| Research Article | ||
Open Vet. J.. 2025; 15(6): 2492-2499 Open Veterinary Journal, (2025), Vol. 15(6): 2492-2499 Research Article Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, IraqKarrar Ali Mohammed Hasan Alsakini1*, Hind H. Al-Ammiri1 and Mustafa Mohammed Touma11University of Baghdad, College of Veterinary Medicine, Microbiology Department, Baghdad, Iraq * Corresponding Author: Karrar Ali Mohammed Hasan Alsakini, University of Baghdad, College of Veterinary Medicine, Microbiology Department, Baghdad, Iraq. Email: karrar [at] covm.uobaghdad.edu.iq Submitted: 14/02/2025 Revised: 10/05/2025 Accepted: 17/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
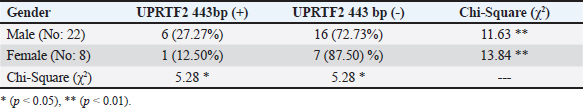
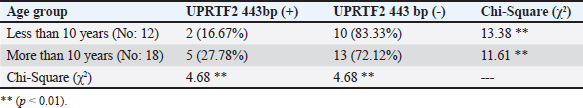
AbstractBackground: A prevalent contagious pathogenic parasite that can lead to major health issues is Toxoplasma gondii. Aim: The present study aimed to detect the parasitic immune response and the existence of genomic DNA in the blood of a T. gondii-positive equine. Methods: Thirty serum samples from horses suspected of having toxoplasmosis were collected from the Al-Rusafa neighborhood in Baghdad. To quantitatively investigate toxoplasma antibody levels in horse serum, an ELISA was used to evaluate immunoglobulin G (IgG) levels. Conventional (PCR) was used to identify T. gondii DNA. Results: The blood levels of IgG immunoglobulin in toxoplasma-infected horses differed significantly (p < 0.01) according to sex and age. Toxoplasma gondii-specific forward and reverse primers were generated using NCBI GenBank software. Toxoplasma genes were amplified using standard PCR. The proposed method can be used as a molecular diagnostic tool for detecting and comparing molecules using a ladder. Conclusion: The findings of this investigation were to ascertain whether the T. gondii genotype (UPRTF2 gene) is present. The size band of 443 bp DNA in the blood of toxoplasma-infected horses was confirmed using serological and molecular assessments. There were no statistically significant differences found by the Chi-square (χ2) test between the age groups or sexes of the seropositive and seronegative horses. Keywords: ELISA, Horse, PCR, Serum, Toxoplasma gondii. IntroductionToxoplasma gondii is a widely distributed intracellular protozoan that induces toxoplasmosis (Innes, 2010; Marzok et al., 2023; Selim et al., 2023). This parasite has been documented from every continent, although its prevalence varies significantly depending on environmental factors (Lass et al., 2019). Toxoplasma gondii primarily infects domestic cats and other members of the Felidae family, making it the primary parasitic host (Dabritz and Conrad, 2010). Only these hosts can release oocysts into the environment, thereby potentially contaminating pastures, food, and water. One cat can transmit approximately 100 million unsporulated oocysts, which become infectious within a period of 1–5 days (Schlüter et al., 2014). Most animals and humans serve as intermediate hosts (Dubey, 2009). Environmental factors and dietary practices can influence infection prevalence. As an illustration, consuming raw or undercooked meat is linked to the transmission of T. gondii, and pig and sheep meat are more susceptible to tissue cysts than cattle meat (Tenter et al., 2000; Tenter, 2009; Stelzer et al., 2019). Transmission of oocysts excreted in cat feces may not always require direct contact with the cat. Domesticated cats are less susceptible to contamination (and oocyst production) than feral or rural cats (Robert-Gangneux and Dardé, 2012; Olsen et al., 2019). Infection primarily occurs via three pathways: transplacental transmission, consumption of infected or undercooked meat, and ingestion of contaminated water (Buxton et al., 2007; Gebremedhin et al., 2014). However, milk from infected livestock is also a secondary source of infection (Tavassoli et al., 2013). In herbivores and equines, T. gondii infection primarily occurs through the ingesting of water or food contaminated with mature oocysts. However, infection can also be transmitted from mother to fetus through the transfer of rapidly multiplying tachyzoite across the placenta (Hill and Dubey, 2002; Tassi, 2007). Toxoplasma is a genus with only one species, T. gondii, which is classified into genotypes I, II, III, and XII and haplotypes X and A. Some species are reserved for wild animals (Attias et al., 2020). Domestication of horses occurred between 5,000 and 6,000 years ago, and since then, people have relied on horses for a wide variety of tasks, including transportation, hunting, warfare, agriculture, and entertainment. Further, horses have been consumed for sustenance since the late Paleolithic period, between 50,000 and 12,000 years ago (Stanciu, 2015; Li et al., 2020). Equestrian meat is still eaten by several nations. The average annual consumption in the EU is approximately 110,000 tones (FAO, 2013). Clinician management, epidemiological research, prevention, and control of toxoplasmosis in animals and humans relies on the diagnosis and genetic characterization of T. gondii (Liu et al., 2015; Gomes et al., 2020; Mohamed, 2020). Independent clinical manifestations are insufficient for the diagnosis of toxoplasmosis (Tenter et al., 2000). Nevertheless, it is difficult to detect and isolate the parasite because of its latent state, which is typically absent in the bloodstream (Robert-Gangneux and Dardé, 2012). Various biochemical, serological, histological, and molecular analyses are typically used to diagnose T. gondii (Pal et al., 2014). Toxoplasma gondii cysts that remain alive have been found in horse and donkey meat using a mouse bioassay (Al-Khalidi and Dubey, 1979; Dubey et al., 2020). The consumption of horse meat has also been linked to human clinical toxoplasmosis (Pomares et al., 2011). Additionally, there is considerable evidence that raw milk from seropositive donkeys consumed by humans could pose a risk of toxoplasmosis (Mancianti et al., 2014). Furthermore, evidence suggests a link between antibodies to T. gondii and clinical equine protozoal myeloencephalitis in horses (James et al., 2017; Schale et al., 2018). This is because scientific evidence shows that horses and other equids are among the most resistant domestic species to clinical toxoplasmosis (Dubey, 2021). In the United States, a horse died of toxoplasmosis (Kimble et al., 2021). Serological tests are the most efficient diagnostic tool for detecting T. gondii in farm animals, including horses. PCR techniques are specific, sensitive, and efficient for identifying parasite DNA in animal tissues (Almeria and Dubey, 2021). Indirect diagnostic approaches have drawbacks because they mostly indicate exposure to the parasite rather than the presence of a current infection. Serological tests are important for survey studies because they help identify animals positive for Toxoplasma and analyze risk factors associated with parasite exposure (Dubey, 2021). A recent global serological study was conducted to investigate the exposure of equids to T. gondii (Dubey et al., 2020). Unfortunately, evidence of the seroprevalence of T. gondii in horses across different regions of Baghdad is limited. This study aimed to assess the prevalence of T. gondii antibodies and identify genetic genes associated with this zoonotic protozoan in horses living in the Al-Rusafa region of Baghdad. Materials and methodsSample collectionThirty blood samples were collected for this study. Practitioners in the field made clinical diagnoses using samples taken from horses in the Al-Rusafa area of Baghdad city between October 2024 and December 2024. The ages of the horses ranged from 5 to 16 years old, with a mean age of 10 years. Three milliliters of blood were transferred to nonadditive tubes for the ELISA test, and 2 ml was transferred to EDTA tubes for molecular testing. After collection, the free tubes were allowed to sit at room temperature for 30 minutes to allow blood to clot. Serum was collected via standard centrifugation at 5,000 rpm for 5 minutes and then kept at -20°C until ELISA analysis. Donated whole blood was stored in EDTA tubes and stored at -20°C for DNA extraction. Enzyme-linked immunosorbent assay (ELISA)Horse serum T. gondii IgG antibodies were detected using an indirect ELISA in accordance with the protocol provided by the manufacturer (IDVet, Montpellier, France). DNA extraction from bloodHorse blood T. gondii DNA was extracted in accordance with the protocol provided by the manufacturer (G-Spin DNA Extraction Kit, Intron Biotechnology, cat. no. 17045, Korean). DNA analysis of DNA using agarose gel electrophoresisElectrophoresis has been used to identify DNA fragments following extraction or to determine the PCR interaction result in the presence of standard DNA, allowing for the differentiation of bundle size on agarose gels. Agarose gel preparationAccording to a previous study (Sambrook et al., 1989), the procedure for preparing the agarose gel involved dispersing 1.5 g of agarose in 100 ml of pre-made TBE (45 mM Tris-borate, 1 mM EDTA) buffer; the resulting concentration was 1.5%. Following boiling, the agarose was subsequently chilled to 45°C–50°C. The gel was transferred to a pour plate, and a comb was used to create wells in the agarose support plate to accommodate the samples. After ensuring that air bubbles did not form, the gel was carefully poured and left to settle for 30 minutes. The comb was carefully extracted from the compacted agarose with great care. The plate was securely fastened to its stand within the horizontal electrophoresis device (CBS, Scientific, USA), which was denoted by the tank employed throughout the electrophoresis procedure. The vessel was filled with TBE buffer, completely submerging the gel surface entirely (Sambrook et al., 1989). Preparation of sampleA volume of 3 μl of the loading buffer for the processor (Intron, Korea) was mixed with 5 μl of the DNA sample to be electrophoresed (loading dye). Following the aforementioned procedure, the gel pores were infused with the resulting mixture using a KAPA Universal DNA Ladder Kit (Kapa Biosystems, cat. no. KK6302, USA). A 7-volt squared electric current was introduced over a period of one to two hours or until the liquid had migrated to the opposite side of the gel. Following immersion in a pool comprising 500 ml of distilled water and 30 μl of Red Safe Nucleic Acid Staining Solution, the gel was subjected to UV-light testing at a wavelength of 336 nm. The purpose of this procedure was to detect nucleic acids in agarose gels (Intron/Korea, cat. no. 21141). Molecular characterization1-PCR amplificationThe PCR reactions were conducted in a duplex manner for each set. Amplifying 100–200 ng of DNA in a 35-cycle, three-step PCR method genotyped all the genes investigated from the DNA samples that were exposed to the PCR amplifications using a Maxime PCR PreMix kit (i-Taq) 20μlrxn (Intron, Korea, cat. no. 25025). 2-Principles used in the interactionThe primers were lyophilized and then dissolved in free ddH2O to provide a stock solution with a final concentration of 100 pmol/μl. A stock solution was kept at 20°C to prepare a working primer suspended at a concentration of 10 pmol/μl. To obtain a final volume of 100 μl, 10 μl of the stock solution was mixed with 90 μl of free ddH2O water. IDT was used to investigate the forward and reverse primers (5’-CCCGATATTCGACAAACGAC-3’) and (5’-GAGCCGTCTGCTTCATGAGC-3’) (Integrated DNA Technologies company, Canada). The primers used in the interaction are listed in Table 1. To obtain the total volume with the master mix, 1 μl was taken from the forward and 1 μl from the reverse, 5 μl from the Taq PCR PreMix, 1.5 μl from the DNA, and 16.5 μl from the deionizer water, resulting in a total volume to 25 μl. This amplification was carried out in a thermal cycler that was configured to satisfy the reaction parameters. After 35 amplification cycles, the products were extended for 7 minutes. Each cycle began with a 3-minutes initial denaturation step (95’c), followed by a 1-minutes denaturation-2 step (95’c), annealing step (56’c), and extension-1 step (72’c). It ended with a 1-minutes extension to step 1. Electrophoresis was performed on the PCR products (amplicons) in a 2.0% agarose gel containing Red Safe Nucleic Acid Staining Solution (20,000x) for the detection of nucleic acids in agarose gels. A DNA ladder assay (100) was also performed to validate the successful amplification of the target gene. Statistical analysisStatistical analysis of T. gondii seroprevalence in Al-Rusafa regions, age, and sex were performed using SPSS software 25.0 (IBM Corp., Armonk, New York, USA), (p < 0.05) and was considered statistically significant. The mean values of variance were compared using kappa. On the other hand, The statistical analysis system (SAS) program (Version 9.3M2 March 2012) was used to examine the influence of differences in study parameters. In this study, the chi-square test (χ2) was used to examine how different numbers were statistically significant. Ethical approvalAll procedures in this study were reviewed and approved by the local ethics committee of the College of Veterinary Medicine, University of Baghdad (P.G 2059), which was issued on 24.10.2024. ResultsResult of ELISAFour out of thirty samples tested positive for ELISA. There was a statistically significant difference between toxoplasma-infected horses based on sex or IgG immunoglobulin levels in their blood (p < 0.01) (see Table 2). Based on age variety, there was a statistically significant difference (p < 0.01) in the serum IgG immunoglobulin level in horses infected with toxoplasma related to the variety of ages (see Table 3). Results of conventional PCRThis study indicated that there is a rate of correlation (p < 0.05) between the results of toxoplasma disease infection and gene identification using the primers for DNA extracted from T. gondii (see Fig. 1). The current study demonstrated that there was a statistically significant difference (p < 0.05) between the presence of UPRTF2 443 bp in the studied blood samples from toxoplasma-infected horses and sex (see Table 4). In addition, there was a statistically significant difference (p < 0.01) between the presence of UPRTF2 443 bp in the blood samples of infected horses and age variation (see Table 5). Table 1. The specific primer of the UPRTF2 gene.
Table 2. The Validity of IgG antibodies in Toxoplasma serum samples infected horses in differentiation with gender.
Table 3. Validity of IgG antibodies in Toxoplasma serum samples infected horses in differentiation with age variation.
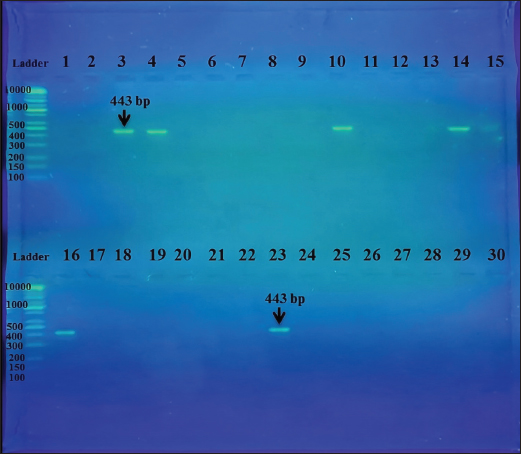
Fig. 1. PCR product the band size 443 bp. The product was electrophoresed on 1.5% agarose at 5 volts/cm2. 1x TBE buffer for 1:30 hours. N: DNA ladder (100). DiscussionThe objective of this study was to determine the prevalence of T. gondii infection in horses residing in the Al-Rusafa district of Baghdad using ELISA and molecular methods. T. gondii, a highly crucial zoonotic pathogen, has emerged as a prominent potential etiological factor for human toxoplasmosis in horses (Dubey, 2021). Regrettably, the epidemiology of T. gondii infection in horses in Iraq is poorly understood, with most extant research focusing on different species (Al-Sim’ani, 2000; Abd-Al-Hameed, 2007; Mikail, 2007; Al-Taie, 2011; Al-Dabagh et al., 2014). In addition, studies on equine toxoplasmosis in Iraq are limited (Alshahery and Mansour, 2012; Mikaeel and Al-Saeed, 2020; Touma et al., 2020). This study revealed an insignificant difference between sex and the prevalence of toxoplasmosis in horses, which is consistent with the results of other studies (Saqib et al., 2015; Sertel and Kirbas, 2022; Jafari-khataylou et al., 2023). In contrast, another study found that T. gondii infection was more common in mares than stallions (Marzok et al., 2023). In this study, the percentage of positive seroprevalence (18.18%) in males compared with females is (0.0%). This may be due to a smaller sample size or low immune responses in females than in males, especially in pregnant women, since there is no correlation between sex and age with immune responses (p < 0.01), especially in females. Table 4. The Validity of UPRTF2 443bp for the studied toxoplasma blood samples infected horses in differentiation with gender.
Table 5. Validity of UPRTF2 443 bp for the study of toxoplasma blood samples infected horses in differentiation with age variation.
Two studies also agreed with this result. They showed that atypical IgG seroconversion with transient IgM levels has been described during the serologic follow-up of seronegative pregnant animals, which makes it harder to understand the results (Fricker-Hidalgo et al., 2013; Hélène Fricker-Hidalgo et al., 2020). These results were also in agreement with a previous study reporting that the percentage of anti-T. gondii IgG antibodies were 21 (15.44%) of 136 cases (Alvarado-Esquivel et al., 2015). On the other hand, these findings disagree with a study that found their comparative study between serological and molecular methods for diagnosis of toxoplasmosis in Egypt, whose results revealed specific IgG in 45.8% and 41.4%, and this may be due to changes in environment and season; it is possible that the sample size of this study is not large enough to elucidate these relationships (Ghoneim et al., 2010). In this study, there was a statistically significant difference (p < 0.05) between the presence of UPRTF2 443bp in the blood samples of toxoplasma-infected horses based on sex, with a higher percentage of positive cases in males (27.27%) compared with females (12.50%) and a Chi-Square (χ2) value of 5.28. However, the observed difference did not reach statistical significance (p < 0.01). In the study of toxoplasma blood samples from infected horses, there was a statistically significant difference between the presence of UPRTF2 443bp with percentages of 16.67% and 20.78% for less than 10 years and more than 10 years, respectively, when controlling for age variation with a Chi-Square (χ2) value of 4.68. ConclusionThe study indicated that 8.8% of horses in the Al-Rusafa region of Baghdad were positive for T. gondii using serological and genetic approaches. These results will help policymakers to better understand the regional epidemiology of T. gondii, which is important for determining the public health hazards associated with this zoonotic disease and developing effective strategies to manage and prevent it. Additionally, additional studies are recommended to determine the frequency of horse toxoplasmosis in various areas in Baghdad. AcknowledgmentsThe authors would like to thank Dr. Nejwa Shihab and Dr. Ban N. Nadhom for their assistance throughout this extensive study. FundingThis research received no specific grant. Authors’ contributionsKarrar Ali Mohammed Hasan Alsakini: study conception, design, data analysis, interpretation of results, and critical revision. Hind H. Al-Ammiri: study conception, data collection, and analysis design. Mustafa Mohammed Touma acquisition of data analysis and critical revision. Conflict of interestThe authors declare no conflicts of interest. Data availabilityThe data supporting the findings of this study are not openly available due to sensitivity reasons. However, they are available from the corresponding author upon reasonable request. ReferencesAbd-Al-Hameed, A. 2007. Seroepidemiological study on ovine toxoplasmosis in Baghdad and Diyala province. M.Sc. Thesis. College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Al-Dabagh, I.I., Jasim, B.M. and Jarjees, M.T. 2014. Seroprevalence of antibodies to toxoplasmosis, brucellosis chlamydiosis in abortive sheep in Nineveh governorate, Iraq. Iraqi J. Vet. Med. 28(1), 21–25. https://doi.org/10.33899/ijvs.2014.89467 Al-Khalidi, N.W. and Dubey, J.P. 1979. Prevalence of Toxoplasma gondii Infection in Horses. J. Parasitol. 65(2), 331–334. doi:10.2307/3280181 Almeria, S. and Dubey, J.P. 2021. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res. Vet. Sci., 135, 371–385. https://doi.org/10.1016/j.rvsc.2020.10.019 Alshahery, M.N. and Mansour, R.S. 2012. Detection of Toxoplasma gondii antibodies in horses in Mosul, Iraq. Iraqi J. Vet. Med., 26(SUPPL.2), 39–41. https://doi.org/10.33899/ijvs.2012.168677 Al-Sim’ani, R. 2000. A serological study to diagnose toxoplasmosis in sheep and human in Ninevah Governorate. M.Sc. Thesis, College of Veterinary Medicine, University of Mosul. Al-Taie, L.H. 2011. Seroprevalance of Toxoplasmosis in sheep and goat: Iraq/ Sulaimania. Iraqi J. Vet. Med., 35(1), 16–24. https://doi.org/10.30539/ iraqijvm.v35i1.599 Alvarado-Esquivel, C., Sánchez-Anguiano, L.F., Hernández-Tinoco, J., Arreola-Cháidez, E., López, J., Salcido-Meraz, K.I., Estrada-Martínez, S., Navarrete- Flores, J.A., Pérez-Álamos, A.R., Hernández-Ochoa, M., Rábago-Sánchez, E. and Liesenfeld, O. 2015. High seroprevalence of Toxoplasma gondii infection in female sex workers: a case-control study. Eur. J. Microbiol. Immunol., 5(4), 285–292. https://doi.org/10.1556/1886.2015.00039 Asal, S.N. 2016. Seroprevelance study of Toxoplasma gondii in horses and camels animal in Wasit province. Iraqi J. Vet. Med. 40(1), 1477–1150 doi: 10.30539/iraqijvm.v40i1.152. Attias, M., Teixeira, D.E., Benchimol, M., Vommaro, R.C., Crepaldi, P.H. and De Souza, W. 2020. The life-cycle of Toxoplasma gondii reviewed using animations. Parasites Vectors, 13(1), 1–13. https://doi.org/10.1186/s13071-020-04445-z Buxton, D., Maley, S.W., Wright, S.E., Rodger, S., Bartley, P. and Innes, E.A. 2007. Toxoplasma gondii and ovine toxoplasmosis: new aspects of an old story. Vet. Parasitol., 149(1–2), 25–28. https://doi.org/10.1016/j.vetpar.2007.07.003 Dabritz, H.A. and Conrad, P.A. 2010. Cats and toxoplasma: implications for public health. Zoonoses Public Health 57(1), 34–52. https://doi.org/10.1111/j.1863-2378.2009.01273.x Dubey, J.P. 2009. Toxoplasmosis in sheep-the last 20 years. Vet. Parasitol, 163(1–2), 1–14. https://doi.org/10.1016/j.vetpar.2009.02.026 Dubey, J.P. 2021. Toxoplasmosis of animals and humans (3rd ed.). Boca Raton, FL: CRC Press. https://doi.org/10.1201/9781003199373 Dubey, J.P., Murata, F.H.A., Cerqueira-Cézar, C.K. and Kwok, O.C.H. 2020. Toxoplasma gondii infections in horses, donkeys, and other equids: the last decade. Res. Vet. Sci. 132, 492–499. https://doi.org/10.1016/j.rvsc.2020.07.005 FAO 2013. Food andAgriculture Organization of the United Nations. La Comisión Europea propone reforzar los controles sobre la carne de caballo y vacuno.Available via http://www.fao.org/agronoticias/agronoticias/detalle/es/?dyna_fef%5Bbackuri%5D=21174&dyna_fef%5Buid%5D=170018 Fricker-Hidalgo, H., Bailly, S., Brenier-Pinchart, M.P., Dard, C., Jean, D., Coston, A.L., Garnaud, C. and Pelloux, H. 2020. How to estimate time of infection with Toxoplasma gondii in pregnant women. Use of specific IgG and IgM kinetics by 7 techniques on 691 sera. J. Clin. Microbiol. 96(4), 114987. https://doi.org/10.1016/j.diagmicrobio.2020.114987 Fricker-Hidalgo, H., Cimon, B., Chemla, C., Darde, M.L., Delhaes, L., L’Ollivier, C., Godineau, N., Houze, S., Paris, L., Quinio, D., Robert-Gangneux, F., Villard, O., Villena, I., Candolfi, E. and Pelloux, H. 2013. Toxoplasma seroconversion with negative or transient immunoglobulin M in pregnant women: myth or reality? A French multicenter retrospective study. J. Clin. Microbiol. 51(7), 2103–2111. https://doi.org/10.1128/JCM.00169-13 Gebremedhin, E.Z., Yunus, H.A., Tesfamaryam, G., Tessema, T.S., Dawo, F., Terefe, G., Di Marco, V. and Vitale, M. 2014. First report of Toxoplasma gondii in camels (Camelus dromedarius) in Ethiopia: bioassay and seroepidemiological investigation. BMC Vet. Res. 10(1), 1–12. https://doi.org/10.1186/s12917-014-0222-7 Ghoneim, N.H., Shalaby, S.I., Hassanain, N.A., Zeedan, G.S.G., Soliman, Y.A. and Abdalhamed, A.M. 2010. Comparative study between serological and molecular methods for diagnosis of toxoplasmosis in women and small ruminants in Egypt. Foodborne Pathog. Dis. 7(1), 17–22. https://doi.org/10.1089/ fpd.2008.0223 Gomes, D.F.C., da Krawczak, F.S., de Oliveira, C.H.S., Júnior, Á.F., Fernandes, É.K.K., Lopes, W.D.Z., da Sevá, A.P. and Gennari, S.M. 2020. Toxoplasma gondii in cattle in brazil: a review. Rev. Bras. Parasitol. Vet. 29(1), 1–16. https://doi.org/10.1590/s1984-29612019106 Hill, D. and Dubey, J. P. 2002. Toxoplasma gondii: Transmission, diagnosis, and prevention. Clin. Microbiol. Infect. 8(10), 634–640. https://doi.org/10.1046/j.1469-0691.2002.00485.x Innes, E.A. 2010. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 57(1), 1–7. https://doi.org/10.1111/j.1863-2378.2009.01276.x Jafari-khataylou, Y., Imani-baran, A. and Akbari, H. 2023. The seroprevalence of Toxoplasma gondii infection among horses in Northwest Iran. J. Zoonotic Dis. 2023, 16897. https://doi.org/10.22034/jzd.2023.16897 James, K.E., Smith, W.A., Packham, A.E., Conrad, P.A. and Pusterla, N. 2017. Toxoplasma gondii seroprevalence and association with equine protozoal myeloencephalitis: a case–control study of Californian horses. Vet. J. 224, 38–43. https://doi.org/10.1016/j.tvjl.2017.05.008 Kimble, K.M., Gomez, G., Szule, J.A., Dubey, J.P., Buchanan, B. and Porter, B.F. 2021. Systemic toxoplasmosis in a horse. J. Comp. Pathol. 182, 27–31. https://doi.org/10.1016/j.jcpa.2020.11.004 Lass, A., Ma, L., Kontogeorgos, I., Zhang, X., Li, X. and Karanis, P. 2019. First molecular detection of Toxoplasma gondii in vegetable samples in China using qualitative, quantitative real-time PCR and multilocus genotyping. Sci. Rep. 9(1), 1–11. https://doi.org/10.1038/s41598-019-54073-6 Li, X., Ni, H.B., Ren, W.X., Jiang, J., Gong, Q.L. and Zhang, X.X. 2020. Seroprevalence of Toxoplasma gondii in horses: a global systematic review and meta-analysis. Acta Trop. 201, 105222. https://doi.org/10.1016/j.actatropica.2019.105222 Liu, Q., Wang, Z.D., Huang, S.Y. and Zhu, X.Q. 2015. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 8(1), 1–14. https://doi.org/10.1186/s13071-015-0902-6 Mancianti, F., Nardoni, S., Papini, R., Mugnaini, L., Martini, M., Altomonte, I., Salari, F., D’Ascenzi, C. and Dubey, J.P. 2014. Detection and genotyping of Toxoplasma gondii DNA in the blood and milk of naturally infected donkeys (Equus asinus). Paras. Vectors 7(1), 2–4. https://doi.org/10.1186/1756-3305-7-165 Marzok, M., AL-Jabr, O.A., Salem, M., Alkashif, K., Sayed-Ahmed, M., Wakid, M.H., Kandeel, M. and Selim, A. 2023. Seroprevalence and risk factors for Toxoplasma gondii infection in horses. Vet. Sci. 10(3), 237. https://doi.org/10.3390/vetsci10030237 Mikaeel, F.B. and Al-Saeed, A.T.M. 2020. Serological and molecular diagnosis of toxoplasma gondii among ewes and horses in Duhok Province-Iraq. Iraqi J. Agric. Sci. 51(4), 1212–1219. Mikail, F.B. 2007. Serodiagnosis of AntiToxoplasma antibodies in aborted ewes in some localities of Duhok Province. Msc. Thesis, College of Veterinary Medicine, University of Duhok, Iraq. Mohamed, K. 2020. Toxoplasmosis in humans and animals in Saudi Arabia: a systematic review. J. Infect. Dev. Ctries., 14(8), 800–811. https://doi.org/10.3855/jidc.12648 Olsen, A., Berg, R., Tagel, M., Must, K., Deksne, G., Enemark, H.L., Alban, L., Johansen, M.V., Nielsen, H.V., Sandberg, M., Lundén, A., Stensvold, C.R., Pires, S. M. and Jokelainen, P. 2019. Seroprevalence of Toxoplasma gondii in domestic pigs, sheep, cattle, wild boars, and moose in the Nordic-Baltic region: a systematic review and meta-analysis. Parasite Epidemiol. Control, 5, e00100. https://doi.org/10.1016/j.parepi.2019.e00100 Pal, M., Alem, B. and Tuli, G. 2014. Toxoplasmosis in animals and humans - its diagnosis, epidemiology and control. Int. J. Livestock Res. 4(2), 1. https://doi.org/10.5455/ijlr.20140608054253 Pomares, C., Ajzenberg, D., Bornard, L., Bernardin, G., Hasseine, L., Dardé, M.L. and Marty, P. 2011. Toxoplasmosis and horse meat, France. Emerg. Infect. Dis. 17(7), 1327–1328. https://doi.org/10.3201/eid1707.101642 Robert-Gangneux, F. and Dardé, M.L. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25(2), 264–296. https://doi.org/10.1128/CMR.05013-11 Sambrook, J., Fritsch, E.R. and Maniatis, T. 1989. Molecular cloning: a laboratory manual (2nd ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. Saqib, M., Hussain, M.H., Sajid, M.S., Mansoor, M.K., Asi, M.N., Al-Kitani, A.F., Zohaib, A., Sial, A.U.R., Muhammad, G. and Ullah, I. 2015. Sero-epidemiology of equine toxoplasmosis using a latex agglutination test in the three metropolises of punjab, pakistan. Trop. Biomed. 32(2), 276–285. Schale, S., Howe, D., Yeargan, M., Morrow, J.K., Graves, A. and Johnson, A.L. 2018. Protozoal coinfection in horses with equine protozoal myeloencephalitis in the eastern United States. J. Vet. Intern. Med. 32(3), 1210–1214. https://doi.org/10.1111/jvim.15127 Schlüter, D., Däubener, W., Schares, G., Groß, U., Pleyer, U. and Lüder, C. 2014. Animals are key to human toxoplasmosis. Int. J. Med. Microbiol., 304(7), 917–929. https://doi.org/10.1016/j.ijmm.2014.09.002 Selim, A., Marawan, M.A., Abdelhady, A. and Wakid, M.H. 2023. Seroprevalence and potential risk factors of Toxoplasma gondii in Dromedary Camels. Agric. 13(1), 10129. https://doi.org/10.3390/agriculture13010129 Sertel, M. and Kirbas, A. 2022. Investigation of seroprevalence of toxoplasmosis in horses and donkeys in Muş Province of Turkey. J. Hell. Vet. Med. Soc., 73(1), 3723–3728. https://doi.org/10.12681/jhvms.25571 Stanciu, S. 2015. Horse meat consumption − between scandal and reality. Procedia Econ. Financ. 23, 697–703. https://doi.org/10.1016/s2212-5671(15)00392-5 Stelzer, S., Basso, W., Benavides Silván, J., Ortega- Mora, L.M., Maksimov, P., Gethmann, J., Conraths, F.J. and Schares, G. 2019. Toxoplasma gondii infection and toxoplasmosis in farm animals: risk factors and economic impact. Food Waterborne Parasitol. 15, e00037. https://doi.org/10.1016/j.fawpar.2019.e00037 Tassi, P. 2007. Toxoplasma gondii infection in horses. A review. Parassitologia 49(1–2), 7–15. Tavassoli, M., Esmaeilnejad, B., Malekifard, F., Soleimanzadeh, A. and Dilmaghani, M. 2013. Detection of toxoplasma gondii DNA in sheep and goat milk in northwest of Iran by PCR-RFLP. Jundishapur J. Microbiol. 6(10), 1–4. https://doi.org/10.5812/jjm.8201 Tenter, A. M. 2009. Toxoplasma gondii in animals used for human consumption. Mem. Inst. Oswaldo Cruz. 104(2), 364–369. https://doi.org/10.1590/S0074-02762009000200033 Tenter, A.M., Heckeroth, A.R. and Weiss, L.M. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30(12–13), 1217–1258. https://doi.org/10.1016/S0020-7519(00)00124-7 Touma, M.M., Jassim, H.S. and Nayyef, H.J. 2020. Molecular and hematological study of Toxoplasma gondii in horses. Biochem. Cell. Arch. 20(2), 4939–4942. | ||
| How to Cite this Article |
| Pubmed Style Alsakini KAMH, Al-ammiri HH, Touma MM. Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. Open Vet. J.. 2025; 15(6): 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 Web Style Alsakini KAMH, Al-ammiri HH, Touma MM. Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. https://www.openveterinaryjournal.com/?mno=241714 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.21 AMA (American Medical Association) Style Alsakini KAMH, Al-ammiri HH, Touma MM. Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. Open Vet. J.. 2025; 15(6): 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 Vancouver/ICMJE Style Alsakini KAMH, Al-ammiri HH, Touma MM. Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 Harvard Style Alsakini, K. A. M. H., Al-ammiri, . H. H. & Touma, . M. M. (2025) Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. Open Vet. J., 15 (6), 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 Turabian Style Alsakini, Karrar Ali Mohammed Hasan, Hind H. Al-ammiri, and Mustafa Mohammed Touma. 2025. Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. Open Veterinary Journal, 15 (6), 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 Chicago Style Alsakini, Karrar Ali Mohammed Hasan, Hind H. Al-ammiri, and Mustafa Mohammed Touma. "Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq." Open Veterinary Journal 15 (2025), 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 MLA (The Modern Language Association) Style Alsakini, Karrar Ali Mohammed Hasan, Hind H. Al-ammiri, and Mustafa Mohammed Touma. "Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq." Open Veterinary Journal 15.6 (2025), 2492-2499. Print. doi:10.5455/OVJ.2025.v15.i6.21 APA (American Psychological Association) Style Alsakini, K. A. M. H., Al-ammiri, . H. H. & Touma, . M. M. (2025) Serologic and molecular survey of Toxoplasma gondii in Baghdad Province, Iraq. Open Veterinary Journal, 15 (6), 2492-2499. doi:10.5455/OVJ.2025.v15.i6.21 |