| Research Article | ||
Open Vet. J.. 2025; 15(6): 2562-2572 Open Veterinary Journal, (2025), Vol. 15(6): 2562-2572 Research Article Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley ratsMai A. Al-Mosaibih1*1Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia *Corresponding Author: Mai A. Al-Mosaibih. Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia. Email: maalmosaibih [at] uj.edu.sa Submitted: 11/02/2025 Revised: 28/04/2025 Accepted: 20/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
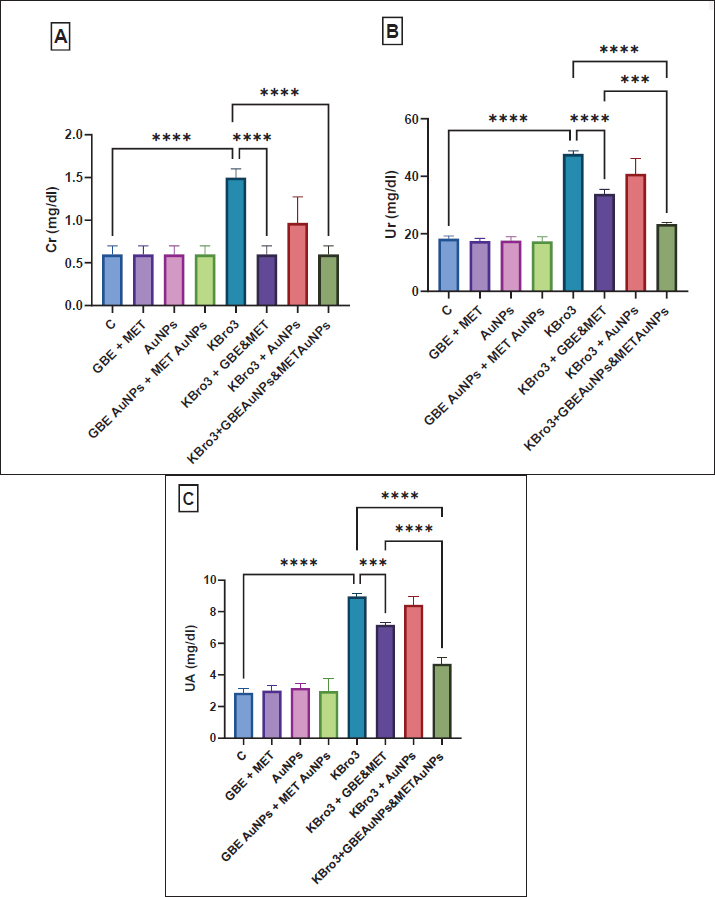
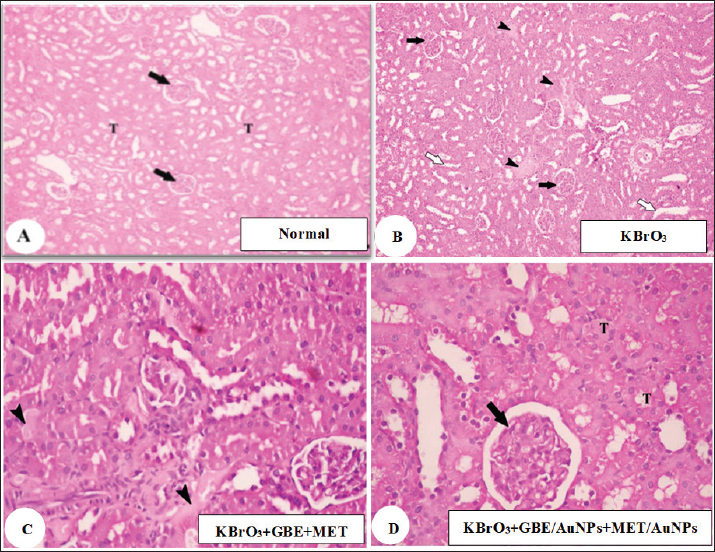
AbstractBackground: Potassium bromate (KBrO3) is a potent oxidizing agent that can initiate the generation of free radicals through xenobiotic metabolic pathways, according to more research. This chemical disturbs cellular redox balance, causing structural and functional damage to particular tissues and macromolecules. Aim: This study explored the therapeutic impact of a blend of Ginkgo biloba extract and metformin, delivered via gold nanoparticles (AuNPs), in reducing the toxicity of KBrO3 in the kidneys. Methods: Rats were categorized into eight groups: a control group, a group receiving G. biloba extract with metformin, a group treated with AuNPs, a group with G. biloba extract and metformin delivered via AuNPs, a KBrO3 group, a KBrO3 group combined with G. biloba extract and metformin, and a KBrO3 group treated with both G. biloba extract and metformin on AuNPs. Results: KBrO3 administration led to a notable increase in levels of creatinine, urea, and uric acid. KBrO3 caused glomeruli shrinkage, expansion of the capsular space, and tubular damage at the histopathological level, and there was a loss of brush borders in the proximal convoluted tubule, an abundance of vacuoles, and glomerular tubules with irregular basement membranes exhibiting significant thickening at the ultrastructural level. Additionally, it increased malondialdehyde, nitric oxide, and reduced superoxide dismutase, and catalase compared to the normal control group. Conclusion: Treatment with G. biloba extract and metformin, and with G. biloba extract and metformin encapsulated in AuNPs reduced the damages caused by KBrO3 in the histopathological and ultrastructural levels of the kidney. Treatment with G. biloba extract and metformin and G. biloba extract and metformin encapsulated in AuNPs improved kidney function tests and kept oxidative stress and antioxidant markers in check. However, G. biloba extract and metformin encapsulated in AuNPs worked better as a treatment option than G. biloba extract and metformin. In conclusion, treatment with G. biloba extract and metformin and with G. biloba extract and metformin encapsulated in AuNPs showed good treatment options against KBrO3 induced-nephrotoxicity in the rat model. Keywords: Ginkgo biloba extract, Gold nanoparticles, Metformin, Nephrotoxicity, Potassium bromate.. IntroductionFor almost a century, potassium bromate (KBrO3) has been utilized as a food additive. It is mainly used during the final stages of dough preparation to improve the strength and elasticity of dough during baking and to help leaven bread. Additionally, beer brewing, cheese production, and fish paste products frequently use KBrO3. In addition, it is used in cosmetics and for cold wave hair treatments (Ahmad and Mahmood, 2016). Many studies have shown that KBrO3 can cause toxicity in multiple organs in both humans and animals (Ahmad et al., 2015). The kidney-damaging effects of KBrO3 are linked to its ability to produce reactive oxygen species (ROS) and cause lipid damage. KBrO3 is particularly deleterious to tissues, most notably those of the central nervous system. The nephrotoxic effects of KBrO3 are associated with its propensity to produce ROS and lipid peroxidation. This process results in significant oxidative damage to DNA and increased levels of 8-hydroxydeoxyguanosine DNA adducts, which act as indicators of oxidative DNA changes, observed in both in vivo and in vitro studies. The oxidative stress caused by KBrO3 greatly overwhelms the cellular antioxidant defense systems, resulting in severe nephrotoxicity in people and animal models and carcinogenic consequences in experimental studies (Ajarem et al., 2016). In humans, the estimated lethal dose of bromates ranges from 5 to 500 mg/kg body weight, with death occurring within 3 to 5 days following ingestion in severe cases. Such acute toxicity further underscores the dangers posed by continued illegal or unregulated use of KBrO3 in food products (Shanmugavel et al., 2020). The use of KBrO3 in food is highly regulated, with the U.S. Food and Drug Administration permitting a maximum concentration of only 0.02 μg/g (0.02 mg/kg) in bread, while several countries—including Nigeria, Canada, Argentina, China, Korea, and Sri Lanka—have completely banned its use due to its carcinogenicity and organ toxicity (Yalçin and Çavuşoğlu, 2022). Similar to many herbal pharmaceuticals, Ginkgo biloba extract contains a multitude of bioactive constituents that are believed to exert synergistic effects. The leaves of G. biloba contain many chemical compounds that have been separated out. These include triterpenes, organic acids, polyprenols, diterpenes, and ginkgo flavonol glycosides (Zhou et al., 2017). Ginkgo biloba extract is known for its antioxidant properties and its role in neutralizing free radicals. It is known that the antioxidant properties, which make it a “radical scavenger,” come from its superoxide dismutase (SOD)-like activity, which lets it get rid of hydroxyl radicals (Zhou et al., 2017). People with type 2 diabetes mellitus (T2DM) are often given the oral drug metformin (N,N-dimethyl biguanide). It is known for being able to control blood sugar levels without causing severe hypoglycemia. Recent guidelines recommend starting metformin treatment at the time of T2DM diagnosis, alongside necessary lifestyle changes. Researchers have observed that metformin treatment significantly restores mRNA levels associated with oxidative stress in streptozotocin-induced diabetic nephropathy. Metformin’s anti-inflammatory and antioxidant benefits have been demonstrated in several studies via a variety of methods. Because of this, metformin could be a good way to treat many conditions where inflammation and oxidative stress play a role in how they work (Hasanpour Dehkordi et al., 2019). Gold nanoparticles (AuNPs) have gotten a lot of attention because they can selectively target cells while being relatively non-toxic to them. They also have surfaces that are easy to change and optical absorption properties that can be changed (Hussein and Saleh, 2018). As was already said, AuNPs are very useful in drug delivery systems because they are biocompatible, have great optical and physicochemical properties, can be made into different monolayers, can be distributed in a controlled way, have a lot of surface area for drug loading, are stable, and are not toxic at all. Due to their biocompatibility, flexibility, and minimal toxicity, AuNPs are excellent carriers for drug delivery. Recent studies indicate that colloidal gold nanorods are especially effective for this purpose (Liu et al., 2017). Despite the known individual therapeutic benefits of G. biloba extract and metformin, there remains a lack of research investigating their combined administration, particularly using nanoparticle-based delivery systems. Given the distinct yet complementary antioxidant and anti-inflammatory actions of G. biloba and metformin, their co-delivery could potentiate therapeutic outcomes against oxidative stress-induced tissue damage. Moreover, encapsulation in AuNPs offers the potential for enhanced bioavailability, targeted delivery, and sustained release, which could overcome the limitations associated with conventional formulations. The present study investigates the therapeutic efficacy of this combination in mitigating KBrO3-induced renal damage through the application of nanotechnology. Materials and MethodsEthical approval and experimental animalsIn this research study, the animal experimental processes were approved by the Committee of the Research Center, University of Jeddah, Saudi Arabia, according to the guidelines and ethical rules. Forty-eight healthy male Sprague Dawley rats (age: 6 to 7 weeks, weight: 90–100 g) were employed for the experimental procedures. Rats were randomly classified into eight groups (n = 6 rats per group). The rats were kept in a standard condition and underwent acclimatization for 1 week. After acclimatization, the eight groups were subjected to the following treatment protocolControl 1 group (n = 6)Rats in this group received no treatment. Group 2 treated with G. biloba extract and metformin (n = 6)These rats were administered both G. biloba extract (100 mg/kg) and metformin (200 mg/kg) via intragastric gavage (i.g.) two times a week for 4 weeks. Group 3 treated with AuNPs (n = 6)Rats were given AuNPs (5 μg Au/Animal) through i.g. administration two times a week for 4 weeks. Group 4 treated with G. biloba extract and metformin delivered via AuNPs'mn'.l;n/., ./,.,m ,...... (n = 6)This group received a combination of G. biloba extract (100 mg/kg) and metformin (200 mg/kg) encapsulated in AuNPs through i.g. administration two times a week for 4 weeks. Group 5 treated with KBrO3 (n = 6)Rats were given KBrO3 (100 mg/kg i.g.) two times a week for 4weeks. Group 6 treated with KBrO3 followed by G. biloba extract and metformin (n = 6)These rats initially received KBrO3 (100 mg/kg i.g.) two times a week for 4 weeks, followed by G. biloba extract (100 mg/kg) and metformin (200 mg/kg) administered via i.g. gavage two times a week for an additional 4 weeks after the cessation of KBrO3 treatment. Group 7 treated with KBrO3 followed by AuNPs (n = 6)Rats were treated with KBrO3 (100 mg/kg i.g.) two times a week for 4 weeks, after which they received AuNPs (5 μg Au/Animal) two times a week for another 4 weeks following the end of KBrO3 treatment. Group 8 treated with KBrO3, G. biloba extract encapsulated in AuNPs, and metformin encapsulated in AuNPs (n = 6)Rats were first given KBrO3 (100 mg/kg i.g.) twice a week for 4 weeks. After that, they were given G. biloba extract (100 mg/kg) and metformin (200 mg/kg) encapsulated in AuNPs i.g. twice a week for another 4 weeks after the removal of the KBrO3 treatment. Chemicals and dosesKBrO3 powder was sourced from El-Gomhouria Chemicals Company in Cairo, Egypt, which was then dissolved in distilled water. We obtained the G. biloba extract from EMA Pharm Company for Pharmaceuticals and Medicinal Plants, located in Cairo, Egypt. This extract was also dissolved in distilled water. The selected dosage of G. biloba extract (100 mg/kg) was based on the previous study by Lebda et al. (2018). AuNPs are available as a suspension that can range from spheroidal to rod shapes. We obtained AuNPs from Nano Gate Company, Cairo, Egypt. The selected dosage of AuNPs was 5 µg per animal, based on findings from a previous study by Ibrahim et al. (2018). The dosage of G. biloba extract encapsulated in AuNPs was set at 100 mg/kg, following the studies of Yallapragada and Velaga (2015). Additionally, the dosage of metformin encapsulated in AuNPs was 200 mg/kg, according to the research conducted by Singh et al. (2016). Formation of AuNPs and structural characterization, including gold concentration and size, was conducted following the methods outlined by Arulkumar and Sabesan (2010), Ibrahim et al. (2018), respectively. Blood collection and tissue preparationWe euthanized the rats 24 hours after the final treatment at the end of the 9-week experiment, having fasted them overnight beforehand. We collected blood samples to obtain the serum. Tissues were preserved in 10% neutral buffered formalin for histological assessment. Kidney function testsUrea (Ur) concentration in the serum was tested using the technique indicated by Mohamed and Ashour (2019). The concentration of creatinine (Cr) was tested in the serum based on (Slot, 1965; Osmic–Husni et al., 2023). Serum uric acid (UA) concentrations were calculated. Oxidative stress and antioxidant markersThe level of malondialdehyde (MDA) was assessed using the technique indicated by Wu et al. (2024). Nitric oxide (NO) production levels were determined following the procedure outlined by Yousef and Hussien (2015). SOD and catalase (CAT) activities were evaluated following the procedures outlined by Misra and Fridovich (1972), Manubolu et al. (2014), respectively Histology examinationKidney samples underwent a dehydration process using progressively higher concentrations of ethyl alcohol (70%, 90%, and 100%). Following dehydration, the specimens were cleared in xylene and subsequently infiltrated with paraffin wax for embedding. Thin sections were cut and then stained with Harris’s Hematoxylin and Eosin to facilitate a comprehensive histological evaluation. Ultra-structural preparationsCut animal kidney tissue into 1 mm³ cubes and put in a 3% glutaraldehyde–formaldehyde solution to stay fixed at 4°C for 18 to 24 hours. The samples were rinsed in phosphate buffer (pH 7.4) and then fixed in 1% osmium tetroxide at 4°C for an hour. To make the area of interest stand out, toluidine blue was used to stain semithin sections. Next, ultrathin sections were made with an ultramicrotome and stained with uranyl acetate and lead citrate. These sections were then examined with a Joel CX 100 transmission electron microscope. Statistical analysisAll statistical analyses were performed using GraphPad Prism 9.0 software. Results are reported as mean ± SD (n = 6). The Shapiro–Wilk test was first used to confirm the normal distribution of the data. A one-way ANOVA was then applied to compare differences among groups, followed by the Bonferroni post hoc test to identify specific group differences. This approach was used for all parameters measured. A difference was deemed significant when the p value was ≤ 0.05, with higher levels of significance also noted. ResultsInfluence of different treatments on renal function testsSerum levels of Cr, Ur, and UA were significantly higher in the group that was treated with KBrO3 compared to the healthy control group (p < 0.0001). Treatment with G. biloba extract and metformin during the parallel administration of KBrO3 significantly reduced serum levels of Cr (p < 0.0001), Ur (p < 0.0001), and UA (p < 0.001) relative to the KBrO3 group. Treatment with G. biloba extract and metformin encapsulated in AuNPs during the parallel administration of KBrO3 significantly lowered serum concentrations of Cr, Ur, and UA compared to the KBrO3 group (p < 0.0001). Additionally, the treatment with G. biloba extract and metformin enclosed in AuNPs worked much better than the treatment with G. biloba extract and metformin alone at lowering Ur (p < 0.001) and UA (p < 0.0001), as shown in Figure 1. No significant improvement in the measured renal function parameters was observed in rats treated with KBrO3 followed by AuNPs compared to the KBrO3 group, indicating that the protective effect in other groups is due to the therapeutic agents rather than the nanoparticles themselves, as shown in Figure 1. Histological examinationsA kidney from the normal group showed a renal cortex with distinct tubules (black arrow) PCT and DCT and renal corpuscles (white arrow) emerged, as shown in Figure (2A). A kidney from the KBrO3 group showed a focal area of necrosis (arrow head), as shown in Figure (2B). A kidney from the group treated with KBrO3, G. biloba extract, and metformin showed a slightly detached brush border of renal tubules (white arrow), as shown in Figure (2C). The kidney from the group treated with KBrO3, G. biloba extract, and metformin encapsulated in AuNPs showed a slightly wide capsular space of renal corpuscle (black arrow) and normal kidney tubules (T), as shown in Figure (2D).
Fig. 1. Kidney function tests (A) Cr, (B) Ur, and (C) UA. All data are reported as mean ± SD (n = 6). The following is an indication of statistical significance: **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. One-way ANOVA and then Bonferroni’s post hoc test were conducted. GBE: Ginkgo biloba extract, MET: Metformin, AuNPs: Gold nanoparticles.
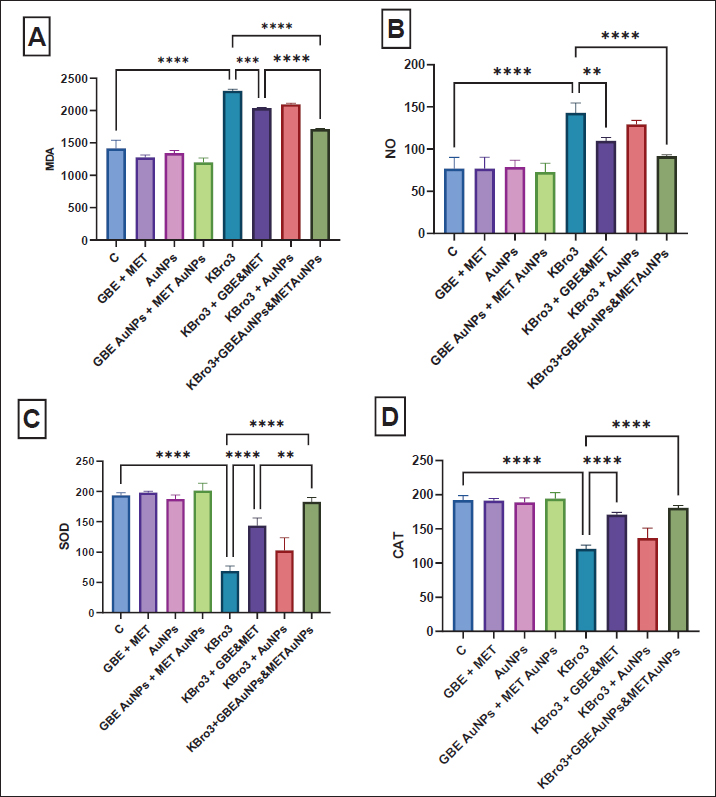
Fig. 2. Histopathological examination (A): kidney from normal group; a renal cortex emerged with renal corpuscles (white arrow) and distinct tubules PCT and DCT (black arrow); (B): kidney from KBrO3 group showed focal area of necrosis (arrow head); (C): kidney from group (KBrO3+GBE+MET) showed renal cortex slightly detached brush border of renal tubules (white arrow); (D): kidney from group (KBrO3+GBE/AuNPs+MET/AuNPs) showed slightly wide capsular space of renal corpuscle (black arrow) and normal kidney tubules (T). GBE: Ginkgo biloba extract, MET: Metformin, AuNPs: Gold nanoparticles. Impact of various treatments on oxidative stress and antioxidant indicatorsMDA and NO levels significantly increased in the KBrO3-administered group in comparison to the normal control group (p < 0.0001). Instead, SOD and CAT activity levels were much lower in the group that received KBrO3 compared to the normal control group (p < 0.0001), as shown in Figure 3. Treatment with G. biloba extract and metformin during the parallel administration of KBrO3 significantly reduced serum levels of MDA (p < 0.001) and NO (p < 0.01) compared to the KBrO3 group. Additionally, this treatment significantly increased the concentrations of SOD and CAT relative to the KBrO3 group (p < 0.0001), as shown in Figure 3. Treatment with G. biloba extract and metformin encapsulated in AuNPs during the parallel administration of KBrO3 significantly lowered the concentrations of MDA and NO relative to the KBrO3 group (p < 0.0001). This treatment also significantly elevated the concentration of SOD and CAT relative to the KBrO3 group (p < 0.0001). Finally, the treatment with G. biloba extract and metformin enclosed in AuNPs worked much better than the treatment with G. biloba extract and metformin alone at lowering MDA (p < 0.0001) and raising SOD (p < 0.01) Figure 3.
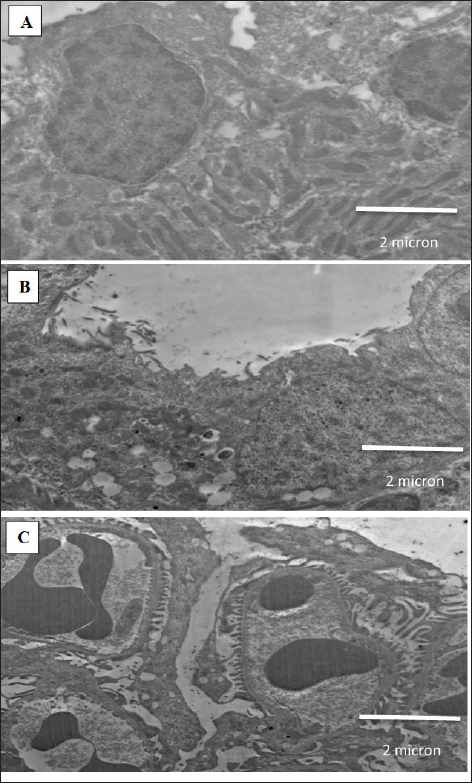
Fig. 3. Oxidative stress and antioxidant markers (A) MDA, (B) NO, (C) SOD, and (D) CAT. All data are reported as mean ± SD (n = 6). The following is an indication of statistical significance: **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. One-way ANOVA and then Bonferroni’s post hoc test were conducted. GBE: Ginkgo biloba extract, MET: Metformin, AuNPs: Gold nanoparticles. No significant improvement in the measured oxidative stress and antioxidant parameters was observed in rats treated with KBrO3 followed by AuNPs compared to KBrO3 group, indicating that the protective effect in other groups is due to the therapeutic agents rather than the nanoparticles themselves Figure 3. Transmission electron microscopyFigure 4A shows a normal kidney showing microvilli and distilled convoluted tubule with normal brush borders. Figure 4B depicts the kidneys of rats treated with KBrO3 showing degeneration of mitochondria and many vacuoles. Figure 4C depicts the kidneys of rats treated with G. biloba extract and metformin encapsulated in AuNPs which were administered in parallel with KBrO3, showing pedicel and thin basement membrane. DiscussionAn extensive array of research has validated that KBrO3 operates as a formidable oxidizing agent capable of initiating free radical production through xenobiotic metabolic routes. This chemical disturbs cellular redox balance, causing structural and functional damage to particular tissues and macromolecules. If these bad effects last for a long time, they can cause many diseases, including cancer, depending on things, such as the amount of exposure, how long it lasts, and the environment where the organisms are exposed (Hassan et al., 2020). This study investigates the prospective therapeutic benefits of combination treatment groups, including G. biloba extract and metformin, as well as G. biloba extract encapsulated in AuNPs combined with metformin encapsulated in AuNPs on KBrO3-induced nephrotoxicity. When Cr, Ur, and UA levels are high, it means that protein metabolism is not working right, which causes proteins to build up in the blood and cause uremia. High Cr levels, a byproduct of creatine phosphate, serve as a marker of advancing renal damage (Akomolafe et al., 2021). In the current study, KBrO3 significantly increased the concentrations of Cr, UA, and Ur relative to the control group, agreeing with (Ali et al., 2018; Akomolafe et al., 2021). The combination-treated groups showed significant improvement in Cr, UA, and Ur levels. Additionally, Chang et al. (2020) found that the increased levels of serum Cr, UA, and Ur caused by streptozotocin were restored to near-normal values through the administration of G. biloba extract. Balamash et al. (2018) reported a similar effect for metformin that decreased levels of serum Cr, UA, and Ur against streptozotocin. Dhas et al. (2016) reported the treated effect of AuNPs that decreased levels of serum Cr, UA, and Ur against alloxan. Similarly, metformin-loaded pectin–chitosan biohybrids reduced these kidney parameters that were altered by streptozotocin reported by Chinnaiyan et al. (2019). Oxidative stress happens when one or more body tissues change in a way that is harmful and continues to do so. This can lead to organ dysfunction, faster aging, and sometimes diseases and death. It is a normal and necessary part of the body’s processes, but over time, it leads to more harmful changes that affect not only cell structures but also molecular parts (Hassan et al., 2020). In the present investigation, KBrO3 notably elevated the concentrations of MDA and NO in comparison to the control cohort. The increases in MDA and NO levels induced by KBrO3 were consistent with findings reported by Hassan et al. (2020). Conversely, the groups subjected to combined treatments exhibited a significant reduction in MDA and NO levels. Furthermore, analogous results were documented by Hassan et al. (2020), who noted that elevated MDA and NO levels provoked by carbon tetrachloride were normalized by G. biloba extract. Similarly, Hassan et al. (2020) documented a comparable effect of metformin that resulted in reduction in the concentrations of MDA and NO in the context of streptozotocin. Additionally, Hassan et al. 2020 reported that elevated MDA and NO levels induced by schistosomiasis returned to baseline levels following treatment with AuNPs. Meanwhile, Hassan et al. (2020) highlighted the efficacy of G. biloba extract, exopleura extract, and chitosan coating in normalizing elevated MDA and NO levels. Moreover, Hassan et al. 2020 observed a reduction in MDA and NO levels through the application of chitosan in conjunction with metformin against streptozotocin. Even so, antioxidant enzymes are very important for getting rid of oxidative damage and setting up a system to protect against ROS. SOD, CAT, GST, and GSH are antioxidants that the body makes to protect itself from oxidative stress and lower or get rid of free radicals that can damage cells. Oxidative stress results from an imbalance between ROS and the antioxidant defense system. The organism’s capacity to synthesize these antioxidants is governed by genetic predisposition and is modulated by environmental exposures, including dietary intake and chemical agents (Hassan et al., 2020). In the present investigation, KBrO3 markedly diminished the levels of SOD and CAT in comparison to the control cohort. The fact that KBrO3 causes SOD and CAT levels to drop is in line with what Hassan et al. (2020) found (Hassan et al., 2020). Conversely, groups subjected to combination treatment exhibited a significant enhancement in SOD and CAT levels. Furthermore, Hassan et al. (2020) noted that the reduction in SOD and CAT levels caused by carbon tetrachloride was reversible to baseline values through treatment with G. biloba extract. Metformin exhibited a similar phenomenon, elevating SOD and CAT levels that streptozotocin reduced (Hassan et al., 2020). Additionally, Hassan et al. (2020) documented that SOD and CAT levels reduced by isoproterenol were restored to normal values through the application of AuNPs. Lastly, Hassan et al. (2020) indicated that SOD and CAT levels diminished due to restraint stress were normalized by G. biloba extract and AuNPs, while Hassan et al. (2020) revealed an increase in SOD and CAT levels through chitosan combined with metformin in response to streptozotocin.
Fig. 4. Electron micrographs. (A) normal kidney showing microvilli and distilled convoluted tubule with normal brush borders, (B) kidney of rats administered KBrO3 showing degeneration of mitochondria and many vacuoles, and (C) kidney of rats treated with G. biloba extract and metformin encapsulated in AuNPs in parallel with KBrO3 showing pedicel and thin basement membrane. In histological examination of kidney, KBrO3 indicated smaller glomeruli, an increased capsular gap, and tubular injury, with similar findings described by Ali et al. (2018). The combination-treated groups improved histological structure changes caused by KBrO3 toward normal. Additionally, Abdul-Hamid et al. 2018 reported improvement in histological structure by G. biloba extract against amiodarone. This report by Janjua et al. (2014) said that metformin improved histological structure changes and inhibited the gentamicin effect. Additionally, Sengani (2017) demonstrates the effect of AuNPs that improved histological structure changes against streptozotocin. Furthermore, Arun et al. (2020) demonstrate the impact of chitosan coupled with metformin that improved histological structure changes against streptozotocin. Furthermore, in the ultrastructural level, KBrO3 caused kidney proximal convoluted tubules to lose brush boundaries with many vacuoles, glomerulus tubules to have uneven basement membranes with considerable thickening and fusion in the pedicel (Kashef et al., 2019). The combination-treated groups improved histological structure changes caused by KBrO3 toward normal. Additionally, Abdul-Hamid et al. (2018) reported improvement in ultrastructure by G. biloba extract against amiodarone. Zhang et al. (2017) reported that metformin ameliorated these ultrastructural changes against streptozotocin. Lopez-Chaves et al. (2018) reported that AuNPs ameliorated these ultrastructural changes against hydrogen peroxide. Chinnaiyan et al. (2019) reported that metformin-loaded pectin-chitosan biohybrids ameliorated these ultrastructural changes against streptozotocin. The observed therapeutic effects of the combination treatments can be attributed to the complementary mechanisms of the individual agents. Ginkgo biloba extract exerts its protective role through its rich content of flavonoids and terpenoids, which scavenge free radicals, and modulate redox-sensitive transcription factors, enhancing the expression of endogenous antioxidants (Zhou et al., 2017). Metformin, beyond its glycemic control, activates AMP-activated protein kinase, which improves mitochondrial function, reduces ROS production, and downregulates inflammatory mediators (Hasanpour Dehkordi et al., 2019). AuNPs further enhance these effects by improving cellular uptake, stabilizing the therapeutic compounds, and directly mitigating oxidative stress via their intrinsic redox-modulating capacity (Liu et al., 2017). Together, these mechanisms help restore redox balance, preserve tissue architecture, and improve renal function following KBrO3-induced nephrotoxicity. One limitation of this study is the lack of a compatibility assessment between G. biloba extract and metformin, particularly in their co-encapsulated nanoparticle form. Future studies are warranted to investigate the compatibility and interaction profile of these agents, as well as to elucidate the detailed molecular mechanisms involved in their nephroprotective actions. Future investigations should also consider extending these findings to other animal models and, ultimately, to human clinical trials, to validate the therapeutic potential and safety of this combination approach in broader biomedical contexts. ConclusionThis study found that combination treatments of G. biloba extract and metformin, as well as G. biloba extract encapsulated in AuNPs combined with metformin encapsulated in AuNPs, represent promising treatment options against KBrO3-induced nephrotoxicity in a rat model. Both treatment combinations reduced the damage caused by KBrO3 at histopathological and ultrastructural levels in the kidneys. Additionally, the treatments with G. biloba extract and metformin, along with G. biloba extract encapsulated in AuNPs and metformin encapsulated in AuNPs, restored kidney function test parameters and regulated oxidative stress and antioxidant markers. However, the combination of G. biloba extract encapsulated in AuNPs and metformin encapsulated in AuNPs was more effective than G. biloba extract and metformin. AcknowledgmentsThe author would like to thank the University of Jeddah. Conflict of interestThe author declares that there is no conflict of interest. FundingNot applicable. Data availabilityAll data are provided in the manuscript. ReferencesAbdul-Hamid, M., Galaly, S.R., Mahmoud, H. and Mostafa, F. 2018. The protective effect of grape seed and Ginkgo biloba against hepatotoxicity induced by the antidysrhythmic drug “amiodarone” in male albino rats. Beni Suef Univ. J. Basic Appl. Sci. 7(2), 223–230. Ahmad, M.K., Khan, A.A., Ali, S.N. and Mahmood, R. 2015. Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA- protein cross-linking and oxidative stress in rat intestine. PLoS One 10(3), e0119137. Ahmad, M.K. and Mahmood, R. 2016. Protective effect of taurine against potassium bromate- induced hemoglobin oxidation, oxidative stress, and impairment of antioxidant defense system in blood. Environ. Toxicol. 31(3), 304–313. Ajarem, J., Altoom, N.G., Allam, A.A., Maodaa, S.N., Abdel-Maksoud, M.A. and Chow, B.K. 2016. Oral administration of potassium bromate induces neurobehavioral changes, alters cerebral neurotransmitters level and impairs brain tissue of swiss mice. Behav. Brain Funct. 12(1), 14. Akomolafe, S.F., Olasehinde, T.A., Adewale, O.O. and Ajayi, O.B. 2021. Curcumin improves biomolecules associated with renal function and attenuates oxidative injury and histopathological changes in potassium-induced toxicity in rats’ kidney. Biol. Trace Elem. Res. 199(1), 197–204. Ali, B.H., Al Za’abi, M., Karaca, T., Al Suleimani, Y., Al Balushi, K.A., Manoj, P., Ashique, M. and Nemmar, A. 2018. Potassium bromate-induced kidney damage in rats and the effect of gum acacia thereon. Am. J. Transl. Res. 10(1), 126. Arulkumar, S. and Sabesan, M. 2010. Behavior and biochemical changes of nanoginkgoba (Ginkgo biloba gold nano-particles) on restraint stress- induced male albino mice. Int. J. Res. Pharm. Sci. 1(4), 533–538. Arun, G., Rajaram, R., Kaleshkumar, K., Gayathri, N., Sivasudha, T. and Kandasamy, S. 2020. Synergistic effect of novel chitosan combined metformin drug on streptozotocin-induced diabetes mellitus rat. Int. J. Biol. Macromol. 153, 1335–1349. Balamash, K.S., Alkreathy, H.M., Al Gahdali, E.H., Khoja, S.O. and Ahmad, A. 2018. Comparative biochemical and histopathological studies on the efficacy of metformin and virgin olive oil against streptozotocin-induced diabetes in Sprague-Dawley rats. J. Diabetes Res. 2018, 4692197. Chang, T.-T., Chen, Y.-A., Li, S.-Y. and Chen, J.-W. 2020. Nrf-2 mediated heme oxygenase-1 activation contributes to the anti-inflammatory and renal protective effects of Ginkgo biloba extract in diabetic nephropathy. J. Ethnopharmacol. 266, 113474. Chinnaiyan, S.K., Deivasigamani, K. and Gadela, V.R. 2019. Combined synergetic potential of metformin loaded pectin-chitosan biohybrids nanoparticle for NIDDM. Int. J. Biol. Macromol. 125, 278–289. Dhas, T.S., Kumar, V.G., Karthick, V., Vasanth, K., Singaravelu, G. and Govindaraju, K. 2016. Effect of biosynthesized gold nanoparticles by Sargassum swartzii in alloxan induced diabetic rats. Enzyme Microb. Technol. 95, 100–106. Hassan, I., Ebaid, H., Alhazza, I.M. and Al-Tamimi, J. 2020. The alleviative effect of vitamin B2 on potassium bromate-induced hepatotoxicity in male rats. Biomed. Res. Int. 2020, 8274261. Hasanpour Dehkordi, A., Abbaszadeh, A., Mir, S. and Hasanvand, A. 2019. Metformin and its anti- inflammatory and anti-oxidative effects; new concepts. J. Renal Inj. Prev. 8(1), 54–61. Hussein, R.M. and Saleh, H. 2018. Promising therapeutic effect of gold nanoparticles against dinitrobenzene sulfonic acid-induced colitis in rats. Nanomedicine (Lond) 13(14), 1657–1679. Ibrahim, K.E., Al-Mutary, M.G., Bakhiet, A.O. and Khan, H.A. 2018. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules 23(8), 1848. Janjua, A., Waheed, A. and Bakhtiar, S. 2014. Protective effect of metformin against gentamicin induced nephrotoxicity in rabbits. Pak. J. Pharm. Sci. 27(6), 1863–1872. Kashef, S.M.I., Abd El Hafez, A.A.A., Sarhan, N.I., El-Shal, A.O., Ata, M.M., Ashour, A.S., Dey, N., Abd Elnaby, M.M. and Sherratt, R.S. 2019. Automated image analysis system for renal filtration barrier integrity of potassium bromate treated adult male albino rat. Multimed. Tools Appl. 79, 7559–7575. Lebda, M.A., Sadek, K.M., Tohamy, H.G., Abouzed, T.K., Shukry, M., Umezawa, M. and El-Sayed, Y.S. 2018. Potential role of α-lipoic acid and Ginkgo biloba against silver nanoparticles-induced neuronal apoptosis and blood-brain barrier impairments in rats. Life Sci. 212, 251–260. Liu, H., Lian, T., Liu, Y., Hong, Y., Sun, D. and Li, Q. 2017. Plant-mediated synthesis of Au nanoparticles: separation and identification of active biomolecule in the water extract of Cacumen Platycladi. Ind. Eng. Chem. Res. 56(18), 5262–5270. Lopez-Chaves, C., Soto-Alvaredo, J., Montes-Bayon, M., Bettmer, J., Llopis, J. and Sanchez-Gonzalez, C. 2018. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine 14(1), 1–12. Manubolu, M., Goodla, L., Ravilla, S., Thanasekaran, J., Dutta, P., Malmlöf, K. and Obulum, V.R. 2014. Protective effect of Actiniopteris radiata (Sw.) Link. against CCl4 induced oxidative stress in albino rats. J. Ethnopharmacol. 153(3), 744–752. Misra, H.P. and Fridovich, I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247(10), 3170–3175. Mohamed, N.E. and Ashour, S.E. 2019. Influence of ethanolic extract of strawberry leaves for abrogating bromate hazards in male rats. J. Basic Appl. Zool. 80(1), 19. Osmic–Husni, A., Hukic, F. and Saric, M.P. 2023. Comparison of Jaffe method and enzymatic method at measuring serum creatinine level, creatinine clearance and estimated glomerular filtration rate. Materia Sociomed. 35(2), 113. Sengani, M. 2017. Identification of potential antioxidant indices by biogenic gold nanoparticles in hyperglycemic Wistar rats. Environ. Toxicol. Pharmacol. 50, 11–19. Shanmugavel, V., Santhi, K.K., Kurup, A.H., Kalakandan, S., Anandharaj, A. and Rawson, A. 2020. Potassium bromate: effects on bread components, health, environment and method of analysis: a review. Food Chem. 311, 125964. Singh, B., Singh, A. and Kumar, V. 2016. Ameliorative effect of adjunct therapy of metformin with atorvastatin on streptozotocin-induced diabetes mellitus in rats. Drug Res (Stuttg). 66(01), 28–32. Slot, C. 1965. Plasma creatinine determination a new and specific Jaffe reaction method. Scand. J. Clin. Lab. Invest. 17(4), 381–387. Wu, H., Kong, Y., Zhao, W. and Wang, F. 2024. Measurement of cellular MDA content through MTBE-extraction based TBA assay by eliminating cellular interferences. J. Pharm. Biomed. Anal. 248, 116332. Yalçin, E. and Çavuşoğlu, K. 2022. Toxicity assessment of potassium bromate and the remedial role of grape seed extract. Sci. Rep. 12(1), 20529. Yallapragada, P.R. and Velaga, M.K. 2015. Effect of Ginkgo biloba extract on lead-induced oxidative stress in different regions of rat brain. J. Environ. Pathol. Toxicol. Oncol. 34(2), 161–173. Yousef, M.I. and Hussien, H.M. 2015. Cisplatin- induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem. Toxicol. 78, 17–25. Zhang, S., Xu, H., Yu, X., Wu, Y. and Sui, D. 2017. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 14(1), 383–390. Zhou, X., Qi, Y. and Chen, T. 2017. Long-term pre- treatment of antioxidant Ginkgo biloba extract EGb- 761 attenuates cerebral-ischemia-induced neuronal damage in aged mice. Biomed. Pharmacother. 85, 256–263. | ||
| How to Cite this Article |
| Pubmed Style Mai A. Al-Mosaibih. Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. Open Vet. J.. 2025; 15(6): 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 Web Style Mai A. Al-Mosaibih. Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. https://www.openveterinaryjournal.com/?mno=242234 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i6.28 AMA (American Medical Association) Style Mai A. Al-Mosaibih. Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. Open Vet. J.. 2025; 15(6): 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 Vancouver/ICMJE Style Mai A. Al-Mosaibih. Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. Open Vet. J.. (2025), [cited January 24, 2026]; 15(6): 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 Harvard Style Mai A. Al-Mosaibih (2025) Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. Open Vet. J., 15 (6), 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 Turabian Style Mai A. Al-Mosaibih. 2025. Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. Open Veterinary Journal, 15 (6), 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 Chicago Style Mai A. Al-Mosaibih. "Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats." Open Veterinary Journal 15 (2025), 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 MLA (The Modern Language Association) Style Mai A. Al-Mosaibih. "Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats." Open Veterinary Journal 15.6 (2025), 2562-2572. Print. doi:10.5455/OVJ.2025.v15.i6.28 APA (American Psychological Association) Style Mai A. Al-Mosaibih (2025) Comparative study for evaluation of Ginkgo biloba extract and gold nanoparticle-encapsulated metformin against nephrotoxicity caused by potassium bromate in Sprague Dawley rats. Open Veterinary Journal, 15 (6), 2562-2572. doi:10.5455/OVJ.2025.v15.i6.28 |