| Short Communication | ||
Open Vet. J.. 2025; 15(6): 2903-2908 Open Veterinary Journal, (2025), Vol. 15(6): 2903-2908 Short Communication Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, IndonesiaJanuaria Maria Seran1, Tri Untari2*, Asmarani Kusumawati3 and Ridha Avicena Ila Salsabila41Master of Veterinary Science, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Department of Microbiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 3Department of Reproduction, Obstetrics, and Gynaecology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Veterinary Sciences Doctoral Program, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Tri Untari. Department of Microbiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: t_untari [at] ugm.ac.id Submitted: 24/02/2025 Revised: 13/05/2025 Accepted: 15/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
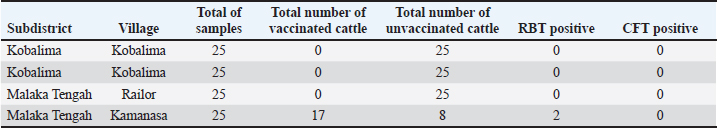
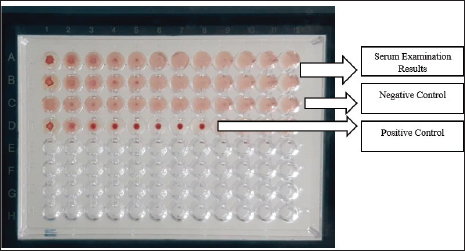
AbstractBackground: One disease that interferes with reproductive performance is brucellosis, which causes significant economic losses. Aim: This study aimed to identify the reactor and evaluate the results of a Brucella abortus vaccination in the Malaka Regency. Methods: This research method involves the first stage, which involves sampling serum from 100 cattle. Serum specimens are derived from blood taken from the jugular or coccygeal vein using a Vacutainer tube filled with silicone and left at room temperature until the blood is separated into serum and clot. Then, for serological tests in the form of the Rose Bengal test (RBT), if the RBT test is positive, followed by the complement fixation test (CFT) test, and the CFT test is also positive, the patient is designated as a brucellosis reactor. At the same time, if the CFT is positive in vaccinated cattle, the vaccination is considered successful, and if it is negative, it is necessary to reevaluate the immunization. Result: The results of 100 samples showed that the RBT and CFT tests yielded two distinct outcomes: a positive RBT and a negative CFT test, indicating that no B. abortus reactors were detected. Conclusion: Cattle in Malacca Regency were not found to be positive for the B. abortus reactor. Vaccination results against Brucella are not optimal, as indicated by the absence of an antibody response, as determined by RBT and CFT tests. Keywords: Brucella abortus, RBT, CFT, Reactor. IntroductionAs one of the most widely consumed animal food products, beef is in increasing demand, but is not accompanied by a corresponding increase in production. According to the Ministry of Agriculture, in 2024, beef production is projected to reach 416.7 million tonnes, with an additional 16.2 million tonnes of buffalo meat, resulting in a total supply of 432.9 million tonnes. Meanwhile, national consumption is estimated to reach 74.2 million tonnes, resulting in a meat deficit of 291.3 million tonnes. The government, through the Ministry of Agriculture, continues to strive to increase beef production through the Special Effort Program for Cattle and Buffalo as National Superior Commodities (SIKOMANDAN), which is a continuation of the Special Effort Program for Mandatory Pregnant Cattle (UPSUS SIWAB). The Indonesian government initiated this program through the Minister of Agriculture’s Regulation No. 17 of 2020, which aims to increase the production of beef and buffalo as superior national commodities to meet the demand for domestic beef consumption (Tagueha, 2020). Problems faced during the implementation of this program include the spread of diseases that attack reproductive organs, which have an impact on reproductive performance and domestic beef production. Brucellosis is a disease found in various parts of the world caused by the bacterium Brucella abortus. This facultative anaerobic Gram-negative bacterium, which does not form spores, is an intracellular bacterium (Ridlo et al., 2024). This disease was first documented in 1935, and it remains prevalent in Indonesia today. Some endemic areas still happen frequently. Brucellosis is common in Java, Sulawesi, and East Nusa Tenggara. The Directorate General of Animal Husbandry and Animal Health plans to eradicate Brucellosis by 2025. The Brucellosis eradication and control program is a disease control plan that operates in stages to manage the disease, progressing from an unknown status to a known prevalence status (Wulandari et al., 2023). The district area endemic for brucellosis, with the highest prevalence in East Nusa Tenggara Province, has a prevalence rate of >2% (Bouk et al., 2022). Meanwhile, the Malaka Regency Agriculture Service reported that the prevalence of brucellosis in the regency in 2023 was 5.31% of the total cattle population. The control program implemented involves vaccination with the B. abortus vaccine using S19. The Malaka Regency Agriculture Service reported that in 2023, the total cattle population was 71,094. Vaccinations up to 2022 accounted for 3.2% (Bouk et al., 2022). This is still far from the minimum figure set by the Directorate of Animal Husbandry and Animal Health of the Ministry of Agriculture, which is 80% of the total population in areas with a brucellosis prevalence rate of 2% or higher. Through the Malaka Regency, the East Nusa Tenggara Provincial Government has made various efforts to eradicate brucellosis in cattle through a conditional slaughter program and vaccination, including examination and destruction. However, in reality, the spread of this disease has increased from year to year. Surveillance is also carried out actively, and measurement and cutting have not significantly reduced the prevalence rate and the number of reactors detected (Kamalasari et al., 2019). Based on this background, vaccination must also be accompanied by identifying and evaluating reactors to determine reactors that are positive for brucellosis and those that are negative for brucellosis (Hadi et al., 2021). Materials and MethodsTo identify reactors, the brucellosis vaccination and abortion history of 100 study-targeted cattle were documented. Additionally, observations were made regarding the animal’s kind, sex, age, and pregnancy status. Using a silicone-coated Vacutainer tube, blood samples were extracted from the coccygeal or jugular veins and allowed to stand at room temperature until they were separated (serum with clot). Testing can be performed as soon as the serum forms. If testing is postponed, the serum can be stored at either a freezer temperature (–20°C) or a refrigerator temperature (4°C). Rose Bengal Test (RBT) and Complement Fixation Test (CFT)The core concept of RBT is that homologous antigens and antibodies are differentiated by agglutination using the Rose Bengal dye. The RBT technique complied with the WOAH (2016) guidelines. According to the testing technique, 25 µl of each serum sample was deposited onto a hemagglutination plate. Twenty-five microliters of antigen was added to the serum samples. Following the homogenization of serum and antigen ose (inoculation loop), they were subjected to a second homogenization using a rotary agitator for 4 minutes at ambient temperature (22°C ± 4°C). The evaluation criteria for RBT are as follows: negative (-), indicating the absence of agglutination, and positive (+), indicating the presence of agglutination, defined by the formation of clumps in the patient. Positive outcomes of the RBT approach are categorized into three classifications. Specifically, assign a positive 1 (+) if the smooth exhibits agglutination and a positive 2 (++) if the aggregate does not resemble sand grains and fails to form aggregate particles. CFT Method: As per WOAH (2018), multiple procedural stages must be executed before testing, including the preparation of 3% sensitized sheep red blood cells (SRBCs) using an equivalent volume of rabbit anti-SRBC serum, typically diluted 2 to 5 times, to yield 100% SRBCs in conjunction with a titration solution of guinea pig complement. The CFT is conducted at 37°C ± 2°C for 30 minutes. The procedure involves inactivating the serum sample for 30 minutes in a water bath at a temperature of 60°C ± 2°C, followed by the addition of 25 µl of CFT buffer to all wells of the 96-well plate, excluding the wells in the first column (A1–G1). Twenty-five microliters of the serum sample was dispensed into the wells of the first column (A1–F1), with well G1 designated as a positive control and well H1 as a negative control for each plate. Multilevel dilution was performed by moving 25 µl of solution from column 1 to column 2, following the homogenization of the solution in the well of column 1, which served as an anticomplementary control. The solution from column 2 was subsequently transferred to column 3, and this process was continued until the last column, with 25 µl of solution disposed of at each step. This procedure yielded consecutive multilevel dilutions throughout nine wells, specifically 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, and 1:1024. The test was conducted in duplicate for each serum sample. Twenty-five microliters of CFT buffer was added to the wells in column 1 to compensate for the absence of antigens. In contrast, 25 µl of antigen was added to all wells except column 1. The plate was incubated at 37°C ± 2°C for 30 minutes or at 5°C ± 3°C overnight. Subsequently, 25 µl of sensitized BCs were added and incubated again at 37°C ± 2°C for 30 minutes, with rotary agglutination placed in the incubator. The plate was extracted from the incubator and stored in the refrigerator for a minimum of 2 hours or overnight. Adverse reactions are defined by the occurrence of full hemolysis (anti-complement control in column 1). The CFT test findings are classified according to the extent of hemolysis: negative or 100% hemolysis. If complete hemolysis occurs, the liquid in the cup becomes crimson, and many red blood cells settle to the bottom, indicating a positive result (1+) or 75% hemolysis. In positive hemolysis 2 (++) or 50% of cases, most hemolysis has transpired, resulting in a crimson fluid with a slightly expanded red blood cell sediment exhibiting flat edges. In positive hemolysis (3 (+++) or 25%), some red blood cells remain intact, resulting in slightly crimson fluid with clearly visible red blood cell debris. A positive result (4 (++++) or 0%) indicates that the liquid is transparent with no hemolysis and that a distinct red blood cell sediment is observable, showing unambiguous demarcations (Wilujeng, 2023). Ethical approvalThe experimental animal treatment approach has obtained ethical approval from the Faculty of Veterinary Medicine Ethics Commission of Universitas Gadjah Mada, Yogyakarta, Indonesia number 00140/EC-FKH/ Int./2024. Results and DiscussionThe test results of 100 serum samples collected from 4 villages in Malaka Regency using the RBT and CFT methods were positive, with 2 RBT tests yielding positive results. In contrast, all CFT tests were negative (Table 1). Positive RBT results indicate the presence of clotting in serum samples mixed with the RBT antigen, forming fine, sand-like clumps (Fig. 1). All CFT tests gave negative results, as shown in Figure 2. In Figure 2, D1–D8 are positive controls, while C1–C2 are negative controls, and A1–A12 and B1–12 are negative sample results. Table 1. RBT and CFT test results for serum cattle in Malaka Regency.
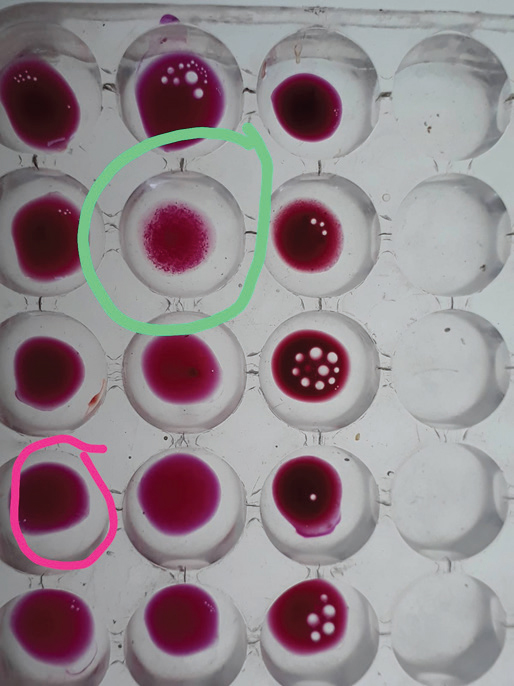
Fig. 1. The RBT test result, with a control positive (red mark) and result sample (green mark), showed 2 (++), indicating 50% agglutination.
Fig. 2. CFT test results of cattle in Malacca Regency. Serum samples A1–A12 and B1–B12 were negative, and the C1–C12 control was also negative. D1–D8: positive control. Based on Table 1, cattle had positive results in the RBT test but negative results in the CFT test. Brucellosis reactors are animals infected with Brucella, as confirmed by diagnostic tests, such as positive RBT and CFT (Tagueha, 2020). The RBT, also known as the RBT, is the first test to detect brucellosis, utilizing the principle of antigen-antibody clotting (Wilujeng, 2023). The RBT test has high sensitivity but low specificity. Several countries worldwide use the RBT as a screening test, followed by the CFT as a confirmatory test to identify the presence of brucellosis (Preena et al., 2024). The CFT is a specific test that can detect immunoglobulin M (IgM) and IgG1 antibodies (Kusumastuti et al., 2021). The CFT test is based on the reaction between the homologous antibody and antigen complex. It attracts complement to bind to the Fc portion of the antibody, thereby lysing red blood cells (Corrente et al., 2015). This makes the CFT the most specific and well-known serological test for diagnosing brucellosis and is recommended for international trade (Stevens et al., 1994). The CFT test is not very sensitive but has reasonable specificity (Dieste-Pérez et al., 2015). The CFT test is difficult to standardize, so during its development, it was replaced by the ELISA methodology (Gwida et al., 2015). The CFT is known to be accurate; however, this test cannot distinguish between antibodies produced by infection and those produced by vaccination (Corrente et al., 2015). In the positive RBT samples, the cows had no history of vaccination. The RBT technique has several weaknesses, notably the potential for cross-reaction with antibodies stimulated by other types of bacteria, such as Yersinia, Bordetella, Salmonella, or Pasteurella, which can lead to false-positive reactions (WOAH, 2016). The RBT test can detect exposure to Brucella sp. bacteria in cows (Preena et al., 2024), which occurs due to an IgM antibody response, as well as the IgG1 and IgG2 isotypes (Gwida et al., 2015; Preena et al., 2024). IgG antibodies are detected 2 weeks after infection and peak at 6–8 weeks (Kartini et al., 2018). IgM is a more reliable indicator of IgM exposure because the response occurs more rapidly. The use of IgM indicators has the potential to cause cross-reactions with other microorganisms, resulting in false-positive reactions in serological tests for Yersinia, Bordetella O:9, Francisella tularensis, Escherichia coli O157, Vibrio cholerae, Stenotrophomonas maltophilia, Salmonella, or Pasteurella (Yanti et al., 2021). The vaccine used in the vaccination program in Malaka Regency is the Brucella strain 19 vaccine produced by the Veterinary Pharmacy Center (PUSVETMA), Ministry of Agriculture, Indonesia. In using the S19 vaccine, false-positive results may be detected because it can stimulate the formation of antibodies against the O-chain antigen, making it challenging to distinguish between antibodies produced by vaccination and those produced naturally through infection. Both vaccines have the potential to cause abortion in pregnant cows. Of the 100 samples, 17 cattle (17% of all samples) were vaccinated. However, 17% of the vaccinated cows were recorded based on routine vaccination history, which was carried out only in Railor Village, while the remaining 83% from other villages had never been vaccinated. Seronegative results in the RBT and CFT tests in vaccinated cows indicate that postvaccination antibodies were not formed. This suggests that the vaccination program implemented has not been effective in protecting against brucellosis at the time of vaccination. The Farma Veterinary Center of the Ministry of Agriculture of Indonesia produced the Brucella vaccine. The vaccine was carried in a cool box and kept cold until it was distributed to the farmers’ cattle in the Malaka Regency. Failure of vaccination requires further research on vaccine quality, cold chain, vaccine application in the field, and livestock health status, which can affect antibody responses (Dorneles et al., 2015; Fernandes et al., 2024). Vaccination coverage in Malaka Regency remains low; according to data from the Agriculture and Food Security Service in 2021, it was only 1.9%, while in 2023, it had increased to 3.2% of the total cattle population in Malaka Regency, which is approximately 80,000 heads. Brucellosis vaccination is considered adequate if the vaccination coverage reaches a minimum of 80% and a maximum of 100% of all vaccinated livestock (Bouk et al., 2022). Factors that can influence low vaccination coverage in Malaka include geography. The proportion of cattle immunized against Brucella abortus falls short of the aim established by the Malaka Regency authority. The majority of the cattle population is in Malaka Regency. The district is unprotected against Brucella abortus, which should be mitigated via vaccination. The factor of geographical conditions of Malaka Regency, the distance of the Agriculture and Food Security Service which is the place for providing vaccines to the village where the cattle will be vaccinated, poor road conditions which hamper the vaccination process, and lack of socialization from officers carrying out vaccinations and farmers’ knowledge of the types of vaccines used. The low vaccination rate in Malaka Regency is also due to the insufficient number of vaccines received by field officers, which fails to cover the number of livestock that need to be vaccinated, resulting in not all livestock being vaccinated. Vaccine distribution is also not carried out routinely every year in each village; thus, not all livestock are vaccinated. One of the causes of the limited number of vaccines is the lack of accurate data on the number of female livestock requiring vaccination. When calculating the number of female livestock, people often fail to report the actual number due to concerns about not receiving livestock assistance from the government (Kurniawati and Trisunuwati, 2010; Bouk et al., 2022). Implementing the vaccination program in Malaka Regency for areas with a prevalence of 2% or more, or regions with severe infections, is not in line with the government’s plan outlined in the Brucellosis Control and Management Roadmap. Vaccination is carried out on all livestock, except for pregnant bulls and cows in areas with a brucellosis prevalence of 2% or higher or severe infections. The second year of vaccination targeted cattle aged 3–9 months and animals that had not received vaccination in the previous year (Horri et al., 2023). The brucellosis control strategy in Indonesia was adopted from the brucellosis control method in Australia, which was declared free of this disease in 1989. The results of this study indicate that the diagnosis of brucellosis is not based only on the RBT test. Decisions regarding the diagnosis of brucellosis should be based at least on RBT and CFT tests and other supporting examinations, such as ELISA, bacterial culture, and PCR, if necessary, as the gold standard for identifying B. abortus reactors. ConclusionThe research in Malaka Regency concluded that no B. abortus reactors were found. The vaccination results in Malaka Regency are not yet optimal, as indicated by the absence of antibodies against Brucella in cattle serum, as determined by the RBT and CFT tests. AcknowledgmentsThe authors would like to thank the Malaka Regency Government, the Faculty of Veterinary Medicine at Universitas Gadjah Mada, and the Disease Investigation Centre, Denpasar, for providing the facilities necessary for this research. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThis research was funded by the authors and the Indonesian Ministry of Finance, LPDP Scholarship, for supporting. Author contributionsJ.M.S., T.U., and A.K. designed the study, conducted the field research, and analyzed the samples in the laboratory. J.M.S., T.U., and R.A.I.S. wrote, edited, read, and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesBouk, A.L., Sanam, M.U.E. and Detha, A.I.R. 2022. Incidence rate, risk factors, and coverage of bruselosis vaccination in Naibone, Sasitamean district, Malaka regency. J. Vet. Nusantara 5(23), 91–101. Corrente, M., Desario, C., Parisi, A., Grandolfo, E., Scaltrito, D., Vesco, G., Colao, V. and Buonavoglia, D. 2015. Serological diagnosis of bovine brucellosis using B. melitensis strain B115. J. Microbiol. Methods 119, 106–109. Dieste-Pérez, L., Blasco, J.M., De Miguel, M.J., Moriyón, I. and Muñoz, P.M. 2015. Diagnostic performance of serological tests for swine brucellosis in the presence of false positive serological reactions. J. Microbiol. Methods. 111, 57–63. Dorneles, E.M., Sriranganathan, N. and Lage, A.P. 2015. Recent advances in Brucella abortus vaccines. Vet. Res. 46(1), 76. Fernandes, C.A.C., Pereira, G.H.S., Pereira, J.R., Alves, D.C., Dias, L.S., Viana, J.H.M. and Drumond, J. 2024. Effectiveness of the RB51 vaccine against brucellosis in adult beef cows. Front. Vet. Sci. 11, 1440599. Gwida, M., El-Ashker, M., Melzer, F., El-Diasty, M., El-Beskawy, M. and Neubauer, H. 2015. Use of serology and real time PCR to control an outbreak of bovine brucellosis at a dairy cattle farm in the Nile Delta region, Egypt. Ir. Vet. J. 69(1), 3. Hadi, S., Sulaxono, R.L. and Siswani. 2021. Kondisi Brucellosis setelah Vaksinasi di Kecamatan Majauleng Kabupaten Wajo Sulawesi Selatan. Prosiding Seminar Nasional Pembangunan dan Pendidikan Vokasi Pertanian 2(1), 114–119. Horri, M., Suyanto. and Jusnita, R.A.E. 2023. Disease control management strategy in Bali cattle. Int. J. Sci. Rev. 5(1), 333–342. Kamalasari, W., Ardhani, F. and Juita, F. 2019. Faktorfaktor yang mempengaruhi pengambilan keputusan peternak dalam melakukan program vaksinasi Jembrana pada Sapi Bali. J. Peternakan Lingkungan Trop. 2(1), 50–62. Kartini, D., Noor, S.M. and Pasaribu, F.H. 2018. Deteksi Brucellosis pada Babi secara Serologis dan Molekuler di Rumah Potong Hewan Kapuk, Jakarta dan Ciroyom, Bandung. Acta Vet. Indones. 5(2), 66–73. Kurniawati, U. and Trisunuwati, P. 2010. Pengaruh vaksinasi brucellosis pada sapi perah dengan berbagai paritas terhadap efisiensi reproduksi. J. Ilmu-Ilmu Peternak. Univ. Brawijaya 20(1), 38–47. Kusumastuti, I., Tyasningsih, W., Praja, R.N., Suwarno, S., Yunita, M.N. and Yudhana, A. 2021. Detection of brucellosis in dairy cattle in Turen District Malang Regency using Rose Bengal Test (RBT) and Complement Fixation Test (CFT) methods. J. Med. Vet. 4(1), 42–47. Preena, P., Ronald, B., South, M., Balakrishnan, S., Murugan, M., Anbu Kumar, K. and Ganesan, P.I. 2024. Serological, bacteriological, and molecular detection of brucellosis in pigs of Tamil Nadu, India. Eme. Anim. Spec. 10, 100041. Ridlo, M.R., Andityas, M., Primatika, R.A., Widantara, H., Loong, S.K. and Nuraini, D.M. 2024. A metaanalysis of livestock brucellosis prevalence in Indonesia. Vet. Q. 44(1), 1–14. Stevens, M.G., Hennager, S.G., Olsen, S.C. and Candeville, N.F. 1994. Serologic responses in diagnostic tests for brucellosis in cattle vaccinated with Brucella abortus 19 or RB51. J. Clin. Microbiol. 32(4), 1065–1066; doi:10.1128/jcm.32.4.1065-1066.1994 Tagueha, A.D. 2020. Identifikasi reaktor brusellosis pada populasi Sapi di Rumah Potong Hewan (RPH) Kota Ambon. J. Liv. Anim. H. 3(2), 39–44. Wilujeng, E. 2023. Serodetection of brucellosis using the Rose Bengal Test (RBT) and Complement Fixation Test (CFT) method in dairy cattle in Banyuwangi. J. B. Med. Vet. 12, 33–39. WOAH. 2016. Manual of diagnostic tests and vaccines for terrestrial animals Brucellosis (Brucella abortus, B. melitensis and B. suis) (Infection with B. abortus, B. melitensis and B. suis). Paris, France: WOAH; 2016. Available via http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.04_BRUCELLOSIS.pdf. (Accessed 11 January 2025>). WOAH. 2018. Manual of diagnostic tests and vaccines for terrestrial animals. Chapters 1.1.2 and 1.1.3. Available via https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/1.01.02_COLLECTION_DIAG_SPECIMENS.pdf (Accessed 11 January 2025). Wulandari, R.A., Wahyuni, A.E.T.H. and Susetya, H. 2023. Risk factor identification and incidence rate measurement on one high-risk farm as brucellosis precautions in Metro City Lampung. J. Adv. Zool. 44(3), 1504–1511. Yanti, Y., Sumiarto, B., Kusumastuti, T.A., Panus, A. and Sodirun, S. 2021. Seroprevalence and risk factors of brucellosis and the brucellosis model at the individual level of dairy cattle in the West Bandung District, Indonesia. Vet. World. 14(1), 1–10. | ||
| How to Cite this Article |
| Pubmed Style Seran JM, Untari T, Kusumawati A, Salsabila RAI. Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. Open Vet. J.. 2025; 15(6): 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 Web Style Seran JM, Untari T, Kusumawati A, Salsabila RAI. Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. https://www.openveterinaryjournal.com/?mno=244458 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.60 AMA (American Medical Association) Style Seran JM, Untari T, Kusumawati A, Salsabila RAI. Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. Open Vet. J.. 2025; 15(6): 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 Vancouver/ICMJE Style Seran JM, Untari T, Kusumawati A, Salsabila RAI. Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 Harvard Style Seran, J. M., Untari, . T., Kusumawati, . A. & Salsabila, . R. A. I. (2025) Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. Open Vet. J., 15 (6), 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 Turabian Style Seran, Januaria Maria, Tri Untari, Asmarani Kusumawati, and Ridha Avicena Ila Salsabila. 2025. Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. Open Veterinary Journal, 15 (6), 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 Chicago Style Seran, Januaria Maria, Tri Untari, Asmarani Kusumawati, and Ridha Avicena Ila Salsabila. "Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia." Open Veterinary Journal 15 (2025), 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 MLA (The Modern Language Association) Style Seran, Januaria Maria, Tri Untari, Asmarani Kusumawati, and Ridha Avicena Ila Salsabila. "Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia." Open Veterinary Journal 15.6 (2025), 2903-2908. Print. doi:10.5455/OVJ.2025.v15.i6.60 APA (American Psychological Association) Style Seran, J. M., Untari, . T., Kusumawati, . A. & Salsabila, . R. A. I. (2025) Reactor identification and evaluation of Brucella abortus vaccination results for cattle in Malaka Regency, East Nusa Tenggara Province, Indonesia. Open Veterinary Journal, 15 (6), 2903-2908. doi:10.5455/OVJ.2025.v15.i6.60 |