats were anesthetized using chloroform, and blood was drawn by heart puncture into 5 ml tubes without anticoagulants and centrifuged (3,000 rpm/15 minutes) to isolate serum, which was frozen at –20°C. Body Weight and Body Weight GainBW was measured on the pretreatment day and on the sampling day. Then, the BW gain was calculated by determining the differences between the pretreatment and final body weight [BW gain (g) = Final BW-pretreatment BW]. Measure the concentration of kidney injury molecule-I (KIM-I), tumor necrotic factor α (TNF-α), and interleukin 6 (IL-6) in the serum sampleThe sandwich ELISA method was utilized to measure KIM-I, TNF-α, and IL-6 (SunLong Biotech Co., LTD). This procedure involves several key steps. In detail, the microELISA strip plate was precoated with the first antibody specific for the target inflammatory markers (KIM-I, TNF-α, and IL-6). The samples or standards containing the target markers were then added to each well. Thereafter, horse-reddish peroxidase-conjugated antibodies for the inflammatory markers were added and incubated. After washing, the substrate was added, resulting in a blue color. Thereafter, a stop solution was applied, which caused the color to change from blue to yellow. Finally, the optical density (OD) was measured at 450 nm, and its values served as an indicator of the concentration of the inflammatory markers. The final concentration of the markers was measured by comparing the samples’ OD values with the standard curve that was produced using the OD values of the standard samples. Determining the Concentration of Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPX)MDA was utilized as an indicator of lipid peroxidation and evaluated according to a previously described method in detail (Yagi, 1998). An ELISA procedure was used to measure the serum concentration of SOD and GPX according to the manufacturer’s protocol (Elabscience Biotechnology Inc., China). Evaluate the Concentration of Urea, Creatinine, and Total ProteinUrea concentration was evaluated in the serum according to the manufacturer’s protocol (bioSystems, Spain; Burtis et al., 1996). Creatinine concentrations were quantified using a specific creatinine kit (BIOLABO SA, Maizy, France). Furthermore, Wu, (2006) described a protocol for measuring total protein in serum samples, which is a colorimetric method that utilizes a special kit (BIOLABO SA, Maizy, France). Statistical AnalysisData analysis was conducted using SigmaStat software 19.0 (SPSS Inc., Chicago, Illinois, USA, 2020). The data are presented as mean and standard deviation (M±SD). Prior to analysis, the normality of data distribution was assessed using the Shapiro-Wilk test. One-way ANOVA was then used to compare differences among groups, with a p-value < 0.05 was considered statistically significant. Additionally, post hoc comparisons were performed for all pairs using the Tukey–Kramer HSD (Honestly Significant Difference) test to identify significant differences between specific groups. Ethical ApprovalThe procedures used in the current study, including animal handling, treatment, anesthesia, and sampling, were reviewed and authorized by the members of the scientific committee at the College of Veterinary Medicine and Surgery, University of Shatrah. ResultsChanges in the Body Weight (BW) of Rats Treated With GentamicinData showed significant differences in the BW and BW gain in rats treated with gentamicin (Table 1). Specifically, the BW of rats in the green tea group (T1) did not show significant differences compared with that of rats in the untreated (control) group. However, BW was significantly affected by gentamicin injection (p Table. 1. Effects of green tea on body weight and weight gain in male rats administered gentamicin.
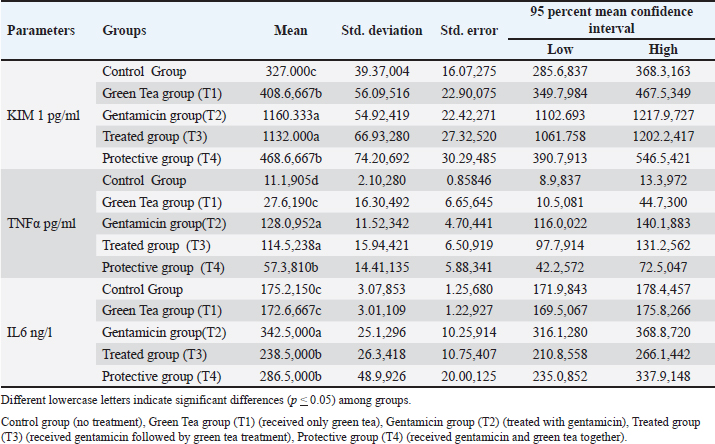
KIM-I, TNF-α, and IL-6 levelsAlterations of inflammatory markers were observed in the experimental groups compared with the untreated group (Table 2). In detail, an injection of gentamicin led to a significant elevation in KIM-I levels despite the oral administration of green tea compared with the control group (p Table. 2. Green tea’s effect on serum levels of KIM, TNFα and IL6 in male rats administered gentamicin.
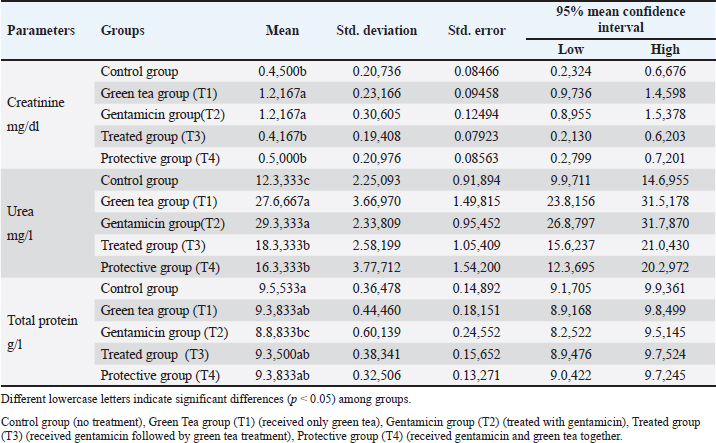
Urea, creatinine, and total protein levelsThe concentration of urea and creatinine was remarkably increased in the T1 (green tea) and T2 (gentamicin) groups compared with the other groups (Table 3; p Table. 3. Green tea’s effect on serum creatinine, urea and total protein levels in male rats administered gentamicin.
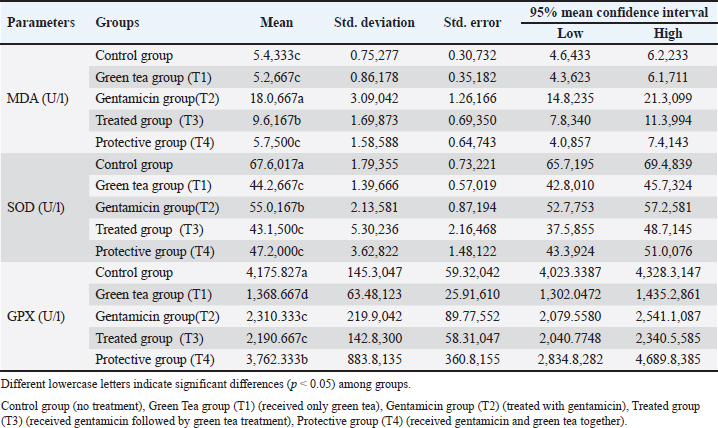
MDA, SOD, and GPX levelsThe MDA levels significantly changed among the experimental groups (Table 4). Specifically, the peak level of MDA was observed in the gentamicin (T2) group (p Table. 4. Effects of green tea on MDA, SOD and GPX in male rats administered gentamicin.
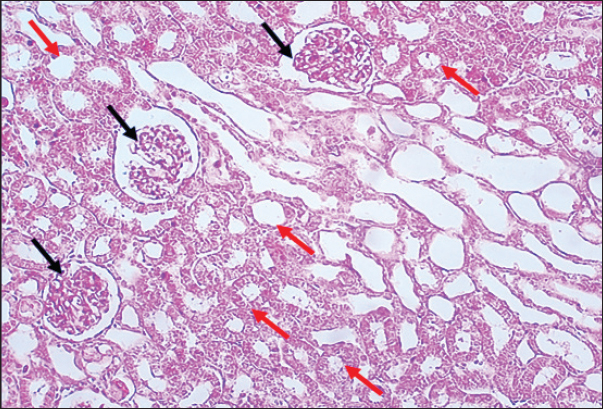
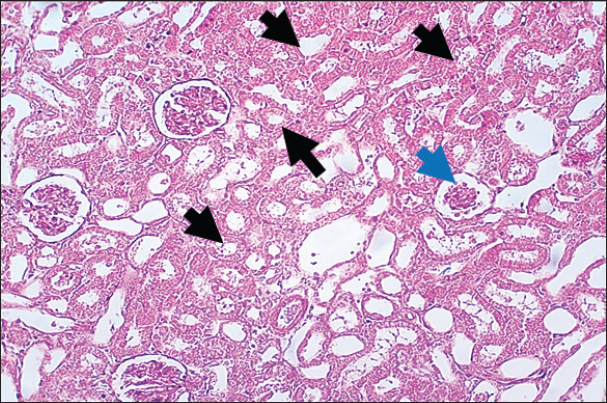
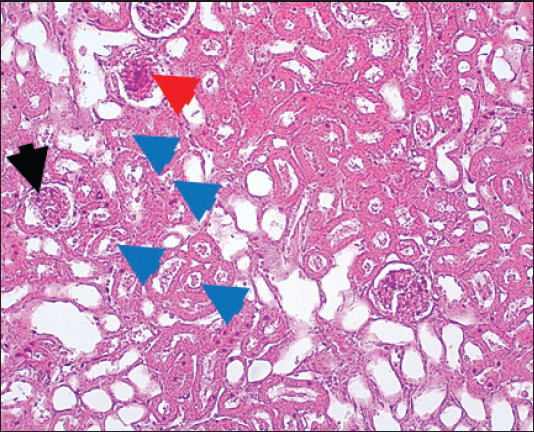
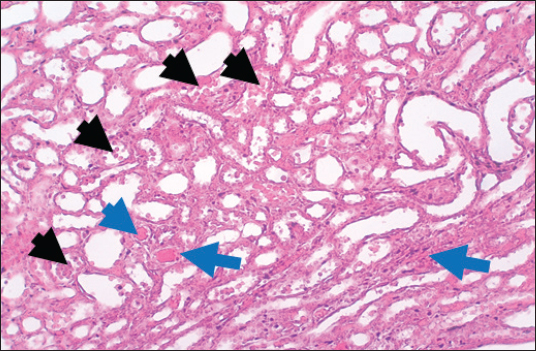
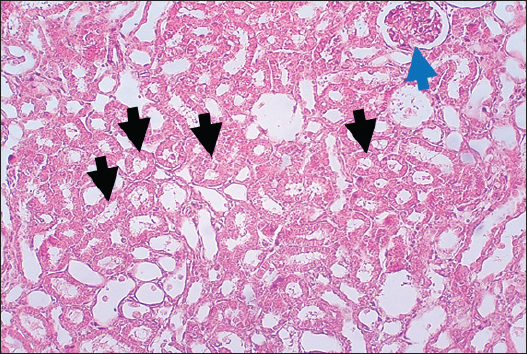
Results of Histopathological ExaminationIn the control group, histological analysis of kidney sections revealed intact glomeruli and renal tubules (Fig. 1). Conversely, Figure 2 presents a kidney section from the green tea group, which exhibited normal renal tubular epithelial cells, a well-preserved tubular lumen, and an overall normal tubular texture, although some glomeruli showed mild atrophy. The renal section of gentamicin showed atrophy of the glomerular tuft, expansion of the globular space, and significant hyperemia lesions in some glomeruli associated with substantial damage to the superficial cell layer (epithelial cells) in the proximal renal tubules, resulting in a reduction of the tubular lumen space (Fig. 3). In contrast, kidney sections from the gentamicin group that received green tea treatment revealed a marked reveal of both glomeruli and renal tubular epithelium, which appeared normal (Fig. 4 and Fig. 5).
Fig. 1. Rat Kidneys of control rat. The section shows the normal texture of renal tissue (glomeruli, Black arrows) and renal proximal tubules (Red arrows) without any significant occupied lesion. H and E (10X).
Fig. 2. Rat kidney treated with green tea extract the section shows normal renal tubular epithelial cells with normal tubular lumen (Black arrows), normal tubular texture, and mild atrophy can be seen in some glomeruli (Blue arrows). H and E (10X).
Fig. 3. Kidney of gentamicin group. The section shows clear glomerular tuft atrophy (Black arrow) with increasing of glomerular space and sever hyperemia lesion can be seen in some glomeruli (Red arrow). This section explains severe epithelial cells damage in the proximal renal tubules with decreased the tubular lumen space (Blue arrows). H and E (10X).
Fig. 4. Kidney of treatment group. The section shows severe hemorrhage (RBCS accumulation in the proximal renal tubules, Black arrows) with ahyperemic lesion in the section of renal tubules (Blue arrows).H and E (10X).
Fig. 5. Kidney of protective group. The section shows normal renal tubular epithelial cells with a normal tubular lumen (Black arrows). A normal tubular texture with mild atrophy can be seen in some glomeruli (Blue arrow). H and E (10X). DiscussionThe current study found that an injection of gentamicin reduced body weight and body weight gain in rats. In comparison, the body weight of the control and green tea groups remained stable, confirming the adverse effects associated with gentamicin treatment. The mechanisms underlying the impact of gentamicin on body weight are multifaceted and include toxic, nutritional, and metabolic effects. Gentamicin induces renal damage, which can disrupt the body’s ability to regulate fluids and minerals or may cause a loss of appetite, resulting in decreased food consumption and subsequent weight loss. Additionally, gentamicin can negatively affect metabolic processes, hindering weight gain, and/or disrupting the balance of gut microbes, thereby affecting nutrient absorption. Furthermore, the use of gentamicin can trigger an inflammatory response in the body, which could further contribute to weight loss. The findings of this study align with other studies (Mahmoud et al., 2021). A marked rise in BW was observed in rats treated with green tea along with gentamicin, which may be due to the antagonist’s effect of GT extract on the toxic effect of gentamicin and subsequent protection of the body from gentamicin toxicity. It is the fact that green tea contains catechins with anti-mutagenic, anti-cancer, and antioxidant properties. Therefore, GT plays a substantial role in the prevention of a number of diseases (Alschuler, 1998; Mukhtar et al., 2000; Khan et al., 2006). The administration of gentamicin, either alone or combined with GT extract, led to a remarkable elevation in the levels of inflammatory markers (KIM 1, TNFα, and IL-6), indicating a potent inflammatory response. In contrast, green tea treatment led to a moderate increase in these markers, yet their levels remained significantly lower than those observed in the T2 group (gentamicin-treated only). The findings of this study are consistent with results found in other publications (Quirós et al., 2011). Gentamicin, an aminoglycoside, is an antimicrobial antibiotic that is used to treat gram-negative bacterial infections (Karahan et al., 2005). However, the potential nephrotoxic effects of gentamicin restrict its widespread clinical application (Maldonado et al., 2003). Although KIM-I is typically undetectable in healthy kidneys; however, it is markedly overexpressed on the apical membrane of proximal tubule cells following ischemic injury and nephrotoxicity (Guo et al., 2012). Elevated levels of NGAL and KIM-I were shown to be associated with severe complications, including vascular damage and impaired functions of proximal tubules (Dobrek et al., 2016). Interestingly, the KIM-I expression in the renal tissues was evaluated to assess the influence of antioxidants on AKI in mice, and found that KIM-I levels in the experimental group were restored to the basal levels, closely resembling those in healthy mice (Ni et al., 2018). Also, using some antioxidants reduced acute renal damage, including tubular cell death and necrosis, and downregulated KIM-I in rats that had ischemia-reperfusion, which enhanced protection against AKI (Zhao et al., 2018). It is well-known that IL-6 has anti-inflammatory and regenerative properties (Scheller et al., 2011). Furthermore, studies have reported that treatment with gentamicin resulted in nephrotoxicity and elevated levels of INF-γ, IL-6, and TNF-α (Erjaee et al., 2015). Recent findings stated that green tea significantly reduced TNF-α levels but had no changes in the levels of C-reactive protein and IL-6 (Assis et al., 2024). The reduction of TNF-α levels was observed in overweight and obese individuals who were supplemented with green tea, supporting the idea that green tea may help decrease fat mass (Baladia et al., 2014; Haghighatdoost and Hariri, 2019), which in turn could influence TNF-α concentrations. It has been shown that gentamicin injected intraperitoneally leads to an increase in creatinine and urea and a decrease in total serum protein in the serum. The increased urea and creatinine levels may suggest renal damage, as evidenced by the data presented (Fig. 3). Studies have found that kidney damage is the primary factor contributing to the rise of urea and creatinine levels in the blood, which can also be influenced by various other factors, including dehydration, dietary intake, and the use of antidiuretic medications (Nwanjo et al., 2005). Additionally, Karunakar et al. (2024) documented an elevation of creatinine and urea levels in groups receiving gentamicin. In addition to the creatinine and urea results, histopathological analysis indicated that gentamicin caused significant renal tissue damage. Consistent with the findings of Vicente et al. (2013), we observed an increase in the urinary excretion of specific proteins in rats treated with gentamicin. Serum-derived proteins are typically excreted in higher quantities in renal diseases that alter the sieving function of the glomerular filtration barrier (Abd-Elrahman et al., 2014). Moreover, an elevation of the MDA levels and a reduction of SOD and GPX levels were observed in the gentamicin-treated rats (Table 4), which was associated with changes in the histological structure of renal tissue. Notably, gentamicin-induced nephrotoxicity was accompanied by a decrease in the activity of oxidative stress biomarkers (GSH-Px, CAT, and SOD) in the renal cortex and an increase in the MDA levels (Sahu et al., 2014). Furthermore, gentamicin can induce hepatotoxicity, which is caused by an imbalance in the redox system (increasing the ROS and decreasing antioxidant defense enzymes), which harms liver cells (Kharkheli et al., 2007). It is possible that biological membranes are damaged by ROS, free radicals, and high MDA levels produced during gentamicin metabolism. Tomşa et al. (2021), Moafa et al. (2023), and Aurori et al. (2023) reported the same results. MDA is often used as an indicator of lipid peroxidation and oxidative stress. Additionally, the body’s sweeping antioxidant capacity is challenged by excessive free radical generation, which prompts the production of uric acid as a compensatory mechanism to neutralize these radicals (Entedhar et al., 2018). The formation of ROS in the mitochondria of renal cortex cells, lipid peroxidation, and protein oxidation induced by gentamicin treatment could lead to both structural and functional deterioration of the kidney. Other studies have shown that ROS production is associated with a decline in the activity of endogenous antioxidant enzymes, including SOD, CAT, and GPx (Balakumar et al., 2010; Randjelović et al., 2017; Bencheikh et al., 2021). Therefore, the administration of gentamicin induces the generation of free radicals and inhibits the activity of these antioxidant enzymes, leading to significant damage to renal tissues in rats. In contrast, the administration of green tea combined with gentamicin in experimental rats resulted in recovery of serum MDA, SOD, and GPX levels. This effect may be due to the ability of green tea to stimulate antioxidant enzymes, including CAT, GPX, and SOD (Tsao, 2010). Furthermore, another study indicated that the oxidative stress-induced production of MDA was reduced following the administration of epigallocatechin-3-gallate, a key compound found in green tea (Gumay et al., 2018). Additionally, research has shown that GT exhibited a protective role against subacute toxicity and helped in the maintenance of antioxidant enzyme levels, such as CAT, SOD, and GSH (Khan and Kour, 2007). Recent publications have reported the same findings as those of the current work (Akter et al., 2024; Lamloum et al., 2024). AcknowledgmentsThe authors thank Dr. Amer M.A. for his essential support in animal handling and Dr. Hassan M.A. for his invaluable contributions to the histopathological study. Conflict of interestThe authors do not have any conflict of interest to declare. FundingThis work did not receive any specific support. Author’s contributionThe author, NMA, planned and conceptualized the research, conducted the animal experiments, performed biochemical and histopathological analyses, analyzed and interpreted the data, and wrote the first draft manuscript. HKJ and FMH wrote the second and final versions of the manuscript. The authors have approved the final draft of the manuscript. Data availabilityData supporting the findings are included in the article. ReferencesAbd-Elrahman, N.M., Farid, A.S. and Fararh, K.M. 2014. Effect of gentamicin and thioacetamide toxicity on serum proteins. Benha Vet. Med. J. 27(2), 456–465. Akter, S., Rafia, S., Huda, R., Haque, R., Paul, S., Sultan, M.T., Kawser, M. and Chowdhury, F.I. 2024. Green tea supplementation prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol- induced Swiss albino mice. Phytomed. Plus. 4(3), 100605; doi: 10.1016/j.phyplus.2024.100605 Alschuler, L. 1998. Green tea: healing tonic. J. Nat. Med. 5(1), 28–31. Al-Timimi, M.L.M., Hassan, E.S., Al-Nafakh, R.T., Alfadhul, S.A.L. and Mohammad, A.R. 2019. Biochemical study of the protective effect of vitamin C and radish on gentamicin-induced nephrotoxicity in rats. IJFMT. 13(4), 424; doi: 10.5958/0973-9130.2019.00327.X Assis, F.S. de O., Vasconcellos, G.L., Lopes, D.J.P., de Macedo, L.R. and Silva, M. 2024. Effect of green tea supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta- analysis. Prev. Nutr. Food Sci. 29(2), 106–117; doi: 10.3746/pnf.2024.29.2.106 Aurori, M., Andrei, S., Dreanca, A.I., Morohoschi, A.G., Cotul, M., Niculae, M., Nan, M.I., Codea,A.R. and Gal, A.F. 2023. The nephroprotective effect of cornelian cherry (Cornus mas L.) and rowanberry (Sorbus aucuparia L.) in gentamicin-induced nephrotoxicity on Wistar rats with emphasis on the evaluation of novel renal biomarkers and the antioxidant capacity in correlation with nitro-oxidative stress. Nutrients 15, 4392; doi: 10.3390/nu1520439 Baladia, E., Basulto, J., Manera, M., Martínez, R. and Calbet, D. 2014. Effect of green tea or green tea extract consumption on body weight and body composition: systematic review and meta- analysis. Nutr. Hosp. 29(3), 479–490; doi: 10.3305/ nh.2014.29.3.7118 Balakumar, P., Rohilla, A. and Thangathirupathi, A. 2010. Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmaco. Res. 62(3), 179–186; doi: 10.1016/j.phrs.2010.03.003 Basu, A. and Lucas, E.A. 2008. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 65(8), 361–375; doi: 10.1111/j.1753-4887.2007.tb00314.x Bencheikh, N., Bouhrim, M., Kharchoufa, L., Al Kamaly, O.M., Mechchate, H., Es-Safi, I., Dahmani, A., Ouahhoud, S., El Assri, S., Eto, B., Bnouham, M., Choukri, M. and Elachouri, M. 2021. The nephroprotective effect of Zizyphus lotus L. (Desf.) fruits in a gentamicin-induced acute kidney injury model in rats: a biochemical and histopathological investigation. Molecules. 26(16), 4806; doi: 10.3390/molecules26164806 Burtis, C.A., Ashwood, E.R., Aldrich, J.E. and Tietz, N.W. 1996. Tietz fundamentals of clinical chemistry, 4th ed. Philadelphia, PA: W.B. Saunders. Campos, M.A.A., de Almeida, L.A., Grossi, M.F. and Tagliati, C.A. 2018. In vitro evaluation of biomarkers of nephrotoxicity through gene expression using gentamicin. J. Biochem. Mol. Toxicol. 32(9), e22189; doi: 10.1002/jbt.22189 Dobrek, B., Skowron, A., Baranowska, A., Malska-Woźniak, P. and Thor, P. 2016. Urinary kidney injury molecule-1 (KIM-1) excretion in rats with experimental cystitis induced by oxazaphosphorines. Przegl. Lek. 73(11), 805–812. Elzoghby, R.R., Hamoda, A.F., Abed-Ftah, A. and Farouk, M. 2014. Protective role of vitamin C and green tea extract on malathion-induced hepatotoxicity and nephrotoxicity in rats. Am. J. Pharmacol. Toxicol. 9(3), 174–185; doi: 10.3844/ajptsp.2014.174-185 Entedhar, R.S., Sarhat, S., Wadi, S.A., Ahmed, M.S., Mustafa, S.N. and Sarhat, T.R. 2018. Evaluation of serum malondialdehyde, glutathione peroxidase, superoxide dismutase, and catalase levels in hormonal contraceptives. MJTU. 24(1), 10–20. Erjaee, H., Azma, F. and Nazifi, S. 2015. Effect of caraway on gentamicin-induced oxidative stress, inflammation and nephrotoxicity in rats. Vet. Sci. Dev. 5, 5896; doi: 10.4081/vsd.2015.5896 Gonsalez, S.R., Cortês, A.L., Silva, R.C. da, Lowe, J., Prieto, M.C. and Silva Lara, L. da. 2019. Acute kidney injury overview: from basic findings to new prevention and therapy strategies. Pharmacol. Ther. 200, 1–12; doi: 10.1016/j.pharmthera.2019.04.001 Gumay, A.R., Bakri, S. and Pudjonarko, D. 2018. The effect of green tea epigallocatechin-3-gallate on spatial memory function, malondialdehyde, and TNF-α level in D-galactose-induced BALB/C mice. Hiroshima J. Med. Sci. 67(2), 41–48. Guo , L., Takino, T., Endo, Y., Domoto, T. and Sato, H. 2012. Shedding of kidney injury molecule-1 by membrane-type 1 matrix metalloproteinase. J. Biochem. 152(4), 425–432; doi: 10.1093/jb/mvs077 Haghighatdoost, F. and Hariri, M. 2019. The effect of green tea on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Phytotherapy Res. PTR. 33(9), 2274–2287; doi: 10.1002/ptr.6432 Jang, M.Y., Lee, Y.L., Long, C.Y., Chen, C.H., Chuang, S.M., Lee, H.Y., Shen, J.T., Wu, W.J. and Juan, Y.S. 2015. The protective effect of green tea catechins on ketamine-induced cystitis in a rat model. Urol. Sci. 26(3), 186–192; doi: 10.1016/j.urols.2015.07.010 Karahan, I., Ateşşahin, A., Yilmaz, S., Ceribaşi, A.O. and Sakin, F. 2005. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology 215(3), 198–204; doi: 10.1016/j.tox.2005.07.007 Karunakar, K., Thanikachalam, V., Dhanalakshmi, S., Kesharwani, P. and Cheriyan, B. 2024. Hinokitiol attenuates gentamicin-induced nephrotoxicity by reversing oxidative stress and inflammation. Pharmacol. Res. Mod. Chin. Med. 10, 100410; doi: 10.1016/j.prmcm.2024.100410 Khan, N., Afaq, F., Saleem, M., Ahmad, N. and Mukhtar, H. 2006. Targeting multiple signaling pathways by green tea polyphenol (−) epigallocatechin-3-gallate. Cancer Res. 66(5), 2500–2505; doi: 10.1158/0008-5472.CAN-05-4382 Khan, S.M. and Kour, G. 2007. Subacute oral toxicity of chlorpyriphos and protective effect of green tea extract. Pestic. Biochem. Physiol. 89(2), 118–123; doi: 10.1016/j.pestbp.2007.04.005 Kharkheli, E., Kevanishvili, Z., Maglakelidze, T., Davitashvili, O. and Schacht, J. 2007. Does vitamin E prevent gentamicin-induced ototoxicity? Georgian Med. News. 146, 14–17. Available via https://pubmed.ncbi.nlm.nih.gov/17595452/ Lamloum, N.S., Soliman, H.A., Ahmed, R.R., Ahmed, O.M. and Zaky, M.Y. 2024.The modulation effect of green tea and pumpkin oils on hyperlipidemia, oxidative stress, and hematological abnormalities in an experimental multiple sclerosis rat model. Clin. Phytosci. 10(1), 6; doi: 10.1186/s40816-024-00365-y Mahi-Birjand, M., Yaghoubi, S., Abdollahpour- Alitappeh, M., Keshtkaran, Z., Bagheri, N., Pirouzi, A., Khatami, M., Sineh Sepehr, K., Peymani, P. and Karimzadeh, I. 2020. Protective effects of pharmacological agents against aminoglycoside- induced nephrotoxicity: a systematic review. Expert Opin. Drug Saf. 19(2), 167–186; doi: 10.1080/14740338.2020.1712357 Mahmoud, A.M., Abd El-Ghafar, O.A.M., Alzoghaibi, M.A. and Hassanein, E.H.M. 2021. Agomelatine prevents gentamicin nephrotoxicity by attenuating oxidative stress and TLR-4 signaling, and upregulating PPARγ and SIRT1. Life Sci. 278, 119600; doi: 10.1016/j.lfs.2021.119600 Maldonado, P.D., Barrera, D., Medina-Campos, O.N., Hernández-Pando, R., Ibarra-Rubio, M.E. and Pedraza-Chaverrí, J. 2003. Aged garlic extract attenuates gentamicin-induced renal damage and oxidative stress in rats. Life Sci. 73(20), 2543– 2556; doi: 10.1016/j.lfs.2003.08.035 Moafa, A., Aldossary, S.A., Al Mohaini, M. and Alsalman, A.J. 2023. Protective effect of aspirin against gentamicin-induced hepatotoxicity in rats model. Biomed. Pharmacol. J. 16(4), 2293–2298; doi: 10.13005/bpj/2805 Mukhtar, H. and Ahmad, N. 2000. Tea polyphenols: prevention of cancer and optimizing health. Am. J. Clin. Nutr. 71(6), 1698S–1702S; doi: 10.1093/ajcn/71.6.1698S Ni, D., Jiang, D., Christopher, J.K., Lai, J., Yan, Y., Todd, E.B., Yu, B., Im, H., Kang, L., Steve, Y.C., Liu, Z., Huang, P., Jonathan, W.E. and Cai, W. 2018. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 9(1), 5421; doi: 10.1038/s41467-018-07890-8 Nwanjo, H.U., Okafor, M.C. and Oze, G.O. 2005. Changes in biochemical parameters of kidney function in rats co-administered with chloroquine and aspirin. J. Clin. Sci. 23(1), 10–12; doi: 10.36478/rjmsci.2007.106.109 Pierson-Marchandise, M., Gras, V., Moragny, J., Micallef, J., Gaboriau, L., Picard, S., Choukroun, G., Masmoudi, K. and Liabeuf, S. 2017. The drugs that mostly frequently induce acute kidney injury: a case—noncase study of a pharmacovigilance database. Br. J. Clin. Pharmacol. 83(6), 1341–1349; doi: 10.1111/bcp.13216 Quirós, Y., Vicente-Vicente, L., Morales, A.I., López- Novoa, J.M. and López-Hernández, F.J. 2011. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol. Sci. 119(2), 245–256; doi: 10.1093/toxsci/kfq357 Randjelović, P., Veljković, S., Stojiljkovic, N., Sokolovic, D. and Ilic, I. 2017. Gentamicin nephrotoxicity in animals: current knowledge and future perspectives. EXCLI J. 16, 388–399; doi: 10.17179/excli2017-165 Sahu, B.D., Tatireddy, S., Koneru, M., Borkar, R.M., Kumar, J.M., Kuncha, M., Srinivas, R., Shyam Sunder, R. and Sistla, R. 2014. Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis, and inflammation in rats: possible mechanism of nephroprotection. Toxicol. Appl. Pharmacol. 277(1), 8–20; doi: 10.1016/j.taap.2014.02.013 Scheller, J., Chalaris, A., Schmidt-Arras, D. and Rose- John, S. 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. BBA, 1813(5), 878–888; doi: 10.1016/j.bbamcr.2011.01.034 Schmidlin, C.J., Dodson, M.B. and Zhang, D.D. 2020. Filtering through the role of NRF2 in kidney disease. Arch. Pharm. Res. 43(3), 361–369; doi: 10.1007/s12272-019-01177-2 Slater, M.B., Gruneir, A., Rochon, P.A., Howard, A.W., Koren, G. and Parshuram, C.S. 2017. Identifying high-risk medications associated with acute kidney injury in critically Ill patients: a pharmacoepidemiologic evaluation. Paediatr. Drugs. 19(1), 59–67; doi: 10.1007/s40272-016-0205-1 Tomşa, A.M., Răchişan, A.L., Pandrea, S.L., Benea, A., Uifălean, A., Parvu, A.E. and Junie, L.M. 2021. Accelerated lipid peroxidation in a rat model of gentamicin nephrotoxicity. Exp. Ther. Med. 22(5), 1218; doi: 10.3892/etm.2021.10652 Tsao, R. 2010. Chemistry and biochemistry of dietary polyphenols. Nutrients 2(12), 1231–1246; doi: 10.1016//2072-6643/2/12/1231 Vicente, L.V., Ferreira, L., Buitrago, J.M., Francisco, J., Hernández, L., López-Nova, J.M. and Morales, A.I. 2013. Increased urinary excretion of albumin, hemopexin, transferrin and VDBP correlates with chronic sensitization to gentamicin nephrotoxicity in rats. Toxicology 304, 83–91; doi: 10.1016/j. tox.2012.11.005 Wu, A.H.B. 2006. Tietz clinical guide to laboratory tests. 4th ed. Louis, MO: Saunders/Elsevier, pp: 1062–1065. Yagi K. 1998. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. (Clifton, N.J.), 108, 101–106; doi: 10.1385/0-89603-472-0:101 Zhao, M., Zhou, Y., Liu, S., Li, L., Chen, Y., Cheng, J., Lu, Y. and Liu, J. 2018. Controlled release of mitochondria-targeted antioxidant by injectable self- assembling peptide hydrogel ameliorates persistent mitochondrial dysfunction and inflammation after acute kidney injury. Drug Deliv. 25(1), 546–554; doi: 10.1080/10717544.2018.1498252 | ||
| How to Cite this Article |
| Pubmed Style Abed NM, Kadhim HJ, Hamed FM. The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. Open Vet. J.. 2025; 15(6): 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 Web Style Abed NM, Kadhim HJ, Hamed FM. The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. https://www.openveterinaryjournal.com/?mno=241380 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.43 AMA (American Medical Association) Style Abed NM, Kadhim HJ, Hamed FM. The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. Open Vet. J.. 2025; 15(6): 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 Vancouver/ICMJE Style Abed NM, Kadhim HJ, Hamed FM. The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 Harvard Style Abed, N. M., Kadhim, . H. J. & Hamed, . F. M. (2025) The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. Open Vet. J., 15 (6), 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 Turabian Style Abed, Nabeel Mahdi, Hakeem Jawad Kadhim, and Fadil Mohsen Hamed. 2025. The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. Open Veterinary Journal, 15 (6), 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 Chicago Style Abed, Nabeel Mahdi, Hakeem Jawad Kadhim, and Fadil Mohsen Hamed. " The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin." Open Veterinary Journal 15 (2025), 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 MLA (The Modern Language Association) Style Abed, Nabeel Mahdi, Hakeem Jawad Kadhim, and Fadil Mohsen Hamed. " The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin." Open Veterinary Journal 15.6 (2025), 2750-2761. Print. doi:10.5455/OVJ.2025.v15.i6.43 APA (American Psychological Association) Style Abed, N. M., Kadhim, . H. J. & Hamed, . F. M. (2025) The protective and therapeutic roles of green tea extract on body weight, inflammatory markers, renal function, oxidative stress, and kidney histopathology in rats treated with gentamicin. Open Veterinary Journal, 15 (6), 2750-2761. doi:10.5455/OVJ.2025.v15.i6.43 |