| Research Article | ||
Open Vet. J.. 2025; 15(6): 2651-2660 Open Veterinary Journal, (2025), Vol. 15(6): 2651-2660 Research Article Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive periodFachruddin Fachruddin1,2, Agik Suprayogi3, Wasmen Manalu3*, Novriyandi Hanif 4,5 and Huda Shalahuddin Darusman3,61Doctoral Student, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 2Department of Biology, Faculty of Science and Technology, Institut Teknologi dan Kesehatan Avicenna, Kendari, Indonesia 3Division of Physiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 4Department of Chemistry, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, Indonesia 5Tropical Biopharmaca Research Center, IPB University, Bogor, Indonesia 6Primate Research Center, IPB University, Bogor, Indonesia *Correspondence to: Agik Suprayogi. Division of Physiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia. Email: agiksu [at] apps.ipb.ac.id Submitted: 20/02/2025 Revised: 13/05/2025 Accepted: 16/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
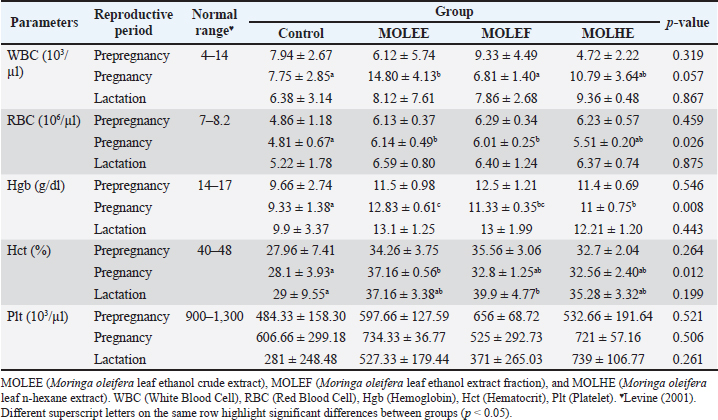
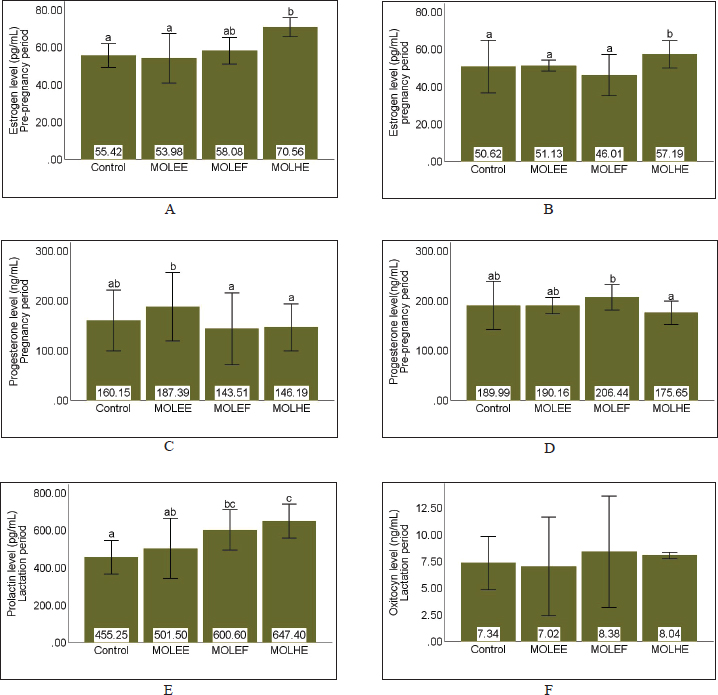
AbstractBackground: Optimal animal health, as indicated by normal hematological values, is essential for improving animal welfare and productivity, including reproductive performance in female mammals. Moringa is an herbal plant with extensive potential for medical applications, including improving and enhancing animal health as well as reproductive performance. Aim: This study aimed to evaluate the effect of Moringa oleifera leaf extract (MOLE) supplementation on the hematological profile and reproductive performance of female rats during the prepregnancy to lactation period. Methods: An experimental study with a completely randomized design was conducted on nulliparous and sexually mature female Sprague–Dawley rats. The study consisted of four treatments and three to five replicates, namely control (feed without MOLE), MOLEE (feed containing MOL ethanol extract, MOLEF (feed containing MOL ethanol fraction), and MOLHE (feed containing MOL n-hexane extract). Observed variables included hematological values, estrogen, progesterone, prolactin, and oxytocin levels, litter size, prenatal mortality rate, birth weight, milk production, and pups’ weight gain. Results: The results showed significant differences in most hematological parameters (white blood cell, red blood cell, hemoglobin, and hematocrit) and reproductive hormones (estrogen, progesterone, and prolactin levels). MOLE supplementation during pregnancy did not affect litter size and birth weight, but the MOLEE and MOLEF groups caused prenatal mortality. In addition, MOLE supplementation significantly increased milk yield but not pups’ weight gain. Conclusion: MOLE supplementation positively affected the hematological values of female rats during the reproductive period and enhanced reproductive performance (reproductive hormone levels and milk production). However, it is essential to be aware of the possible adverse effects of prenatal mortality, which require further investigation to uncover the underlying causes and mechanisms. Keywords: Hematology, Milk production, Moringa oleifera, Prenatal mortality, Reproductive hormones.. IntroductionAnimal health is a fundamental aspect that influences the welfare and productivity of animals. Healthy animals are defined by a body condition free of disease, absence of stress, adequate nutrition, and optimal development. Consequently, these animals are happier, have a longer productive lifespan, and can produce higher quality products (Colditz et al., 2023). An important parameter that can be reliably used to assess the physiological health status of animals is hematological examination (Purwar et al., 2019; Pessini et al., 2020). Hematological profiles provide detailed insights into the overall health condition of animals, particularly regarding blood composition and immune system function (Khalil et al., 2018; McClain et al., 2022). Changes in hematological values reflect the pathophysiological responses of animals, and deviations from normal hematological values may indicate health issues (Etim, 2014). The significance of hematology is often associated with reproductive performance (Bezerra et al., 2017), especially in female mammals that exhibit physiological complexity. Normal hematological values indicate that healthy female mammals exhibit optimal reproductive performance. Conversely, abnormal hematological values can diminish reproductive performance (Saleh et al., 2019; Baimishev et al., 2020). For instance, anemia has been reported to adversely affect estrogen hormone levels, disrupt estrous cycles, reduce fertility rates, and decrease the likelihood of successful conception (Hasan et al., 2022). Overall, female mammals in good health during the reproductive period are more likely to have high chances of conception, optimal intrauterine environmental conditions, and well-developed mammary glands. These factors contribute to increased productivity and population sustainability. Efforts to enhance health status and animal reproductive performance can be achieved by applying various approaches, including supplementation with natural product extracts, such as Moringa leaf extract. Moringa leaf exhibits significant potential and applications in nutrition and medicine (Patil et al., 2022; Trigo et al., 2023). Moringa leaf is rich in nutrients, vitamins, and minerals (Dasat et al., 2020; Rubio-Sanz, 2020; Asekunowo et al., 2022). In addition, various bioactive compounds, including flavonoids, tannins, steroids, and other pharmacologically beneficial phytoconstituents, are commonly found in Moringa leaf (Kalauni et al., 2023; Fachruddin et al., 2024; Hasim et al., 2025). In the context of animal health and reproductive performance, previous studies have demonstrated significant impacts of utilizing Moringa leaf extract on improving hematological parameters such as hemoglobin (Hgb), red blood cell (RBC) counts, and other relevant values in anemic conditions across various animal models (Hassan et al., 2020; Safitri and Retnaningsih, 2021). Furthermore, Moringa leaf extract has been reported to enhance reproductive performance, including the modulation of reproductive hormones such as follicle-stimulating hormone, luteinizing hormone estrogen, and progesterone (Ogunsola et al., 2017). Nevertheless, a study has yet to address the effects of various Moringa leaf extracts on female rats’ health status and reproductive performance across different reproductive periods. Therefore, this research aims to evaluate the hematological values and reproductive performance of female rats supplemented with various Moringa leaf extracts from the preconception period through lactation. Materials and MethodsPlant materials and extractionThe samples of the Moringa leaf were collected from Kokalukuna district, Baubau City, Southeast Sulawesi at the following geographical coordinates: 05o 25′41.68″ S 122o 39′12.47″ E or 51M 461611 939987. The plant was identified by a curator at the Herbarium Bandungense, Department of Biology, Institut Teknologi Bandung, Bandung, Indonesia. The voucher specimen was deposited in this herbarium (number 3027/IT1.C11.2/TA.00/2025). The extraction of Moringa leaf using 70% ethanol was conducted based on our previous research (Fachruddin et al., 2024). The ethanol fraction was obtained by separating the crude ethanol extract using n-hexane, whereas the n-hexane extract was derived from the direct maceration of Moringa leaf powder. Supplemented feed preparation, research subjects, and experimental designThe resulting extract was freeze-dried and incorporated into commercial rat feed (CitraFeed RatBio). The dosage determination was referenced and adjusted according to prior research by Setiasih et al. (2019), resulting in doses of 53.5 mg for crude ethanol extract (MOLEE), 36.5 mg for ethanol fraction (MOLEF), and 26 mg for n-hexane extract (MOLHE) per 20 g of feed. This study was an experimental investigation employing a completely randomized design using female Rattus norvegicus of the Sprague–Dawley strain, aged 10–12 weeks, weighing 150–200 g, and nulliparous (having never mated or given birth). A total of 44 rats were randomly divided into four groups: the control group, MOLEE, MOLEF, and MOLHE, with each group comprising 11 rats. Supplementation protocolsAfter acclimatization, the animals were supplemented with feed enriched with different Moringa extracts at 20 g per rat per day starting from the preconception period (3 estrous cycles ≈ 15 days). On day 16, three estrous rats from each group were euthanized. Feeding (30 g per rat per day) was continued during the gestation period (from day 5 until parturition), with another three rats from each group being euthanized on day 16. The feed (30 g per rat per day) was continued for lactating mothers from day 1 postpartum until day 18, and on day 19, five remaining rats from each group were euthanized. Hematology testBlood collection was performed via intracardiac puncture when the rats were euthanized at each reproductive phase. Fresh blood samples were collected in EDTA tubes and immediately analyzed using the Alovet Alovision HM560v Hematology Analyzer. The observed hematological parameters constitute the core components of routine hematology, encompassing the white blood cell (WBC) count, RBC count, Hgb concentration, hematocrit (Hct), and platelet (Plt) count. Measurement of reproductive hormone levelsIn addition to hematological analysis, a significant portion of the collected blood was centrifuged at 2,000 rpm for 20 minutes. Plasma was collected in Eppendorf tubes and analyzed for estrogen (Rats Estradiol Kit BT Lab, cat: EA0011Ra), progesterone (Rats Progesterone Kit BT Lab, cat: EA0063Ra), prolactin (Rats Prolactin Kit BT LAB, cat: E2212Ra), and oxytocin levels (Rats Oxytocin Kit BT LAB, cat: E1476Ra) using an ELISA Reader (Diatek DR-200BC). Calculation of litter size, prenatal mortality rate, and birth weight of offspringAt parturition, the number of offspring born was counted immediately after birth (both live and stillborn) to obtain data on litter size and prenatal mortality rate. In addition, all offspring were weighed immediately after birth using a mini digital scale (MH-Series Pocket Scale) with a precision of 0.01 g to obtain birth weight data. Measurement of milk production by lactating rat mothers and offspring weight gainMilk production was measured using the pup weight method, referencing Fachruddin et al. (2017), with slight modifications to the number of measurement sessions. Milk production was measured every 3 days from day 3 to 18 postpartum, with three measurement sessions conducted every 8 hours, specifically at 16:00 (afternoon), 00:00 (midnight), and 08:00 (morning). On the day of milk production measurements, pups were initially separated from their dams for 4 hours. Following this fasting period, the pups were weighed (w1) and then reunited with the dams for a 1-hour suckling period, after which a second weighing was performed (w2). Milk production was calculated as the difference between the pups’ body weights after (w2) and before suckling (w1). This difference exhibits a positive correlation with the dam’s milk volume. The total milk production for each measurement day was obtained by calculating the area under the curve from the three measurement sessions. Meanwhile, the weight gain of the offspring was calculated from the difference in weight before fasting on day 18 of lactation compared to their weight before fasting on day 3 of lactation. Statistical analysisThe data obtained were processed using IBM SPSS 30 software. The analysis commenced with tests for normality and homogeneity. Data that followed a normal distribution were analyzed using parametric ANOVA, while non-normally distributed data were analyzed using the nonparametric Kruskal–Wallis test. Duncan’s test followed results indicating significant differences (p < 0.05) at a 95% confidence interval. Ethical approvalThe procedures for handling and maintaining the experimental animals in this study have been approved by the Animal Ethics Committee of the School of Veterinary Medicine and Biomedical Sciences, IPB University (approval number: 187/KEH/SKE/III/2024). Animal treatment was conducted with minimum pain or discomfort, following the guidelines established by the Institutional Animal Ethics Committee. ResultsHematological profileThe hematological profiles of female rats administered with Moringa leaf extract during the reproductive period are presented in Table 1. This table indicates that supplementing various Moringa leaf extracts has differing effects on the hematological values across different reproductive periods. The data revealed that the MOLEE group (14.80 ± 4.13 103/µl) has a significantly (p = 0.05) higher mean WBC count than the control (7.75 ± 2.85 103/µl) and MOLEF groups (6.81 ± 1.40 103/µl) during pregnancy. Regarding the RBC parameter during pregnancy, the MOLEE (6.14 ± 0.49 106/µl) and MOLEF (6.01 ± 0.25 106/µl) groups exhibited significantly (p = 0.02) higher mean values than the control group (4.81 ± 0.67 106/µl). Furthermore, during pregnancy, the mean Hgb levels in all treatment groups are significantly (p = 0.00) higher than in the control group. In addition, for the Hct parameter, the MOLEE group (37.16% ± 0.56%) during pregnancy and the MOLEF group (39.9% ± 4.77 %) during lactation each showed mean values that were significantly (p < 0.05) higher than the control group during pregnancy (28.1% ± 3.93%) and lactation (29% ± 9.55%), respectively. For the Plt parameter, all treatment groups, except for the MOLEF group during pregnancy, demonstrate higher Plt counts across all reproductive periods. However, these differences were not statistically significant (p > 0.05). Reproductive hormone levelsThe estrogen and progesterone levels during the prepregnancy and pregnancy periods, as well as the prolactin and oxytocin levels during lactation, are presented in Figure 1. The data indicate that during the prepregnancy and pregnancy periods, the estrogen levels in the MOLHE group (70.56 ± 0.00 and 57.19 ± 0.00 pg/ml, respectively) differ significantly (p = 0.00) from the control group (55.52 ± 0.00 and 50.62 ± 0.00 pg/ml, respectively) compared with the other treatment groups (Fig. 1A and B). Furthermore, in assessing progesterone levels, the data revealed significant differences (p < 0.05) among all groups during the prepregnancy and pregnancy periods. However, distinct responses were observed between the two treatment groups across the two periods, with the highest progesterone levels found in the MOLEF group (206.44 ± 0.00 ng/ml) during the prepregnancy period and in the MOLEE group (187.39 ± 0.00 ng/ml) during the pregnancy period. During the lactation period, the data showed significant differences (p = 0.00) in prolactin levels among all groups, whereas oxytocin levels did not differ significantly (p = 0.05). The prolactin levels in all supplemented groups appeared to be higher than those of the control group, with the MOLHE group (647.40 ± 0.00 pg/ml) recording the highest levels. Table 1. Table 1. Effects of MOLE on hematological values.
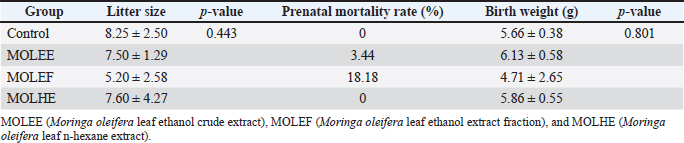
Milk production by lactating rat mothers and offspring weight gainThe average estimated milk production of lactating rat mothers and the percentage of body weight gain in their offspring are presented in Table 2. The data indicate significant differences (p = 0.00) in the average milk production every 3 days over the 15-day measurement period among all groups. The MOLEE group recorded the highest average estimated milk production at (34.23 ± 12.79 g), followed by the MOLEF group (30.82 ± 11.63 g) and the MOLHE group (29.62 ± 12.84 g). Furthermore, the data also showed no significant differences (p = 0.35) in the body weight gain of the offspring among the groups. Nevertheless, the supplemented groups exhibited higher weight gains than the control group. Litter size, prenatal mortality rate, and birth weight Data regarding litter size, prenatal mortality rates, and birth weights of the offspring from rat mothers consuming diets containing Moringa leaf extract during the prepregnancy and pregnancy periods are presented in Table 3. The results indicate no significant differences (p > 0.05) in litter size and birth weight among all groups. Litter size ranged from 5.20 to 8.25 offspring per mother, whereas the birth weight of the offspring was between 4.71 and 6.13 g. The data also show instances of prenatal mortality in the MOLEF (18.18%) and MOLEE (3.44%) groups, whereas the MOLHE and control groups exhibited a fetal survival rate of 100%. DiscussionFemale mammals undergo physiological changes throughout their reproductive lifespan, including the estrous cycle, ovulation, pregnancy, and lactation. These changes affect various aspects, including hormone levels (particularly reproductive hormones), appetite, body weight, and immunity. In response to these changes, female mammals must maintain optimal health conditions and superior reproductive performance to sustain productivity and produce high-quality offspring. The present study reveals that female rats consuming Moringa leaf extract can maintain and even enhance most hematological parameters (WBC, RBC, Hgb, and Hct), approximating the normal range during the reproductive period (Table 1). A more significant increase was observed in the MOLEE group during pregnancy. These results suggest the potential for beneficial effects in improving the health status of rats across all reproductive phases. The increase in hematological value is strongly suspected to be influenced by the synergistic activity of bioactive compounds, such as flavonoids and polyphenols, as well as vitamins and minerals in Moringa leaf extract. The positive effects on hematological values can be explained through the mechanisms of action of the bioactive compounds involved in several pathways, such as modulation of oxidative stress and enhancement of hematopoietic function (Olukanni et al., 2023), modulation of immune response (Olaniran et al., 2019), modulation of iron metabolism, stimulation of erythropoiesis, and increased osmotic resistance, as well as engagement of antioxidant activity (Médard et al., 2023). Moreover, this finding is consistent with several previous studies that have indicated that the administration of Moringa leaf can improve the hematological value of various research subjects in both healthy or normal conditions, such as albino rats and rabbits (Osman et al., 2012) and quail (Coturnix cotrunix japonica) (Salam et al., 2025), as well as in pathological conditions, such as benzene-induced leukemia Wistar rats (Akanni et al., 2014), Plasmodium berghei berghei-infected mice (Olaniran et al., 2019), phenylhydrazine-induced anemic Wistar rats (Olukanni et al., 2023), hemolytic anemic Wistar rats (Médard et al., 2023), and women with iron deficiency anemia (Suzana et al., 2017).
Fig. 1. Effects of MOLE on reproductive hormone levels. (A) Estrogen pre-pregnancy; (B) Estrogen pregnancy; (C) Progesterone pre-pregnancy; (D) Progesterone pregnancy; (E) Prolactin lactation; and (F) Oxitocyn lactation. MOLEE (Moringa oleifera leaf ethanol crude extract), MOLEF (Moringa oleifera leaf ethanol extract fraction), and MOLHE (Moringa oleifera leaf n-hexane extract). Different letters on the same bar highlight significant differences between groups (p < 0.05). Table 2. Effects of MOLE on averaged estimate milk yield and pups weight gain.
Table 3. Effects of MOLE on litter size, prenatal mortality rate, and birth weight.
The composition of bioactive compounds in each Moringa leaf extract or fraction may influence differences in reproductive hormone levels observed in this study. For instance, the increase in estrogen levels (Fig. 1A and B) in the MOLHE group is attributed to the activity of phytosterol compounds. These compounds, such as lathosterol, campesterol, stigmasterol, and β-sitosterol, are present in the hexane fraction of Moringa leaf (Setiasih et al., 2019). The nonpolar nature of these compounds’ ring structure and long carbon chains facilitates their solubility in n-hexane solvent (Uddin et al., 2018). Due to their cholesterol-like structure in animals, these compounds have been reported to be precursors to estrogen hormones via the steroidogenesis pathway (Setiasih et al., 2021). Furthermore, this result is consistent with the findings of Setiasih et al. (2021), who reported that the hexane fraction of Moringa leaf enhances estrogen levels in rabbits before mating. Meanwhile, the elevated progesterone levels (Fig. 1C and D) in the MOLEF group (prepregnancy) and MOLEE group (pregnancy) may be associated with isoflavone compounds reported to be present in Moringa leaf. These compounds are predominantly polar and are thus also found in polar extracts, such as ethanol. Moringa leaf isoflavone may regulate the balance of reproductive hormones through their influence on steroidogenesis by inhibiting the activity of the aromatase enzyme, which is crucial in estrogen synthesis. Consequently, steroidogenesis may only progress to the formation of progesterone, dehydroepiandrosterone sulfate, or androstenedione. Isoflavones can inhibit the activity of cytochrome P450 17α-hydroxylase (CYP17A1), essential for producing androgen precursors (Swart et al., 2019). This inhibition may explain why progesterone levels in the MOLEF (pre-pregnancy) and MOLEE (pregnancy) groups are significantly higher than in the control group. The synthesis and secretion of prolactin occur in the lactotroph cells of the anterior pituitary gland and are regulated by a complex interplay of various factors and pathways. Dopamine is the primary regulator inhibiting prolactin release. At the same time, several hormones (e.g., estrogen and thyrotropin-releasing hormone) and other factors (e.g., serotonin) can stimulate its release (Freeman et al., 2000). In this study, our finding indicates that prolactin levels during lactation in all treatment groups appear to be higher than in the control group, with the MOLHE group showing the highest levels (Fig. 1E). The increase in prolactin levels in the MOLHE group was likely closely related to the high estrogen levels during pregnancy (Fig. 1B). The significant role of estrogen in the synthesis and secretion of prolactin occurs through various mechanisms that collectively contribute to regulating prolactin levels. These mechanisms include the modulation of ion channels in lactotroph cells responsible for prolactin secretion (Sánchez et al., 2017), inhibition of the interaction between Gi3 and Gs proteins regulated by the D2S receptor, leading to increased prolactin production and lactotroph cell proliferation (Sengupta and Sarkar, 2012), and direct influence on prolactin gene expression through binding to specific DNA regions upstream of the prolactin transcription start site (Oseko et al., 1993). In the MOLEF and MOLHE groups, although estrogen levels were not as high as those in the MOLHE group, the effects of increasing prolactin levels may occur through the role of phytoestrogen compounds that exhibit estrogenic effects, although with a weaker influence compared to endogenous estrogen due to their lower affinity for estrogen receptors. In addition to estrogen regulation, further studies are needed on other bioactive compounds that may influence the synthesis and secretion of prolactin through different mechanisms, such as their effects on the dopaminergic or serotonergic pathways. Furthermore, our finding indicates that incorporating Moringa leaf extract into the diet of female rats, consumed before pregnancy through lactation, significantly enhances milk production and optimizes weight gain in offspring (Table 2). Nonpolar and polar extracts contribute to the galactagogue effects. Prior research has indicated that Moringa leaf’s galactagogue effect primarily occurs through hormonal action mechanisms, increasing prolactin levels. All supplemented groups experienced increased prolactin (Fig. 1E). Interestingly, the MOLEE group, which had lower prolactin levels, exhibited greater milk yield than the MOLHE and MOLEF groups, which had higher prolactin levels (Table 2). These observations suggest that although prolactin is the primary regulator of milk synthesis and secretion, it is not the sole determining factor in milk production. The synthesis and secretion of milk in the alveoli of the mammary glands is a complex process regulated by a combination of local, hormonal, and molecular mechanisms (Biswas et al., 2022). Therefore, the significant increase in milk production in the MOLEE group warrants consideration of other factors. The research indicates that the MOLEE group experienced increased proliferation of mammary epithelial cells, as evidenced by a greater number of alveoli and larger diameters (unpublished data). The number and size of alveoli directly affect milk accumulation and production capacity by enhancing overall milk synthesis and storage capacity (Pandey et al., 2018). The MOLEE group also exhibited higher prolactin receptor expression in the mammary glands than the other groups (unpublished data). These findings are supported by previous research, which has revealed that enhancing the diameter and number of mammary gland alveoli, increasing prolactin levels, and upregulating PRLR gene expression, all of which positively contribute to milk production parameters (Salahshoor et al., 2018). Another factor that plays a role is that the bioactive compounds in Moringa leaf can either decrease or increase prolactin sensitivity. Our findings demonstrate that Moringa leaf extract has galactagogue properties that can address insufficient milk production in lactating mothers and weight deficiency in offspring. This study revealed both the beneficial effects of Moringa leaf extract on reproductive performance and its concomitant adverse consequences. Our finding indicates that consuming Moringa leaf extract, particularly polar extracts such as MOLEE and MOLEF, during pregnancy can negatively affect fetal development (Table 3). The cases of prenatal mortality in the groups receiving polar extracts of Moringa leaf may be attributed to polar and/or semipolar bioactive compounds, the types and underlying mechanisms of which require further investigation. Several previous studies have reported Moringa leaf extracts’ adverse effects on fetal development. The teratogenic and embryotoxic effects of water extracts from Moringa leaf in a zebrafish embryo model can lead to prenatal mortality and severe morphological abnormalities (David et al., 2016). Another study revealed that using water extracts in Wistar rats reported occurrences of conception prevention, induced abortion, and increased uterine contractions in the myometrium (Attah et al., 2020). Moreover, using 70% ethanol extract in pregnant Wistar rats strengthens the suspicion of potential adverse effects of polar compounds during pregnancy (Abdu et al., 2023). The study revealed an increase in fetal resorption, a decrease in fetal and placental weight, and the absence of proximal hind limb digits. All the findings mentioned provide substantial evidence regarding the toxic effects of polar extracts from Moringa leaf on fetal development and the potential for prenatal mortality. This research has several limitations that may affect the discussion’s analysis, interpretation, and depth. The limitations of this study include the small sample size, which necessitates caution when generalizing the findings. In addition, the research variables used to assess the health status of the animals were limited to hematological examinations. The study did not include other parameters, such as blood chemistry, physical examinations, and behavioral assessments. Furthermore, although this study demonstrated a significant increase in prolactin levels in lactating rats, further investigation is required to explore other detailed mechanisms related to the role of active compounds in regulating prolactin synthesis and secretion, aside from the critical role of estrogen. Similarly, potentially toxic effects on the pups, particularly concerning physical developmental parameters such as nasoanal length, cranial diameter, cranial length, and tail length on postnatal days, were also not observed, in addition to prenatal mortality parameters. Moreover, the types of polar/semipolar compounds in Moringa leaf extract and the underlying mechanisms of the adverse effects (prenatal mortality in fetuses) they cause need to be further researched. Therefore, future studies should be conducted using larger sample sizes, examining physical health, physiological and behavioral variables, and physical development parameters of pups on postnatal days, and performing in silico studies to predict which active compounds may influence prolactin regulation and the toxic effects of Moringa leaf on fetal development. ConclusionIt can be concluded that supplementing Moringa leaf extract from prepregnancy through lactation can optimize blood parameters, enhance reproductive hormone levels, and increase milk production in lactating rats. However, the administration during the pregnancy period requires careful consideration because of potential adverse effects on fetal development. AcknowledgmentsThe authors express their gratitude to the Indonesian Education Scholarship (BPI), Center for Higher Education Funding and Assessment, Ministry of Higher Education, Science, and Technology of the Republic of Indonesia, and the Endowment Fund for Education Agency (LPDP), Ministry of Finance of the Republic of Indonesia, for their generous financial support. Conflict of interestAll authors declare that they have no conflicts of interest. FundingThe research was supported by a grant from the Indonesian Education Scholarship (BPI), Center for Higher Education Funding and Assessment, Ministry of Higher Education, Science, and Technology of Republic of Indonesia, and the Endowment Fund for Education Agency (LPDP), Ministry of Finance of Republic of Indonesia, with contract number 1987/ J5.2.3./BPI.06/10/2021. Author’s contributionsF.F.: proposed the idea, collected, analyzed, and interpreted the data, and drafted the original manuscript. A.S., W.M., N.H., and H.S.D.: conceptualized the idea, refined the research methodology, interpreted the data, and contributed to writing, reviewing, and editing. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data are available in the manuscript. ReferencesAbdu, H., Ergete, W., Tadele, A., Woldekidan, S., Abebe, A. and Seyoum, G. 2023. Toxic effects of 70% ethanol extract of Moringa stenopetala leaf (Baker f.) Cufod. (Moringaceae) on fetus and placenta of pregnant Wistar rats. BMC Complement. Med. Ther. 23(1), 105. Akanni, E.O., Adedeji, A.L., Adedosu, O.T., Olaniran, O.I. and Oloke, J.K. 2014. Chemopreventive and anti-leukemic effects of ethanol extracts of Moringa oleifera leaves on Wistar rats bearing benzene induced leukemia. Curr. Pharm. Biotechnol. 15(6), 563–568. Asekunowo, A.K., Ebabhi, A.M. and Ogundajo, A.L. 2022. Comparative study of the phytochemical profile, proximate content and antioxidant properties of leaves, seeds and pods of Moringa oleifera. Ann. Sci. Technol. 7(2), 62–68. Attah, A.F., Moody, J.O., Sonibare, M.A., Salahdeen, H.H., Akindele, O.O., Nnamani, P.O., Diyaolu, O.A. and Raji, Y. 2020. Aqueous extract of Moringa oleifera leaf used in Nigerian ethnomedicine alters conception and some pregnancy outcomes in Wistar rat. S. Afr. J. Bot. 129, 255–262. Baimishev, M., Baimishev, R., Eremin, S. and Baimishev, H. 2020. Hematological parameters that determine the course of labor and the postpartum period in highly productive Holstein cows. BIO Web Conf. 27, 16–20. Bezerra, L.R., Oliveira, W.D.C., Silva, T.P.D., Torreão, J.N., Marques, C.A., Araújo, M.J. and Oliveira, R.L. 2017. Comparative hematological analysis of Morada Nova and Santa Inês ewes in all reproductive stages. Pesqui. Vet. Bras. 37(4), 408–414. Biswas, S.K., Banerjee, S., Baker, G.W., Kuo, C.Y. and Chowdhury, I. 2022. The mammary gland: basic structure and molecular signaling during development. Int. J. Mol. Sci. 23(7), 3883. Colditz, I.G., Smith, E.G., Ingham, A.B. and Dominik, S. 2023. Indicators of functional integrity in production animals. Anim. Prod. Sci. 63(9), 825–843. Dasat, G.S., Danjuma, G. and Chundusu, E.S. 2020. Evaluation of the nutritionally valuable mineral composition of Moringa oleifera leaf. Eur. J. Nutr. Food Saf. 12(10), 46–53. David, C.R.S., Angeles, A., Angoluan, R.C., Santos, J.P.E., David, E.S. and Dulay, R.M.R. 2016. Moringa oleifera (Malunggay) water extracts exhibit embryo-toxic and teratogenic activity in zebrafish (Danio rerio) embryo model. Der. Pharm. Lett. 8, 163–168. Etim, N. 2014. Haematological parameters and factors affecting their values. Agric. Sci. 2(1), 37–47. Fachruddin, F., Suprayogi, A. and Hanif, N. 2017. Addition of hexane fraction from Sauropus androgynus leaves Zanzibar variety for increasing milk yield and performance of female and rat pups. J. Vet. 18(2), 289–296. Fachruddin, F., Suprayogi, A., Manalu, W., Hanif, N. and Darusman, H.S. 2024. Data on characteristics of simplicia, phytoconstituents, and antioxidant activity of Moringa oleifera leaves ethanol extract. Data Br. 54, 1–11. Freeman, M.E., Kanyicska, B., Lerant, A. and Nagy, G. 2000. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80(4), 1523–1631. Hasan, M.M., Soares, M.R.J., Garnett, S.P., Fatima, Y., Tariqujjaman, M., Pervin, S., Ahmed, S. and Mamun, A.A. 2022. Anaemia in women of reproductive age in low-and middle-income countries: progress towards the 2025 global nutrition target. Bull. World Health Organ. 100(3), 196–204. Hasim, H., Tunnisa, F., Faridah, D.N., Saraswati, S. and Slameut, F. 2025. Antioxidant, lipase inhibitory potential, and UHPLC profile of different fractions of Moringa oleifera leaves. Trop. J. Nat. Prod. Res. 9(1), 31–36. Hassan, I.M., Saidu, B., Ishaq, J.A., Bello, M.B., Abubakar, A.A. and Baraya, Y.S. 2020. Changes in haematological parameters following toxicity study with 80% methanol extract of Moringa oleifera in Wistar rats. Am. J. Appl. Chem. 8(6), 143–151. Kalauni, S.K., Khanal, L.N., Thapa, P. and Kunwor, K. 2023. Phytochemical screening, antioxidant, antibacterial, and α-amylase inhibitory activity of Moringa oleifera Lam. leaves. JNCS 43(2), 141–150. Khalil, A.M., Shanab, O.M., Zannoni, G.F. and Heygazy, E.M. 2018. Assessment of hematological and biochemical markers in the early post-burn period for predicting recovery and mortality in animals with burn injuries. J. Clin. Exp. Pathol. 08(02), 8–12. Levine, B.S. 2001. Animal clinical pathology. In Handbook of toxicology second edition (chapt. 18). Eds., Derelanko, M.J. and Hollinger, M.A. Boca Raton, FL: CRC Press. McClain, A.M., Whitmer, E.R., Rios, C., Jensen, E.D., Stacy, N.I. and Johnson, S.P. 2022. Evaluation of the hemoCue® WBC system as a point of care diagnostic tool for white blood cell quantification in Pinnipeds. Oceans 3(1), 72–83. Médard, S.F., Pascal, T.A., Espérance, M.S., Félicienne, A., Yollande, A., Roxane, K., Ezéchiel, L.J., Alban, H., Amegnon, A., Djimon, G.B., Lamine, B. and Maximin, S. 2023. Aqueous leaf extract of Moringa oleifera (Moringaceae) effectively treats induced hemolytic anemia in Wistar rats. J. Biosci. Med. 11(8), 154–168. Ogunsola, A.O., Joshua, O. and Sunday, F.O. 2017. Moringa plant parts consumption had effects on reproductive functions in male and female rat models. IOSR J. Dent. Med. Sci. 16(10), 82–86. Olaniran, O., Adetuyi, F., Omoya, F., Odediran, S.A., Hassan-olajokun, R.E., Awoyeni, E.A., Odetoyin, B.W., Adesina, A., Awe, A., Bejide, R.A., Odujoko, O., Akinyemi, L.E., Oyetoke, O.O. and Afolayan, D.O. 2019. Antiplasmodial, antipyretic, haematological and histological effects of the leaf extracts of Moringa oleifera in Plasmodium berghei berghei infected mice. JAMMR 29(4), 1–13 Olukanni, A., Minari, J. and Okpuzor, J. 2023. Evaluation of antioxidant and anti-anaemic potential of Moringa oleifera parts on phenylhydrazine-induced haematoxicity in male Wistar albino rats. JASEM 27(10), 2323–2329. Oseko, F., Morikawa, K., Nakano, A. and Taniguchi, A. 1993. Biosynthesis and secretory regulation of pituitary prolactin. Nihon Rinsho 51(10), 2592–2597. Osman, H.M., Shayoub, M.E. and Babiker, E.M. 2012. The effect of Moringa oleifera leaves on blood parameters and body weights of albino rats and rabbits. JJBS 5(3), 147–150. Pandey, Y., Taluja, J.S., Vaish, R., Pandey, A., Gupta, N. and Kumar, D. 2018. Gross anatomical structure of the mammary gland in cow. J. Entomol. Zool. Stud. 6(4), 728–733. Patil, S.V., Mohite, B.V., Marathe, K.R., Salunkhe, N.S., Marathe, V. and Patil, V.S. 2022. Moringa tree, gift of nature: a review on nutritional and industrial potential. Curr. Pharmacol. Rep. 8(4), 262–280. Pessini, P.G.S., de Souza, P.R.K., Chagas, C.S., Sampaio, E.G., Neves, D.S., Petri, G., Fonseca, F.L.A. and da Silva, E.B. 2020. Hematological reference values and animal welfare parameters of BALB C-FMABC (Mus musculus) inoculated with Ehrlich tumor kept in the vivarium at ABC Medical School. Anim. Model Exp. Med. 3, 32–39. Purwar, V., Cherryl, D., Singh, S., Kumar, J., Khare, A. and Thorat, G. 2019. Assessment of haematological parameters during different climatic seasons. J. Pharmacogn. Phytochem. 8(1), 1741–1744. Rubio-Sanz, L. 2020. Comparativa nutricional del cultivo de Moringa oleifera en España. Cienc y Tecnol. 13(2), 17–22. Safitri, R. and Retnaningsih, R. 2021. Role of Moringa oleifera leaf extract in increasing hemoglobin levels in pregnant rats with anemia. J. Health Sci. 14(1), 8–13. Salahshoor, M.R., Mohammadi, M.M., Roshankhah, S. and Jalili, C. 2018. Effect of Falcaria vulgaris on milk production parameters in female rats’ mammary glands. JFRH 12(4),177–183. Salam, A., Ulupi, N. and Maheshwari, H. 2025. Suplementary Moringa leaf meal for treating anemia in quail. IOP Conf. Ser. Earth Environ. Sci. 1484, 012030. Saleh, A., Adamu, S.S., Lawan, M.K., Isa, I., Habibu, B., Enam, S.J. and Idris, S.Y. 2019. Trend in occurrence of diseases causing abnormal haematological values in domestic animals based on haematological records of cases in Zaria and its environs. Niger. Vet. J. 40(1), 44–55. Sánchez, M., Suárez, L., Cantabrana, B. and Bordallo, J. 2017 Estradiol-modified prolactin secretion independently of action potentials and Ca 2+ and blockade of outward potassium currents in GH 3 cells. Naunyn Schmiedebergs Arch. Pharmacol. 390(1), 95–104. Sengupta, A. and Sarkar, D.K. 2012. Estrogen inhibits D2S receptor-regulated Gi3 and Gs protein interactions to stimulate prolactin production and cell proliferation in lactotropic cells. J. Endocrinol. 214(1), 67–78. Setiasih, Wahjuningsih, S., Winarsih, S. and Hendrawan, S. 2019. The effects of adding Moringa oleifera leaves extract on rabbit does’ milk production and mammary gland histology. Russ. J. Agric. Socio-Economic Sci. 92(8), 296–304. Setiasih, Wahjuningsih, S., Winarsih, S. and Hendrawan, S. 2021. The concentrations of cholesterol and reproduction hormones in serum of rabbits doe that consumed Moringa oleifera leaf extracts. Int. J. Curr. Sci. Res. Rev. 04(12), 1697–1703. Suzana, D., Suyatna, F.D., Azizahwati, Andrajati, R., Sari, S.P. and Mun’im, A. 2017. Effect of Moringa oleifera leaves extract against hematology and blood biochemical value of patients with iron deficiency anemia. J. Young Pharm. 9(1), s79–s84. Swart, A.C., Johannes, I.D., Sathyapalan, T. and Atkin, S.L. 2019. The effect of soy isoflavones on steroid metabolism. Front. Endocrinol. 10(229), 1–10. Trigo, C., Castelló, M.L. and Ortolá, M.D. 2023. Potentiality of Moringa oleifera as a nutritive ingredient in different food matrices. Plant Foods Hum. Nutr. 78(1), 25–37. Uddin, M.S., Ferdosh, S., Akanda, M.J.H., Ghafoor, K., Rukshana, A.H., Ali, M.E., Kamaruzzaman, B.Y., Fauzi, M.B., Hadijah, S., Shaarani, S. and Sarker, M.Z.I. 2018. Techniques for the extraction of phytosterols and their benefits in human health: a review. Sep. Sci. Technol. 53(14), 2206–2223. | ||
| How to Cite this Article |
| Pubmed Style Fachruddin F, Suprayogi A, Manalu W, Hanif N, Darusman HS. Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. Open Vet. J.. 2025; 15(6): 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 Web Style Fachruddin F, Suprayogi A, Manalu W, Hanif N, Darusman HS. Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. https://www.openveterinaryjournal.com/?mno=243683 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.34 AMA (American Medical Association) Style Fachruddin F, Suprayogi A, Manalu W, Hanif N, Darusman HS. Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. Open Vet. J.. 2025; 15(6): 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 Vancouver/ICMJE Style Fachruddin F, Suprayogi A, Manalu W, Hanif N, Darusman HS. Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 Harvard Style Fachruddin, F., Suprayogi, . A., Manalu, . W., Hanif, . N. & Darusman, . H. S. (2025) Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. Open Vet. J., 15 (6), 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 Turabian Style Fachruddin, Fachruddin, Agik Suprayogi, Wasmen Manalu, Novriyandi Hanif, and Huda Shalahuddin Darusman. 2025. Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. Open Veterinary Journal, 15 (6), 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 Chicago Style Fachruddin, Fachruddin, Agik Suprayogi, Wasmen Manalu, Novriyandi Hanif, and Huda Shalahuddin Darusman. "Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period." Open Veterinary Journal 15 (2025), 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 MLA (The Modern Language Association) Style Fachruddin, Fachruddin, Agik Suprayogi, Wasmen Manalu, Novriyandi Hanif, and Huda Shalahuddin Darusman. "Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period." Open Veterinary Journal 15.6 (2025), 2651-2660. Print. doi:10.5455/OVJ.2025.v15.i6.34 APA (American Psychological Association) Style Fachruddin, F., Suprayogi, . A., Manalu, . W., Hanif, . N. & Darusman, . H. S. (2025) Hematology and reproductive performance of female rats supplemented with kelor (Moringa oleifera Lam.) leaf extract during the reproductive period. Open Veterinary Journal, 15 (6), 2651-2660. doi:10.5455/OVJ.2025.v15.i6.34 |