| Research Article | ||
Open Vet. J.. 2025; 15(6): 2671-2681 Open Veterinary Journal, (2025), Vol. 15(6): 2671-2681 Research Article Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatmentMykola Zhelavskyi1*, Mykola Maryniuk2, Maryna Drobot2, Vitalii Kostenko2, Nataliia Boiko2 and Tetiana Paliukh21Department of Surgery, Therapy, Virology, and Biotechnology of Animal Reproduction and Nutrition, Faculty of Veterinary Medicine, Vinnytsia National Agrarian University, Vinnytsia, Ukraine 2Department of Internal Animal Diseases, Faculty of Veterinary Medicine, National University of Life and Environmental Sciences of Ukraine, Kyiv, Ukraine *Corresponding Author: Mykola Zhelavskyi. Department of Surgery, Therapy, Virology, and Biotechnology of Animal Reproduction and Nutrition, Faculty of Veterinary Medicine, Vinnytsia National Agrarian University, Vinnytsia, Ukraine. Email: nicoladoctor [at] gmail.com Submitted: 26/02/2025 Revised: 06/05/2025 Accepted: 11/05/2025 Published: 30/06/2025 © 2025 Open Veterinary Journal
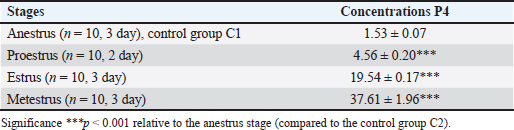
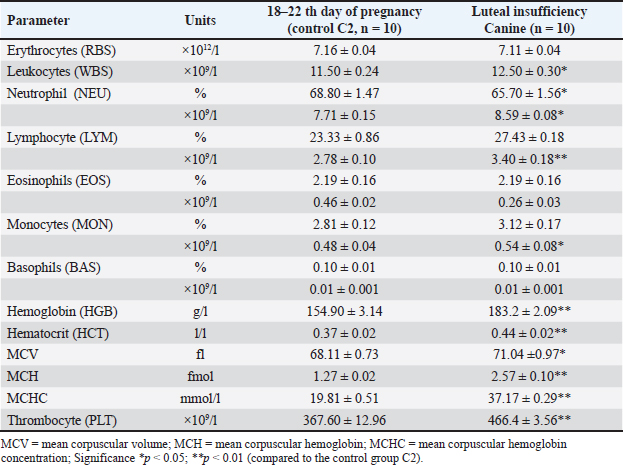
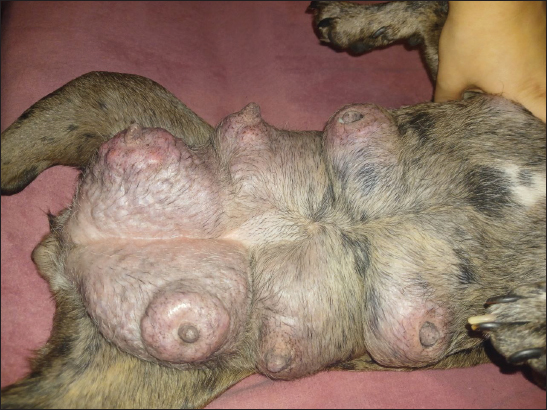
AbstractBackground: Progesterone, primarily produced by the corpus luteum, is essential for maintaining pregnancy in dogs by promoting endometrial receptivity and supporting fetal development. Hypoluteoidism, defined as inadequate progesterone production, significantly increases the risk of pregnancy loss and reproductive failure. This study evaluated the role of progesterone in canine pregnancy, the risks associated with hormonal deficiency, and the effectiveness of therapeutic interventions. Aim: This study emphasized the role of progesterone supplementation combined with magnesium and tocopherol in supporting pregnancy maintenance and restoring hormonal homeostasis. Methods: Progesterone levels were monitored across the estrous cycle in French Bulldog bitches (n = 20). The control group was divided into two subsets. In the first stage of the study, control group C1 (n = 10) consisted of dogs in the anestrus phase. In the second stage, control group C2 (n = 10) included dogs on days 18–22 of pregnancy. Animals in both control groups (C1 and C2) did not receive any pharmacological treatment throughout the experiment. The experimental group (dogs with luteal insufficiency, days 18–22 of pregnancy; n = 10) exhibited significantly lower serum progesterone levels (9.90 ± 0.19 nmol/l) compared with the control group (55.60 ± 2.23 nmol/l, p < 0.001). Serum progesterone levels were measured using the Immulite® system (Siemens Health Care Diagnostics GmbH, Eschborn, Germany). The experimental group received treatment with exogenous progesterone [medroxyprogesterone acetate (MPA), Perlutex® 5 mg, Dechra, UK], administered orally at a dose of 0.1 mg/kg/day. The treatment was combined with tocopherol acetate (2.0 ml, 500 IU) for 21 days and MagiCalm® (1 tablet per 10–25 kg/day for 10–12 days). Blood sampling for hematological and hormonal analysis was conducted as follows: for the control group on gestational days 18–22. In the experimental group (luteal insufficiency), samples were collected on the day treatment was initiated (day 0; gestational days 18–22, prior to treatment initiation), and subsequently on treatment days 5 (23–27 days–20.03 ± 0.75 nmol/l; p < 0.001), 10 (33–37 days–37.76 ± 0.40 nmol/l; p < 0.001), 20 (43–47 days–43.25 ± 0.57 nmol/l; p < 0.001), and 30 (53–57 days–33.37 ± 0.87; p < 0.001). Results: During the anestrus phase (control group C1), the progesterone concentration was minimal (1.53 ± 0.07 nmol/l). Progesterone levels showed significant variation across different phases of the estrous cycle, measuring 4.56 ± 0.20 nmol/l (p < 0.001) in proestrus, 19.54 ± 0.17 nmol/l in estrus (p < 0.001), and peaking at 37.61 ± 1.96 nmol/l (p < 0.001) in metestrus. On gestational days 18–22, healthy bitches in control group C2 exhibited a plasma progesterone concentration of 55.60 ± 2.23 nmol/l, indicating normal luteal function. In contrast, the experimental group (bitches with luteal insufficiency on days 18–22 of gestation) demonstrated a significant decrease in progesterone levels (9.95 ± 0.27 nmol/l; p < 0.001), accompanied by clinical signs such as restlessness, dark red vaginal discharge, and increased abdominal muscle tone. Throughout the treatment period, no critical clinical symptoms indicative of pregnancy loss were observed in animals with luteal deficiency. The clinical condition of all patients remained satisfactory. Pregnancies progressed with a dynamic normalization of plasma progesterone levels and culminated in normal, complication-free parturition. Conclusion: Progesterone therapy, when combined with tocopherol and magnesium, effectively addresses luteal insufficiency in dogs, improving implantation success rates and reducing pregnancy loss. This study highlights the importance of progesterone in canine reproduction and supports its supplementation as a viable treatment for hormonal imbalance. Keywords: Canine reproduction, Hormonal therapy, Hypoluteoidism, Luteal insufficiency, Progesterone. IntroductionPregnancy is accompanied by a complex set of physiological, biochemical, and hormonal changes that affect various organs and systems. Hormonal homeostasis ensures fetal development throughout gestation (Kautz et al., 2015; Feng et al., 2017; Graubner et al., 2020; De Geyter et al., 2024). Among the key regulators of pregnancy is the steroid hormone progesterone, which plays a central role in initiating pregnancy, maintaining gestation, and creating optimal conditions for embryonic development (Kautz et al., 2014; Graubner et al., 2017; Kowalewski et al., 2020b). Primarily synthesized by the corpus luteum and to a lesser extent in the uterus, progesterone regulates numerous biological functions. It induces changes in the uterine lining, preparing it for implantation and fetal growth, and stimulates mammary gland development (Ren et al., 2019; Zhelavskyi, 2021). While its essential role in pregnancy has been well established, current studies continue to investigate optimal concentration thresholds and their impact on outcomes. Understanding these levels is crucial for managing infertility, recurrent pregnancy loss, and other complications linked to hormonal imbalances (Krachudel et al., 2013; Salazar et al., 2016; Graubner et al., 2018; Zhelavskyi et al., 2020; Kowalewski et al., 2021a). Veterinary research highlights that a serum progesterone level above 15.9 nmol/l after day 30 of gestation is critical for sustaining pregnancy (Hinderer et al., 2021). However, successful pregnancies have been observed below this threshold, suggesting that compensatory mechanisms may support gestation under suboptimal conditions (Nowak et al., 2017; Kowalik et al., 2019; Pecci and Marengo, 2021; Zhelavskyi et al., 2024a). This variability underscores progesterone’s dynamic role in the reproductive system. It is involved in endometrial preparation, gestational support, and pregnancy stability (Friel et al., 2015; Tavares et al., 2022a). Its interaction with other hormonal and cellular factors maintains a delicate balance essential for reproduction. Clarifying these interactions through ongoing research is vital for developing targeted reproductive therapies (Salazar et al., 2016; Graubner et al., 2018). Additionally, factors such as immune, metabolic, and placental adaptations may contribute to gestational maintenance despite low progesterone levels. This highlights significant gaps in our understanding of its mechanisms and thresholds, stressing the need for further study (Friel et al., 2015; Garrido-Gomez et al., 2020; Diessler et al., 2023). Greater insight could improve diagnostic and therapeutic approaches, enhance pregnancy outcomes, and address hormonal imbalance-related complications. This study was motivated by ongoing clinical challenges related to luteal insufficiency (hypoluteidism) in canine pregnancy (Granger et al., 2018; Lyzikova et al., 2020; Kowalewski et al., 2021b; Kazemian et al., 2023). Inadequate progesterone levels in the corpus luteum increase the risk of early embryonic loss, preterm labor, and pregnancy termination. Overcoming these challenges requires accurate diagnostics and precise therapeutic interventions (McElrath et al., 2020; Zhelavskyi et al., 2023a). Advancing these protocols is essential for reducing progesterone-related risks and improving reproductive management (Kaluka et al., 2015; Guo et al., 2016; Ives et al., 2020; Kazemian et al., 2023). Tocopherol plays an important role in reproductive health. Hormonal imbalances, exacerbated by free radicals (reactive oxygen species), can damage reproductive tissues, including gametes and the endometrium. Antioxidants like tocopherol neutralize free radicals, maintain cellular balance, and support fertility (Tan et al., 2019; Shastak et al., 2023). It is hypothesized that monitoring and managing progesterone levels can mitigate the risks associated with luteal insufficiency, thereby ensuring successful pregnancy outcomes. This study emphasizes the role of progesterone supplementation combined with magnesium and tocopherol in supporting pregnancy maintenance and restoring hormonal homeostasis. By addressing these objectives, this study aimed to enhance the management of pregnancy in veterinary practice and reduce associated risks. Materials and MethodsThe control group was divided into two subsets. In the first stage of the study, control group C1 (n = 10) consisted of dogs in the anestrus phase. In the second stage, control group C2 (n = 10) included dogs on days 18–22 of pregnancy. Animals in both control groups (C1 and C2) did not receive any pharmacological treatment throughout the experiment. The subjects were French Bulldog bitches aged 3–5 years, weighing 9.5–11.2 kg. The research was conducted at a veterinary clinic in Kamianets-Podilskyi, Ukraine, between 2021 and 2025. The animals were subjected to regular deworming (Drontal Plus, Bayer HealthCare LLC, Germany) and vaccination with combination vaccines (Nobivac® DHPP, Merck & Co., Inc., Rahway, NJ). Before breeding, the bitches underwent a breeding evaluation, including an examination of external genitalia, vaginoscopy, vaginal cytology, bacterial culture, ultrasound of the uterus and ovaries, and serological testing [detection of Canine Herpesvirus (CHV, Qiagen, Germany) and Mycoplasma (IDEXX Mycoplasma PCR, USA)]. Clinical and laboratory examinationsClinical and laboratory examinations were conducted during anestrus, proestrus, estrus, and metestrus. Treatment was started on the day of pregnancy on the 18th–21st day of pregnancy (day 0 of treatment). The criteria for diagnosis were the detection of vaginal discharge, increased abdominal tone, and determination of progesterone (P4 9.95 ± 0.27 nmol/l). Blood samples were collected during the first phase of the study at various stages of the reproductive cycle: anestrus (day 3), proestrus (day 2), estrus (day 3), and metestrus (day 3). In the second phase, samples were collected on the day of treatment initiation (day 0, corresponding to days 18–22 of gestation) and subsequently on days 5 (gestation days 22–27), 10 (33–37), 20 (43–47), and 30 (53–57) of treatment. Progesterone concentrations were measured using Immulite® (Siemens Health Care Diagnostics GmbH, Eschborn, Germany). Ovulation was based on serum progesterone levels of 15.9–25.44 nmol/l (5–8 ng/ml). Bitches were mated once or twice within 1–3 days postovulation with a fertile male of the same breed. The first ultrasound for pregnancy diagnosis was conducted 22 days postovulation (Concannon, 2011). Laboratory research methodsAt the initiation of the study, a blood sample volume of 50 ml was collected from each subject via the cephalic vein (v. cephalica), following strict aseptic techniques to prevent contamination and ensure sample integrity. The blood samples were subjected to detailed biochemical profiling to evaluate a comprehensive range of physiological parameters. Hematological analysis included erythrocyte count, leukocyte count, leucogram values, hemoglobin concentration, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and thrombocyte count using the Abaxis Vetscan HM5 Hematology Analyzer (USA). Furthermore, the concentration of progesterone (P4, nmol/l) was meticulously evaluated to monitor the reproductive status and hormonal dynamics (Conley et al., 2023). This was achieved using the Catalyst Dx Chemistry Analyzer (IDEXX Laboratories, Inc., USA), a reliable platform widely recognized for its precision in hormone assays (Constantin and Posastiuc, 2024). The microbiological assessment included the culture of meat peptone agar and meat peptone broth (Merck KGaA, Germany) to identify bacterial spectra. Visual diagnosticsUltrasound diagnostics were conducted using the Sonosite SonoSite 180 Plus (FUJIFILM Sonosite, USA) with a C60/5-2 transducer and Parker Laboratories Ultrasound Gels (USA). Radiography was performed using a Vet Ray Digital DR (Vet Ray Technology, by IDEXX, USA). Differential diagnoses included abortion, vaginitis, pyometra, endometrial hyperplasia, endometritis, ovarian cysts, canine herpesvirus infection, mycoplasmosis, brucellosis, and vaginal/vulvar tumors, such as leiomyomas, fibromas, lipomas, and canine transmissible venereal tumors. TreatmentMPA (Perlutex® 5 mg, Dechra, UK) was administered to stabilize pregnancy. We used blood progesterone levels of 9.90 ± 0.19 nmol/l (day 0). The medication was given orally at a dose of 0.1 mg/kg body weight once daily (Romagnoli et al., 2024). Treatment was initiated on the day of luteal insufficiency detection. Treatment initiation and dose adjustment were based on laboratory analysis of blood progesterone levels (experimental group). Subsequently, from day 48 of pregnancy, the dose was gradually reduced (0.05–0.025 mg/kg) over the final week of treatment, concluding by day 52 of pregnancy. Dogs in the experimental group were orally administered tocopherol acetate (vitamin E for dogs, Ivitamins, USA) at a dose of 2.0 ml (500 IU) for 21 days. The complex supplement MagiCalm® (Healthspan, Holistic Pet Organics, USA) was used to reduce uterine and abdominal muscle tone. The formulation contained magnesium (magnesium citrate) 200 mg, taurine 50 mg, valerian extract 30 mg, and vitamin B6 (pyridoxine) 5 mg. The dosage was one tablet per 10–25 kg body weight, administered orally once daily for 10–12 days until muscle tone normalized (Humphrey et al., 2015). Statistical analysisResults were expressed as mean ± SD to summarize variability within each dataset (MANOVA). A p-value of less than 0.05 was used as the criterion for statistical significance, ensuring a reliable interpretation of the data. All computations and analyses were conducted using Statistica® 12.6 software (StatSoft, USA), a comprehensive tool for statistical and data visualization tasks. Ethical approvalThe clinical investigations were carried out in compliance with the Law of Ukraine «On Protection of Animals from Cruel Treatment» (21/02/2006, 3447-IV) and followed the European Commission’s guidelines on the treatment of vertebrates, ensuring protection from thirst, hunger, malnutrition, discomfort, fear, pain, and suffering. All studies adhered to bioethics standards, with written owner consent, local animal protection guidelines, and national legislation. ResultsProgesterone concentrations varied significantly depending on the stage of the estrous cycle (Table 1). During anestrus, baseline progesterone levels were measured at 1.53 ± 0.07 nmol/l. In the proestrus phase, progesterone concentrations gradually increased, reaching approximately 4.56 ± 0.20 nmol/l in peripheral blood (p < 0.01). During estrus (3-day stage), a significant rise in progesterone levels was observed (19.54 ± 0.17 nmol/l; p < 0.01), indicating the onset of luteal activity after ovulation. At this point, the corpus luteum begins to actively synthesize progesterone to support the early phases of a potential pregnancy. The highest progesterone concentrations were recorded during the metestrus phase (3-day stage), reaching 37.61 ± 1.96 nmol/l. Hematological analysis (Table 2) revealed significant alterations in blood parameters in dogs with luteal insufficiency. In the experimental group, a decline in progesterone levels was associated with a moderate but statistically significant increase in leukocyte count (12.50 ± 0.30 × 109/l; p < 0.05). There was also a moderate increase in neutrophils (8.59 ± 0.08 × 109/l; p < 0.05) and a marked elevation in both the absolute count (3.40 ± 0.18 × 109/l; p < 0.01) and percentage (27.43 ± 0.18%; p < 0.01) of lymphocytes. Additionally, significant increases in hemoglobin (183.2 ± 2.09 g/l; p < 0.01) and hematocrit values (0.44 ± 0.02 l/l; p < 0.01) were observed, reflecting altered oxygen transport capacity. Other hematological parameters such as MCV, MCH, MCHC, and platele counts also changed notably, with a significant increase in platelet levels in peripheral blood (466.4 ± 3.56 ×109/l; p < 0.001). In pregnant dogs in the experimental group (18–22 days of gestation), clinical signs indicative of luteal insufficiency included restlessness, dark-red vaginal discharge, and increased abdominal tone. Hormonal analysis showed (Table 3) significantly lower progesterone levels in this group (9.90 ± 0.19 nmol/l) compared with controls (55.60 ± 2.23 nmol/l; p < 0.001), which was considered a risk factor for pregnancy loss (day 0 of treatment). These animals received a treatment regimen including MPA and tocopherol. Clinical improvement was observed during therapy: vulvar discharge subsided, and abdominal tone normalized. Progressive increases in progesterone concentrations following MPA with tocopherol and magnesium supplementation were documented. No signs of uterine hypertonicity or vaginal discharge were recorded in treated pregnant dogs. In all cases, the pregnancy progressed without complications. However, two dogs developed mammary gland hyperplasia (Fig. 1), requiring dose adjustments of MPA with tocopherol and magnesium supplementation (Table 4). Table 1. Plasma concentrations of progesterone (P4, nmol/l) during the estrous cycle of the canines [mean (±SЕ)].
Table 2. Hematological parameters of luteal insufficiency canines [mean (±SЕ)].
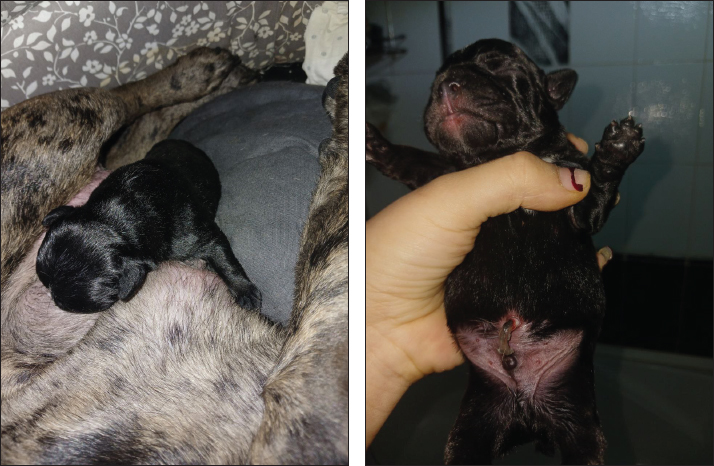
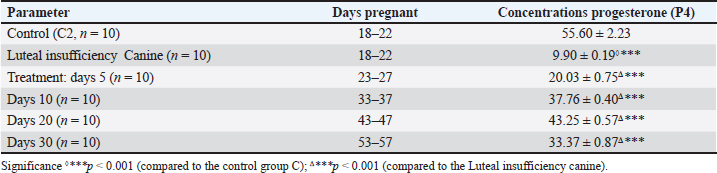
The mean serum progesterone (P4) concentrations in canines during treatment for luteal insufficiency were evaluated. In the control group (C2), the progesterone concentration at 18–22 days of gestation was 55.60 ± 2.23 nmol/l. In the group with luteal insufficiency (n=10), the concentration was significantly lower at 9.90 ± 0.19 nmol/l (p < 0.001 compared to control). Following treatment, progesterone levels increase progressively. On treatment day 5 (gestational days 23–27), the concentration rose to 20.03 ± 0.75 nmol/l (p < 0.001 compared to untreated luteal insufficiency group). On day 10 (33–37 days of gestation), progesterone levels reached 37.76 ± 0.40 nmol/l (p < 0.001). By day 20 (43–47 gestational days), the concentration further increased to 43.25 ± 0.57 nmol/l (p < 0.001). On day 30 (53–57 gestational days), a decrease in progesterone concentration to 33.37 ± 0.87 nmol/l was observed (p < 0.001). Deliveries occurred on days 62–63 of gestation, and all litters consisted of anatomically and physiologically normal puppies (Fig. 2). DiscussionHypoluteoidism is a reproductive dysfunction characterized by inadequate production or premature decline of progesterone, a hormone critical for maintaining early pregnancy. This endocrinological deficiency typically results from suboptimal activity of the corpus luteum following ovulation, impairing its ability to sustain the hormonal environment necessary for embryo implantation and gestational progression (Kowalik et al., 2018; Kowalewski et al., 2020c; Gualdoni et al., 2022; Zhelavskyi et al., 2024b). In canines, the corpus luteum is the primary source of progesterone during the luteal phase of the estrous cycle (Peluso, 2022). A failure to achieve or sustain adequate progesterone concentrations during this phase can compromise endometrial receptivity, ultimately leading to implantation failure and early embryonic loss (Wetendorf and Demayo, 2014; Gram et al., 2015; Castelnovo et al., 2019; Conley et al., 2023). Progesterone is indispensable in modulating the uterine environment, notably by transforming the endometrial lining to a state conducive to embryo attachment and implantation (Gram et al., 2014; Kowalewski et al., 2020a; Zhelavskyi et al., 2020; Garrido-Gomez et al., 2022). Its role in the reproductive cycle of dogs cannot be overstated because it ensures favorable conditions for embryo development. In cases in which progesterone levels are inadequate, the endometrium may not reach the necessary receptivity, potentially resulting in failed implantation (Monsivais et al., 2017; Nowak et al., 2019). This hormonal insufficiency can also contribute to early pregnancy losses or miscarriages because the uterine environment becomes unsupportive of the developing embryo (Kowalik et al., 2014; Ponikwicka-Tyszko et al., 2019; Mistry et al., 2020; Nöthling et al., 2022; Zhelavskyi et al., 2023b). Table 3. Plasma concentrations of progesterone (P4) in luteal insufficiency canines [mean (±SЕ)].
Fig. 1. Signs of mammary gland hyperplasia in a 2-year-old French bulldog.
Fig. 2. Newborn (2 days old) French bulldog. Table 4. Mean (±SЕ) serum progesterone (P4) concentration (nmol/l) during dynamic treatment for luteal insufficiency of canines.
Hypoluteoidism, characterized by insufficient plasma progesterone levels, is a poorly documented cause of embryo or fetal loss in dogs. Experimental studies suggest that a plasma progesterone concentration of at least 6–9 nmol/l (2–3 ng/ml) is required to maintain pregnancy, and levels below this threshold for more than 24–48 hours result in pregnancy loss (Zedda et al., 2017 ; Kreis et al., 2019). In experimental settings, dogs have been observed to maintain pregnancy with plasma progesterone concentrations of 2–5 ng/ml. However, in clinical practice, it is often safer to anticipate potential pregnancy loss by initiating progesterone supplementation when levels decline (Conley et al., 2023). This approach is typically adopted without concrete evidence that the pregnancy is terminated without supplementation. Clinicians and breeders prefer to avoid waiting until progesterone levels drop to 2–3 ng/ml, as preserving pregnancy at this stage may no longer be feasible (Thomas et al., 2014; Sarkar and Mondal, 2020; Hinderer et al., 2021). Hypoluteoidism is challenging to document clinically, but it is considered a potential cause of pregnancy loss in dogs. Cases in which pregnancy loss is directly linked to low plasma progesterone levels raise questions about whether the failure is solely due to low progesterone levels or other underlying causes affecting the corpus luteum. Hypoluteoidism has been observed around 25–35 days of pregnancy, a period normally marked by increased progesterone secretion in pregnant animals through mechanisms associated with placentation (Kazemian et al., 2022; Kowalewski, 2023). In cases with low progesterone levels and imminent pregnancy loss risk, progestin supplementation may be recommended, albeit with empirical dosing (Thomas et al., 2014; Sarkar and Mondal, 2020). Progesterone use should be restricted to diagnosed or suspected cases of luteal insufficiency because improper use may lead to adverse effects, including mammary fibroadenoma development (Zhelavskyi, 2024; Zedda et al., 2017) and masculinization of female pups. Studies have demonstrated that tocopherol can improve oocyte quality and reduce the risk of miscarriage in dogs with luteal insufficiency (Peluso et al., 2014; Gram et al., 2016; Cottrell et al., 2017; Beal et al., 2021). It also mitigates the oxidative stress associated with hormone therapy, thereby enhancing reproductive outcomes. Tocopherol is a natural antioxidant that improves the fertility and functioning of the gonads in both males and females (Krachudel et al., 2013). Magnesium is critical for numerous biochemical processes, including hormone synthesis and regulation. Magnesium is a vital cation that plays numerous essential roles in the body, particularly in the nervous and muscular systems. Additionally, magnesium is crucial for muscle function because it is involved in muscle contraction and relaxation processes by interacting with calcium and potassium. Beyond these functions, magnesium also contributes to energy production, as it is a cofactor for many enzymes involved in ATP synthesis and supports bone health by promoting calcium absorption. A magnesium deficiency can lead to a range of issues, including muscle cramps, fatigue, and neurological disturbances, highlighting its importance for maintaining overall health (Kolanu et al., 2020). Magnesium deficiency can impair ovarian function, negatively affecting reproductive capabilities. Antistress properties of magnesium are particularly valuable for dogs experiencing stress or anxiety during pregnancy or estrus. Stress can impair reproductive function, underscoring the importance of providing comfortable living conditions and supporting the emotional well-being of animals (Jaripur et al., 2022). These findings highlight the intricate regulation of progesterone secretion throughout the estrous cycle and reinforce its pivotal role in orchestrating reproductive events (Kautz et al., 2014; Kowalewski et al., 2021a). Hormonal fluctuations in progesterone are tightly linked to sequential physiological transformations that facilitate successful reproduction (Guo et al., 2016; Graubner et al., 2020). Notably, therapeutic strategies involving progesterone supplementation, particularly in conjunction with antioxidant and mineral cofactors such as tocopherol (vitamin E) and magnesium, have shown promise in producing synergistic effects (Tan et al., 2019; Shastak et al., 2023). These combined treatments are associated with improved implantation outcomes and decreased incidence of spontaneous pregnancy loss. Emerging evidence underscores the clinical importance of restoring adequate progesterone levels in individuals diagnosed with luteal phase deficiency, as a prerequisite for achieving a favorable intrauterine environment conducive to embryo implantation and early gestational development (Mistry et al., 2020; Nöthling et al., 2022). The necessity of rigorous hormonal monitoring and prompt therapeutic intervention upon detection of progesterone insufficiency is further emphasized in the literature. Experimental studies have validated the efficacy of progesterone-based regimens in mitigating endocrine-related causes of recurrent miscarriage. In the broader context of fertility, luteal insufficiency, which is characterized by deficient progesterone output, remains a critical barrier to conception (Thomas et al., 2014; Hinderer et al., 2021). A suboptimal hormonal milieu disrupts the morphofunctional preparation of the endometrial lining, undermining its receptivity to embryonic implantation and thereby elevating the risk of infertility and early pregnancy termination (Zedda et al., 2017; Tavares et al., 2022b). Clinical investigations support the application of targeted hormonal therapies to correct such deficiencies, demonstrating significant improvements in pregnancy retention rates and highlighting the essential role of early diagnosis and timely hormonal correction in reproductive management (Carr et al., 2020; Khalphallah et al., 2022; Kapper et al., 2024). ConclusionProgesterone therapy when combined with supplementary agents, such as tocopherol and magnesium, shows considerable promise in addressing luteal insufficiency in dogs. The synergistic effects of these treatments improve implantation success rates and reduce the likelihood of pregnancy loss, providing an effective approach to correcting hormonal imbalances. Future research should aim to refine progesterone-based therapies and investigate innovative supplement combinations to enhance outcomes in animals with reproductive disorders. Such advancements will deepen our understanding of hormonal regulation and improve clinical approaches to managing infertility and pregnancy-related complications in canines. AcknowledgmentsThe authors would like to express their sincere gratitude to the faculty and staff of the Vinnytsia National Agrarian University, Vinnytsia, Ukraine, and the National University of Life and Environmental Sciences of Ukraine, Kyiv, Ukraine, for their invaluable support and assistance throughout this research. Their contributions were essential to the successful completion of this study. Conflicts of interestThe authors declare that they have no conflicts of interest. FundingThis research was not funded by any funding organization. Authors’ contributionsMZ: responsible for conceptualizing the study, data acquisition, analyzing the results, and initial manuscript drafting. MM: supervised the overall research process, conducted the analysis of laboratory data, and actively participated in the preparation and revision of the manuscript. DM and VK: conducted the analysis and interpretation of clinical data, and assisted in drafting and refining the manuscript. NB: the author guided the research process and played a key role in manuscript development and revision. TP: statistical analysis was performed and supported the writing and editing of the manuscript. All authors critically reviewed, revised, and approved the final manuscript for submission. Data availabilityAll data were provided in the manuscript. ReferencesBeal, R., Alonso-Carriazo Fernandez, A., Grammatopoulos, D.K., Matter, K. and Balda, M.S. 2021. ARHGEF18/p114RhoGEF coordinates PKA/CREB signaling and Actomyosin remodeling to promote trophoblast cell-cell fusion during placenta morphogenesis. Front. Cell Dev. Biol. 9, 658006. Carr, S., Jia, Y., Crites, B., Hamilton, C., Burris, W., Edwards, J.L., Matthews, J. and Bridges, P.J. 2020. Form of supplemental selenium in vitamin- mineral premixes differentially affects early luteal and gestational concentrations of progesterone, and postpartum concentrations of prolactin in beef cows. Animals 10, 967. Castelnovo, L.F., Canesi, L., Bacigaluppi, M. and Butti, E. 2019. Expression of membrane progesterone receptors (mPRs) in rat peripheral glial cell membranes and their potential role in the modulation of cell migration and protein expression. Steroids 141, 56–62. Concannon, P.W. 2011. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 124, 200–210. Conley, A.J., Gonzales, K.L., Erb, H.N. and Christensen, B.W. 2023. Progesterone analysis in canine breeding management. Vet. Clin. Small Anim. Pract. 53, 931–949. Constantin, N.T. and Posastiuc, F.P. 2024. Progesterone: an essential diagnostic resource in veterinary. In Progesterone—basic concepts and emerging new applications. Ed.,. Wang, Z. London, UK: IntechOpen. Cottrell, H.N., Wu, J., Rimawi, B.H., Duran, J.M., Spencer, J.B., Sidell, N. and Rajakumar, A. 2017. Human endometrial stromal cell plasticity: reversible sFlt1 expression negatively coincides with decidualization. Hypertens. Pregnancy 36, 204–211. De Geyter, I., Kowalewski, M.P. and Tavares Pereira, M. 2024. Applying a novel kinomics approach to study decidualization and the effects of antigestagens using a canine model. Biol. Reprod. 110, 583–598. Diessler, M.E., Hernández, R., Gomez Castro, G. and Barbeito, C.G. 2023. Decidual cells and decidualization in the carnivoran endotheliochorial placenta. Front. Cell Dev. Biol. 11, 1134874. Feng, Y., Ma, X., Deng, L., Yao, B., Xiong, Y., Wu, Y., Wang, L., Ma, Q. and Ma, F. 2017. Role of selectins and their ligands in human implantation stage. Glycobiology 27, 385–391. Friel, A.M., Zhang, L. and Tilly, J.L. 2015. Progesterone receptor membrane component 1 deficiency attenuates growth while promoting chemosensitivity of human endometrial xenograft tumors. Cancer Lett. 356, 434–440. Garrido-Gomez, T., Castillo-Marco, N., Cordero, T. and Simon, C. 2022. Decidualization resistance in the origin of preeclampsia. Am. J. Obstet. Gynecol. 226, S886–S894. Garrido-Gomez, T., Quinonero, A., Dominguez, F., Rubert, L., Perales, A., Hajjar, K.A. and Simon, C. 2020. Preeclampsia: a defect in decidualization is associated with deficiency of Annexin A2. Am. J. Obstet. Gynecol. 222, 376 e371. Gram, A., Boos, A. and Kowalewski, M.P. 2014. Uterine and placental expression of canine oxytocin receptor during pregnancy and normal and induced parturition. Reprod. Domest. Anim. 49, 41–49. Gram, A., Hoffmann, B., Boos, A. and Kowalewski, M.P. 2015. Expression and localization of vascular endothelial growth factor a (VEGFA) and its two receptors (VEGFR1/FLT1 and VEGFR2/FLK1/ KDR) in the canine corpus luteum and utero- placental compartments during pregnancy and at normal and induced parturition. Gen. Comp. Endocrinol. 223, 54–65. Gram, A., Trachsel, A., Boos, A. and Kowalewski, M.P. 2016. Elevated utero/placental GR/NR3C1 is not required for the induction of parturition in the dog. Reproduction 152, 303–311. Granger, J.P., Spradley, F.T. and Bakrania, B.A. 2018. The endothelin system: a critical player in the pathophysiology of preeclampsia. Curr. Hypertens. Rep. 20, 32. Graubner, F.R., Boos, A., Aslan, S., Kucukaslan, I. and Kowalewski, M.P. 2018. Uterine and placental distribution of selected extracellular matrix (ECM) components in the dog. Reproduction 155, 403–421. Graubner, F.R., Gram, A., Kautz, E., Bauersachs, S., Aslan, S., Agaoglu, A.R., Boos, A. and Kowalewski, M.P. 2017. Uterine responses to early pre-attachment embryos in the domestic dog and comparisons with other domestic animal species. Biol. Reprod. 97, 197–216. Graubner, F.R., Pereira, M.T., Boos, A. and Kowalewski, M.P. 2020. Canine decidualization in vitro: extracellular matrix modification, progesterone mediated effects and selective blocking of prostaglandin E2 receptors. J. Reprod. Dev. 66, 319–329. Gualdoni, G., Gomez Castro, G., Hernández, R., Barbeito, C. and Cebral, E. 2022. Comparative matrix metalloproteinase-2 and -9 expression and activity during endotheliochorial and hemochorial trophoblastic invasiveness. Tissue Cell 74, 101698. Guo, M., Xu, J. and Yu, S. 2016. Progesterone receptor membrane component 1 mediates progesterone- induced suppression of oocyte meiotic prophase I and primordial folliculogenesis. Sci. Rep. 6, 36869. Hinderer, J., Lüdeke, J., Riege, L., Haimerl, P., Bartel, A., Kohn, B., Weber, C., Müller, E. and Arlt, S.P. 2021. Progesterone concentrations during canine pregnancy. Animals 11, 3369. Humphrey, S., Kirby, R. and Rudloff, E. 2015. Magnesium physiology and clinical therapy in veterinary critical care. J. Vet. Emerg. Crit. Care 25, 210–225. Ives, C.W., Sinkey, R., Rajapreyar, I., Tita, A.T.N. and Oparil, S. 2020. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J. Am. Coll. Cardiol. 76, 1690–1702. Jaripur, M., Ghasemi-Tehrani, H., Askari, G., Gholizadeh-Moghaddam, M., Clark, C.C. and Rouhani, M.H. 2022. The effects of magnesium supplementation on abnormal uterine bleeding, alopecia, quality of life, and acne in women with polycystic ovary syndrome: a randomized clinical trial. Reprod. Biol. Endocrinol. 20, 110. Kaluka, D., Batth, I.S. and Zang, T. 2015. Spectroscopic and mutagenesis studies of human PGRMC1. Biochemistry 54, 1268–1280. Kapper, C., Oppelt, P., Ganhör, C., Gyunesh, A.A., Arbeithuber, B., Stelzl, P. and Rezk-Füreder, M. 2024. Minerals and the menstrual cycle: impacts on ovulation and endometrial health. Nutrients 16, 1008. Kautz, E., de Carvalho Papa, P., Reichler, I.M., Gram, A., Boos, A. and Kowalewski, M.P. 2015. In vitro decidualisation of canine uterine stromal cells. Reprod. Biol. Endocrinol. 13, 85. Kautz, E., Gram, A., Aslan, S., Ay, S.S., Selcuk, M., Kanca, H., Koldaş, E., Akal, E., Karakaş, K., Findik, M., Boos, A. and Kowalewski, M.P. 2014. Expression of genes involved in the embryo- maternal interaction in the early-pregnant canine uterus. Reproduction 147, 703–717. Kazemian, A., Tavares Pereira, M., Aslan, A., Payan- Carreira, R., Reichler, I.M., Agaoglu, R. A. and Kowalewski, M.P. 2023. Membrane-bound progesterone receptors in the canine uterus and placenta; possible targets in the maintenance of pregnancy. Theriogenology 210, 68–83. Kazemian, A., Tavares Pereira, M., Hoffmann, B. and Kowalewski, M.P. 2022. Antigestagens mediate the expression of decidualization markers, extracellular matrix factors, and connexin 43 in decidualized dog uterine stromal cells. Animals 12, 798. Khalphallah, A., Elmeligy, E., Zakaria, A.M., Ghallab, R.S., Abdulkarim, A. and Mohamed, R.H. 2022. Comparative study of efficacy of prepartum injection of multivitamins and selenium-vitamin E (&aacgr;-tocopherol)-combination on post-partum clinical findings, serum steroids, calf and placental weights, and milk antioxidant biomarkers changes in female dromed. Open Vet. J. 12, 657–667. Kolanu, B.R., Vadakedath, S., Boddula, V. and Kandi, V. 2020. Activities of serum magnesium and thyroid hormones in pre-, peri-, and post-menopausal women. Cureus 12, e6554. Kowalewski, M.P. 2023. Advances in understanding canine pregnancy: endocrine and morpho- functional regulation. Reprod. Domest. Anim. 58, 163–175. Kowalewski, M.P., Gram, A. and Boos, A. 2020a. Canine conceptus-maternal communication during maintenance and termination of pregnancy, including the role of species-specific decidualization. Theriogenology 150, 329–338. Kowalewski, M.P., Gram, A. and Boos, A. 2020b. Progesterone receptor blockers: historical perspective, mode of function and insights into clinical and scientific applications. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 48, 126–139. Kowalewski, M.P., Gram, A. and Boos, A. 2021a. Canine endotheliochorial placenta: morpho- functional aspects. Adv. Anat. Embryol. Cell Biol. 231, 1–131. Kowalewski, M.P., Kazemian, A., Klisch, K., Gysin, T., Tavares Pereira, M. and Gram, A. 2021b. Canine endotheliochorial placenta: morpho-functional aspects. Adv. Anat. Embryol. Cell Biol. 234, 155– 179. Kowalewski, M.P., Tavares Pereira, M.T., Papa, P. and Gram, A. 2020c. Progesterone receptor blockers: historical perspective, mode of function and insights into clinical and scientific applications. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 48, 433–440. Kowalik, M.K., Kotwica, J. and Rekawiecki, R. 2018. Expression of membrane progestin receptors (mPRs) in the bovine corpus luteum during the estrous cycle and first trimester of pregnancy. Domest. Anim. Endocrinol. 63, 1–9. Kowalik, M.K., Kotwica, J. and Rekawiecki, R. 2019. Expression of membrane progestin receptors (mPRs) alpha, beta and gamma in the bovine uterus during the oestrous cycle and pregnancy. Theriogenology 132, 105–113. Kowalik, M.K., Rekawiecki, R. and Kotwica, J. 2014. Expression and localization of progesterone receptor membrane component 1 and 2 and serpine mRNA binding protein 1 in the bovine corpus luteum during the estrous cycle and the first trimester of pregnancy. Theriogenology 82, 1088–1097. Krachudel, J., Bondzio, A., Einspanier, R., Einspanier, A., Gottschalk, J., Kuechenmeister, U. and Muennich, A. 2013. Luteal insufficiency in bitches as a consequence of an autoimmune response against progesterone? Theriogenology 79, 1278–1283. Kreis, N.N., Louwen, F. and Yuan, J. 2019. The multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation, migration and cancer therapy. Cancers 11, 1220. Lyzikova, Y.A., Tsyganov, A.M. and Bayramova, S.A. 2020. Increase in FoxP3, CD56 immune cells and decrease in glands PGRMC1 expression in the endometrium are associated with recurrent miscarriages. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 113–118. McElrath, T.F., Cantonwine, D.E., Gray, K.J., Mirzakhani, H., Doss, R.C., Khaja, N., Khalid, M., Page, G., Brohman, B., Zhang, Z., Sarracino, D. and Rosenblatt, K.P. 2020. Late first trimester circulating microparticle proteins predict the risk of preeclampsia $<$35 weeks and suggest phenotypic differences among affected cases. Sci. Rep. 10, 17353. Mistry, H.D., Ogalde, M.V.H., Broughton Pipkin, F., Escher, G. and Kurlak, L.O. 2020. Maternal, fetal, and placental selectins in women with pre-eclampsia; association with the renin-angiotensin-system. Front. Med. 7, 270. Monsivais, D., Clementi, C., Peng, J., Fullerton, P.T. Jr., Prunskaite-Hyyrylainen, R., Vainio, S.J. and Matzuk, M.M. 2017. BMP7 induces uterine receptivity and blastocyst attachment. Endocrinology 158, 979–992. Nöthling, J.O., Joonè, C.J. and De Cramer, K.G.M. 2022. Use of serum progesterone and prostaglandin F2α metabolite levels to predict onset of parturition in the bitch. Reprod. Domest. Anim. 57, 635–642. Nowak, M., Chlopek, J., Kotwica, J. and Rekawiecki, R. 2019. Gene expression profiling of the canine placenta during normal and antigestagen-induced luteolysis. Gen. Comp. Endocrinol. 276, 80–89. Nowak, M., Gram, A., Boos, A., Aslan, S., Ay, S.S., Onyay, F. and Kowalewski, M.P. 2017. Functional implications of the utero-placental relaxin (RLN) system in the dog throughout pregnancy and at term. Reproduction 154, 415–431. Pecci, A. and Marengo, B. 2021. The emerging role of progesterone receptor membrane component 1 (PGRMC1) in cancer. Front. Cell Dev. Biol. 9, 648484. Peluso, J.J. 2022. Progesterone signaling and mammalian ovarian follicle growth mediated by progesterone receptor membrane component family members. Cells 11, 468. Peluso, J.J., Griffin, D. and Musci, T.J. 2014. Progesterone receptor membrane component 1 and its role in ovarian follicle function. Fertil. Steril. 101, 704–710. Ponikwicka-Tyszko, D., Kajta, M. and Makarevich, A.V. 2019. Molecular mechanisms underlying mifepristone’s agonistic action on ovarian cancer progression. EBioMedicine 40, 251–261. Ren, J., Tang, Y. and Liang, Z. 2019. Genomic sequence analyses of classical and non-classical lamprey progesterone receptor genes and the inference of homologous gene evolution in metazoans. BMC Evol. Biol. 19, 83. Romagnoli, S., Krekeler, N., de Cramer, K., Kutzler, M., McCarthy, R. and Schaefer-Somi, S. 2024. WSAVA guidelines for the control of reproduction in dogs and cats. J. Small Anim. Pract. 65, 424–559. Salazar, M., Lerner, M.R. and Brackett, D.J. 2016. Progestin-mediated activation of MAPK and AKT in nuclear progesterone receptor negative breast epithelial cells: the role of membrane progesterone receptors. Gene 591, 190–196. Sarkar, D. K. and Mondal, M. 2020. Membrane progesterone receptors and their role in breast cancer. Front. Endocrinol. 11, 586. Shastak, Y., Obermueller-Jevic, U. and Pelletier, W. 2023. A century of vitamin E: early milestones and future directions in animal nutrition. Agriculture 13, 1526. Tan, W., Ye, L., Fang, Y. and Yu, Z. 2019. Induction of sperm hypermotility through membrane progestin receptor alpha (mPRα): a teleost model of rapid, multifaceted, nongenomic progestin signaling. Gen. Comp. Endocrinol. 277, 67–76. Tavares Pereira, M., Kazemian, A., Rehrauer, H., and Kowalewski, M.P. 2022a. Transcriptomic profiling of canine decidualization and effects of antigestagens on decidualized dog uterine stromal cells. Sci. Rep. 12, 21890. Tavares Pereira, M., Papa, P., Reichler, I.M., Aslan, S. and Kowalewski, M.P. 2022b. Luteal expression of factors involved in the metabolism and sensitivity to oestrogens in the dog during pregnancy and in non-pregnant cycle. Reprod. Domest. Anim. 57, 86–97. Thomas, P., Pang, Y., Dong, J. and Groenen, P. 2014. Role of membrane progestin receptors in mediating progestin-induced oocyte maturation in teleosts. Front. Endocrinol. 5, 81. Wetendorf, M. and Demayo, F.J. 2014. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int. J. Dev. Biol. 58, 95–106. Zedda, M.T., Bogliolo, L., Antuofermo, E., Falchi, L., Ariu, F., Burrai, G.P. and Pau, S. 2017. Hypoluteoidism in a dog associated with recurrent mammary fibroadenoma stimulated by progestin therapy. Acta Vet. Scand. 59, 1–6. Zhelavskyi, M.M. 2021. The role of neutrophils in subclinical mastitis in cows. Pol. J. Nat. Sci. 36, 107–115. Zhelavskyi, M.M. 2024. Apoptosis of neutrophils, monocytes, and lymphocytes in the peripheral blood of cows during lactation. Pol. J. Nat. Sci. 39, 5–14. Zhelavskyi, M.М., Kernychnyi, S.P. and Betlinska, T.V. 2023a. Effects of hydroxychloroquine and tacrolimus on discoid facial lupus erythematosus in a dog. World Vet. J. 13, 360–364. Zhelavskyi, M., Kernychnyi, S. and Betlinska, T. 2023b. Hematological and biochemical parameters of macropod progressive periodontal disease in wild western gray kangaroos. World Vet. J. 13, 630–635. Zhelavskyi, M., Maryniuk, M. and Drobot, M. 2024a. Sebaceous adenitis in an Akita: symptoms and therapeutic approaches. World Vet. J. 14, 637–644. Zhelavskyi, M., Maryniuk, M. and Drobot, M. 2024b. The role of neutrophils and NETosis in local immunity of feline inflammatory aural polyps. World Vet. J. 14, 137–144. Zhelavskyi, M., Shunin, I. and Midyk, S. 2020. Extracellular antibacterial defense mechanisms of neutrophil granulocytes and their role in the pathogenesis of pyometra in cats. Pol. J. Nat. Sci. 35, 363–378. | ||
| How to Cite this Article |
| Pubmed Style Zhelavskyi M, Maryniuk M, Drobot M, Kostenko V, Boiko N, Paliukh T. Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. Open Vet. J.. 2025; 15(6): 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 Web Style Zhelavskyi M, Maryniuk M, Drobot M, Kostenko V, Boiko N, Paliukh T. Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. https://www.openveterinaryjournal.com/?mno=244775 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i6.36 AMA (American Medical Association) Style Zhelavskyi M, Maryniuk M, Drobot M, Kostenko V, Boiko N, Paliukh T. Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. Open Vet. J.. 2025; 15(6): 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 Vancouver/ICMJE Style Zhelavskyi M, Maryniuk M, Drobot M, Kostenko V, Boiko N, Paliukh T. Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. Open Vet. J.. (2025), [cited January 25, 2026]; 15(6): 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 Harvard Style Zhelavskyi, M., Maryniuk, . M., Drobot, . M., Kostenko, . V., Boiko, . N. & Paliukh, . T. (2025) Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. Open Vet. J., 15 (6), 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 Turabian Style Zhelavskyi, Mykola, Mykola Maryniuk, Maryna Drobot, Vitalii Kostenko, Nataliia Boiko, and Tetiana Paliukh. 2025. Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. Open Veterinary Journal, 15 (6), 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 Chicago Style Zhelavskyi, Mykola, Mykola Maryniuk, Maryna Drobot, Vitalii Kostenko, Nataliia Boiko, and Tetiana Paliukh. "Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment." Open Veterinary Journal 15 (2025), 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 MLA (The Modern Language Association) Style Zhelavskyi, Mykola, Mykola Maryniuk, Maryna Drobot, Vitalii Kostenko, Nataliia Boiko, and Tetiana Paliukh. "Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment." Open Veterinary Journal 15.6 (2025), 2671-2681. Print. doi:10.5455/OVJ.2025.v15.i6.36 APA (American Psychological Association) Style Zhelavskyi, M., Maryniuk, . M., Drobot, . M., Kostenko, . V., Boiko, . N. & Paliukh, . T. (2025) Luteal insufficiency in canines: Assessment of progesterone dynamics and efficacy of combined hormonal treatment. Open Veterinary Journal, 15 (6), 2671-2681. doi:10.5455/OVJ.2025.v15.i6.36 |