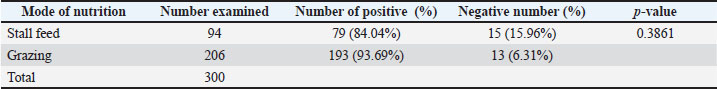| Research Article | ||
Open Vet. J.. 2025; 15(10): 5284-5293 Open Veterinary Journal, (2025), Vol. 15(10): 5284-5293 Research Article Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, PakistanMashael A. Aldamigh1, Wajid Ali2, Wali Khan2*, Azizu Ur Rahman3, Yousef Abdal Jalil Fadladdin4, Muhammad Yousaf5,Zaira Ahmad6 and Patricio R. De los Ríos-Escalante71Department of Biology, College of Science, Majmaah University, Al-Majmaah, Saudi Arabia 2Department of Zoology, University of Malakand, Lower Dir, Pakistan 3Department of Pharmacy, University of Malakand, Lower Dir, Pakistan 4Department of Biological Sciences, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia 5Centre for Animal Science and Fisheries, University of Swat, Swat, Pakistan 6Department of Environmental Science, Lahore College for Women University, Lahore, Pakistan 7Facultad de Recursos Naturales, Departamento de Ciencias Biológicas y Químicas Casilla, Universidad Católica de Temuco, Temuco, Chile *Corresponding Author: Wali Khan. Department of Zoology, University of Malakand, Lower Dir, Pakistan. Email: walikhan.pk [at] gmail.com Submitted: 03/03/2025 Revised: 01/08/2025 Accepted: 12/08/2025 Published: 31/10/2025 © 2025 Open Veterinary Journal
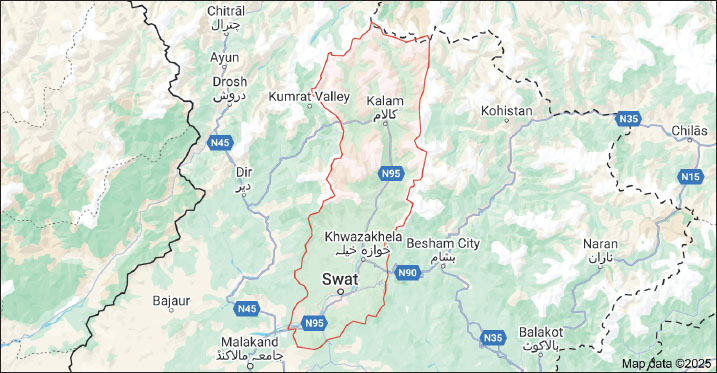
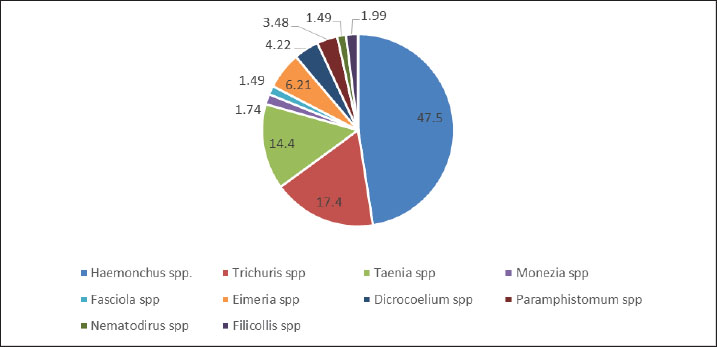
AbstractBackground: Gastrointestinal tract parasites (GIT) pose a significant economic constraint and public health challenges worldwide, including in Pakistan. Aim: This study was conducted to assess the effect of the prevalence and associated risk factors on the prevalence of GIT parasites found in sheep of lower and higher altitudes in the district of Swat, Pakistan. Methods: Fecal samples from the rectum were collected by means of gloved fingers and then placed in plastic bottles containing 70% ethanol. The collected fecal specimens were transported to the parasitology laboratory of Malakand University in the Zoology Department for investigating gastrointestinal parasites. General laboratory techniques were applied to detect parasitic infections. Results: Of the 300 fecal samples, 90.4% (n=272/300) were parasitized. The most prevalent species was Haemonchus spp 47.5% (n=191), followed by Trichuris spp 17.4%(n=70), Taenia spp 14.4% (n=58), Moniezia spp 1.74% (n=7), Fasciola spp 1.49% (n=6), Eimeria spp 6.21% (n=25), Dicrocoelium spp 4.22% (n=17), Paramphistomum spp 4.67% (n=14), Nematodirus spp 2% (n=6), and Filicollis spp 2.67% (n=8). Different risk factors, such as gender, age, health status, grazing behavior, drinking water sources, treatment, and nature of parasitism, were investigated. Sex-wise prevalence demonstrated that females were more parasitized than males. The association between helminth parasitic infection was noted statistically significant with mode of nutrition, body condition, age, altitudes, and status of females (p < 0.05). However, the prevalence rate of the infection was higher in younger animals than in older ones. The animals at higher altitudes were more infected than those at lower altitudes (p > 0.05). In winter, the sheep were found to be more infected (94.8%) than in other seasons of the year (p > 0.05). The number of eggs per gram for Haemonchus spp was 50–1,600epg, Trichuris spp 50-300epg, Taenia spp 50–250epg, Fasciola spp 50–200epg, Moniezia spp 50–150epg, Paramphistomum spp 100–150epg, Dicrocoelium 100–150epg, Filicollis spp 50–150epg, Eimeria spp (oocysts) 50–100epg, and Nematodirus spp 50–100epg, were detected. Conclusion: The current study evidenced higher rates of helminth parasitic infection that can be prevented by ensuring antihelminthic treatments for the sheep population at different intervals. Keywords: Gastrointestinal parasites, helminthiasis, prevalence, risk factors, sheep. IntroductionSmall ruminants are the leading sources of protein, milk, meat, horns, bones, wool, religious sacraments, and medication, and they are recognized as the cheapest sources of economic development in remote parts of the world (Farooq et al., 2015; Yohannes and Alemu 2019). In Pakistan, livestock production is a significant component of the agricultural industry, making a considerable economic contribution and offering job opportunities to rural populations. Pakistan’s livestock accounts for 14.04% of Gross Domestic Product and 61.89% of the country’s agricultural output, as reported as 2.38% (Pakistan Economic Survey 2021–22). There are 28 sheep breeds in Pakistan that are well adapted to the various agro-ecological conditions of the country. Exporting and importing live sheep and goats across countries is a significant source of income (Shime 2018). In comparison to other cattle farms, running goat and sheep farms is simpler because there are fewer requirements for food, expenses, investment, and farm maintenance (Caroprese et al., 2016). Their nutritional needs can be easily met using bushes, grasses, fodder crops, and two types of agricultural industrial waste (Sarwar et al., 2002). Parasitism remains a source of association among hosts that poses a significant danger to the global livestock industry (Vercruysse and Claerebout 2001). Farmers have a serious problem with parasitism in sheep and goats. Parasitic gastroenteritis continues to cause serious health risks and restrict the output of small ruminants because of the associated morbidity, mortality, treatment cost, and control efforts at the clinical and subclinical levels (Nwosu et al., 2007). The health of goats, sheep, and other ruminants is severely threatened by endoparasites, which can potentially cause their demise, particularly in young animals, resulting in irreparable economic loss (Getachew et al., 2016). In terms of the economy, gastrointestinal parasites can cause weight loss of up to 50% and a 15% decline in profitability (Shahnawaz et al., 2011). Because of some endoparasites, ruminants might lose 3%–8% of their body weight (Niguse et al., 2014). Thus, parasite infection is viewed as a significant concern to the economy and health of cattle globally (Elemo and Geresu 2017). Gastrointestinal worms are a major effect on ruminant growth and development. These worms are dependent on factors such as geographic location, pasture management, grazing habitats, nutritional deficiency, immune status of ruminants, season, presence of intermediate hosts and vectors, climatic conditions, and the quantity of infectious eggs or larvae in the environment. This may slow down small ruminant development, reduce output, and raise mortality and morbidity rates (Husen et al., 2018). Moreover, helminth infections raise the risk of underweight attainment, poor food utilization, decreased fertility, and decreased milk, meat, and wool production (Lashari et al., 2015). Due to the increasing resistance of parasites to various anthelmintics, intestinal parasites in small ruminants have become increasingly challenging to control (Eke et al., 2019). Helminth infection is influenced by a number of variables, such as the host’s age, the type of parasites present, the species of the host, and other epidemiological manifestations, including the animals’ health, temperature, precipitation, humidity, vegetation, and weather, which are climate-related factors that can also have an impact on the quantity of eggs deposited and the health of the larvae (Mohammed et al., 2015). The small ruminant’s industry’s economics is harmed by gastrointestinal parasite illnesses (Qudoos et al., 2017). The ability of small ruminants to breed, as well as growth and wool production, can all be somewhat hampered by infections caused by gastrointestinal helminths (Walkden-Brown and Kahn 2002). Considering the available information, there is a scarcity of research on gastrointestinal helminth parasites in sheep in the district of Swat, Pakistan. Therefore, the current research work aimed to examine the prevalence and risk factors associated with the GIH parasitic fauna in sheep at lower and higher altitudes in the district of Swat, Pakistan. Materials and MethodsStudy areaThis research was conducted out the Swat district, located in the Malakand division of Khyber Pakhtunkhwa, approximately 165 km away from the capital city of Peshawar, Pakistan. The Swat is surrounded by Chitral and Ghizer valleys to the North, Kohistan, and Shangla districts to the East, Buner, and Malakand to the South, and the district Dir to the West (Khan et al., 2015). It is situated in the North-western parts of Pakistan, with geographical coordinates ranging from 34° 34ʹ to 35° 55ʹ North latitude and 72° 08ʹ to 72° 50ʹ East longitude. Swat experiences varying temperatures throughout the year, with summer temperatures ranging from 33°C to 37°C, while winter temperatures can drop to as low as −2°C. The region is characterized by mountainous terrain with elevations ranging from 600 meters to 6,000 meters above sea level. The total area of Swat is approximately 5,337 km2 (Fig. 1).
Fig. 1. Map showing the lower and higher altitudes of the study area. Data collectionThe fecal samples from each of the sheep at higher and lower altitudes were collected directly from the rectum with gloved fingers; then, the feces were put in the hygienic sampling plastic bottles in which ethanol was added, and the samples were numbered accordingly. Age, gender, locality, and collection date were recorded during collection. The collected data were transported to the Laboratory of Parasitology, Department of Zoology, University of Malakand Lower Dir, Khyber Pakhtunkhwa, for parasitic infection analysis. Field surveysA questionnaire-based interview was conducted that comprises the subsequent parameters: categories of ruminants, gender, geographical location, grazing types and locations, age of ruminants, stall feeding, health position, and drinking water source. The questionnaire was distributed among the sheep farmers, and the questions were mostly asked in their own dialects, “Gujri dialects” mostly. Macroscopic examinationThe collected samples were kept and scattered via glass rods in the petri dish and were carefully examined with the naked eye for the existence of various structures of the helminthic parasites, such as segments of tapeworms, adults and larval stages of nematode parasites, and eggs of trematodes. Microscopic examinationA small quantity of the preserved feces was placed on a clean glass slide. To dissolve the feces, a few drops of distilled water were added and mixed thoroughly. Careful removal of any debris was performed. Next, a cover slip was gently placed on the slide, streaked back and forth, and then examined under the microscope to identify helminth ova, eggs, larvae, and adult worms. Each sample was processed through sedimentation, flotation, centrifugation procedure and techniques. Data analysisThe raw data were entered into Microsoft Excel spreadsheets and analyzed using SPSS statistical software version 20, and descriptive statistics were used to summarize the data. One-way analysis of variance and t-test were performed to calculate the variations among seasons, altitudes, populations, and parasite species within the 95% confidence interval. The relative prevalence of different helminth species or groups was calculated using the following equation, which was previously reported by Raza et al. (2014). Prevalence (%)=Number of samples infected × 100/number of samples examined. Ethical approvalNot needed for this study. ResultsPrevalence of gastrointestinal parasites in sheepThe most prevalent species were Haemonchus spp 47.5% (n=191, Trichuris spp 17.4% (n=70), Taenia spp 14.4% (n=58, Moniezia spp 1.74% (n=7), Fasciola spp 1.49% (n=6), Eimeria spp 6.21% (n=25), Dicrocoelium spp 4.22% (n=17), Paramphistomum spp 4.67% (n=14), Nematodirus spp 2% (n=6), and Filicollis spp 2.67% (n=8). (p-value=0.0001) Figure 2.
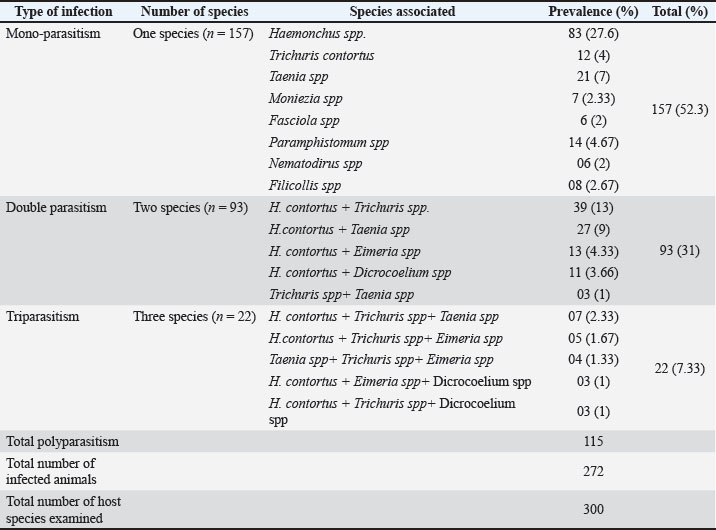
Fig. 2. Overall prevalence of helminths in the sheep population of the study area. Pattern of infection in the sheep population of Swat, PakistanA total of 300 sheep were screened for helminth parasitic infections. Out of the 272 screened, 272 were found to have been infected with one or more than one gastrointestinal parasite species. Of the infected number of sheep, 52.3%(n=157/300) (Table 1) were noted with single species of parasitic infection, 31% (n=93/300) with two species of parasitic infection, 7.33% (n=22/300) with three species of parasites Figure 2. Table 1. Mono- and polyparasitism of gastrointestinal parasitic infections in sheep hosts of hilly areas of Swat, Pakistan.
Mode of nutrition and GIT parasitesThe fecal samples were collected from two different sources: household-raised sheep and grazing-raised sheep production systems. Household-based animals primarily consume food such as leaves, vegetable wastes, and agricultural byproducts. These animals are kept in small numbers within houses. On the other hand, grazing-recovered ruminants are grazed by nomads and serve as the main source of income for them. Table 2 presents the data, with 94 stall-fed and 206 grazing animals included in the study. Among the households, 84% (n=79) were found to be infected by certain helminths, while among the grazing animals, the prevalence was higher at 93.6% (n=193) being infected. Table 2. Gastrointestinal parasitic infection between households and grazing animals.
Age-wise prevalence of gastrointestinal parasitic infection in sheepThe fecal samples from different-aged sheep were collected and arranged into two groups. The first group was sub-adults containing from birth to 1 year, and the second group was adults having more than 1 year. In sheep, the rate of helminth prevalence was 57/62 (91.94%) in sub-adults and 215/238 (90.34%) in adults (p-value=0.6998) (Table 3). Table 3. Age-wise prevalence of gastrointestinal parasitic infection in sheep.
In relation to sex, female sheep were found to be more infected, 92.2% (n=219/238), compared to male sheep, 85.4% (n=53/62). The statistical analysis shows a 0.115 p value (Table 4). Table 4. Sex-based helminthiasis in small ruminants.
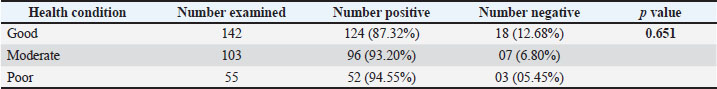
Association between helminth parasites and health conditions in sheepThe studied animals were categorized into three groups: those with good health conditions, moderate health conditions, and poor health condition. Out of 142 animals with good health, 124 (87.3%) were infected with helminth parasites. Similarly, out of 103 animals with moderate health conditions, 96 (93.2%) were found to be infected, and out of 55 animals with poor health condition, 52 (94.5%) were infected with helminth parasites (Table 5). These findings highlight that helminth parasites have a significant impact on the health of small ruminants in the study area, as they were prevalent across all three health/body condition categories (p >0.05). Table 5. Sex-based helminthiasis in small ruminant sheep.
Prevalence of gastrointestinal parasites in relation to altitudesAt higher altitudes, the prevalence rate of gastrointestinal helminths in sheep was higher, 94.5% as compared to lower altitude, 87.7% (p < 0.05) (Table 6). Table 6. Prevalence of gastrointestinal parasitic infection in sheep at higher and lower altitudes.
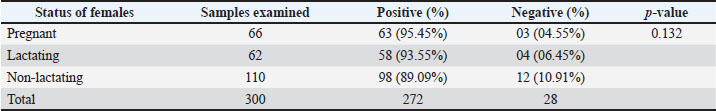
Prevalence of gastrointestinal parasites in different status of female sheepA total of 238 females were divided into three categories: one group contains pregnant animals, the second group contains lactating animals, and the third group contains dry female animals. Out of the 66 pregnant sheep, 63 (95.45%) showed positive infection for helminth parasites; in total lactating animals, 62 animals 58 (93.5%) were found positive, and in the remaining 110 dry females, 98 (89%) showed positive prevalence for helminth parasites (p > 0.05) (Table 7). Table 7. Prevalence of gastrointestinal parasitic infection in different status of female sheep.
Season-wise prevalenceData demonstrate that in winter season, sheep were found more to be infected as 94.8% followed by autumn 92.9% (n=79/85), summer 90.1% (n=55/61), while samples collected in spring were least infected as 86.4% (n=83/96) (Table 8). The association was found p > 0.05. Table 8. Season-wise prevalence of helminth parasites in sheep and goats.
DiscussionThe current research work revealed a 90.6% prevalence of parasitic infection in sheep, which is comparable with the studies conducted by Beskawy et al. (2018). The 80.8% fecal specimens were noted to be infected with helminth parasites. Sangma et al. (2012) examined 190 ovine hosts and found that 81.1% were infected with one or more than one species of helminth parasites. A similar rate of prevalence might be due to the climatic factors, nutritional status, and mode of living of the host population. A low prevalence rate of 63% was noted by Lemma and Abera (2013), who studied the prevalence of gastrointestinal helminths in ovine hosts in and around Asella, South Eastern Ethiopia. They have reported 68.8% prevalence. Seyoum et al. (2018) calculated 57.5% prevalence of nematode infections in sheep rearing in district Dabat, the northwest Ethiopia, which is lower than the findings of current study. Rahman et al. (2017) reported the prevalence of GIT worms in sheep from different areas of Bangladesh, with a prevalence of 63.4% in sheep hosts. The higher prevalence rate in the present study compared with the previous studies indicates environmental contamination of the pasture with eggs of parasitic worms and development of the infective stages. Haemonchus spp was the rank-first reported as 47.5% helminth infection in sheep of the study area. A higher prevalence rate of 82% was investigated in sheep in Kashmir (Lone et al., 2012). Sheep from Rawalpindi and Islamabad, Pakistan, were screened and reported 80.6% prevalence of Haemonchus contortus (Asif et al., 2008). A higher prevalence of this nematode was investigated in sheep in countries other than Pakistan as 88.9% (Radavelli et al., 2014) in Brazil and 76.6% in Slovakia (Babjak et al., 2017). Similar findings, 61.1% were also observed by Khajuria et al. (2013) in sheep of Jammu Kashmir. Some low rate of prevalence of H. contortus in sheep was reported as 23.4% (Rizwan et al., 2017) Sialkot, Pakistan; 34% (Sohail et al., 2017) Peshawar, Pakistan; 21.73% in sheep (Ruhoollah et al., 2021) Dir, Upper, Pakistan; 20% (Ayaz et al., 2013) Punjab, Pakistan; 27.4% (Rizwan et al., 2019) Punjab, Pakistan; 6.50% (Lashari and Tasawar, 2011) southern Punjab, Pakistan; 31.2% (Rahman et al., 2017) Bangladesh; 23.3% (Islam et al., 2017) Bangladesh; 5% (Maimadu et al., 2020) Nigeria; 43.7% (Sivajothi and Reddy, 2018) India; and 0.6% (Awaludin and Nusantoro, 2020) India. This study reports the prevalence of Trichuris spp as 17.4%, which is low compared with findings such as 32.2% (Asif et al., 2008), Rawalpindi, Islamabad; 40% (Gadahi et al., 2009) Rawalpindi-Islamabad; and 46% (Vohra et al., 2018) in India. Some low rate of prevalence of Trichuris spp 17.3% (Ruhoollah et al., 2021) in upper Dir; 3.08% (Yadav et al.,2006) Jammu Kashmir; 12.1% (Khajuria et al., 2013) Jammu Kashmir; 18.61% (Rizwan et al., 2019) Punjab; 8.72% (Sivajothi and Reddy, 2018) India; 7.8% (Babjak et al., 2017) Slovakia; 0.52% (Fayisa et al., 2020) Ethiopia; and 2.9% (Awaludin and Nusantoro, 2020) India. The prevalence of Taenia spp was 14.4%, which is comparable to findings such as 20.7% (Aliye and Deressa, 2017) Ethiopia; 26.4% of Egypt’s (Amer et al., 2017); 23.6% (Hughes et al., 2019) Tanzania; and 18.3% (Anwar et al., 2013) Egypt. Some low prevalence rates were observed as follows: 4.7% (Deressa et al., 2012) in Ethiopia; 4.9% (Mengistu et al., 2017) Ethipia; and 3.5% (Beskawy et al., 2018) Egypt. The current research demonstrated that the prevalence of Moniezia spp 1.74% which is lower than the findings such as 18.22% (Hassan et al., 2019) in Egypt and 15% (Vohra et al., 2018) in India. The current study findings were comparable with prevalence of Trichuris spp, which was 3.39% (Sivajothi and Reddy, 2018) India; 2.65% (Babjak et al., 2017) Slovakia; 2.5% (Fayisa et al., 2020) Ethiopia; 1.1% (Awaludin and Nusantoro 2020) Indonesia; 0.5% (Maimadu et al., 2020) Nigeria; and 6.7% (Juszczak et al., 2019) in Poland. In the current study, the prevalence of Fasciola spp was 1.49% which is comparable to findings such as 4.38% (Asif et al., 2008) in Rawalpindi and Islamabad; 8.2% (Khajuria et al., 2013) in Jammu Kashmir; and 4.4% (Gadahi et al., 2009) in Rawalpindi and Islamabad. Some higher prevalence rates were noted: 15% (Mehmood et al., 2013) in Lahore; 21.41% (Lashari and Tasawar 2011) in Southern Punjab; 13.58% (Ruhoollah et al., 2021) in Dir Upper; and 5.5% (Koinari et al., 2013) in Papua New Guinea. The prevalence of Eimeria spp oocysts was 6.21%, which is comparable to findings such as 0.26% (Fayisa et al., 2017) Ethiopia; 1% (Sohail et al., 2017) Peshawar; and 6.73% (Yadav et al., 2006) Jammu Kashmir. Higher prevalence rates, such as 18.22% (Rizwan et al., 2017) in Sialkot, Pakistan; 15.40% (Sivajothi and Reddy, 2018) in India; and 17.3% in sheep (Koinari et al., 2013) in Papua New Guinea. In the current study, the prevalence of Paramphistomum spp was 3.48% similar to the findings such as 5.9% (Al-Robaiee et al., 2019) Iraq, 1.1% (Awaludin and Nusantoro 2020) Indonesia, 0.5% (Maimadu et al., 2020) Nigeria, 5% (Ayaz et al., 2013) Punjab; and 8.91% (Sivajothi and Reddy, 2018) India. In this study, the prevalence of Nematodirus 1.49% (n=6) which is comparable to findings such as 1.8% (Koinari et al., 2013) in Papua New Guinea. Based on the pattern of infection, in the current study, more than half of the sheep (52.3%) were found infected with a single infection, while 38.3% were reported infected with multiple parasitic infections. Among the multiple parasitic infections, 31% with two species of parasitic infection and 7.33% with three species of parasitic infection were investigated; however, 9.33% were found without any parasitic infection. The present study results of double parasitic infection were similar to those of the study investigated by Ruhoollah et al. (2021) in small ruminants and greater than those of the study conducted by Rafiullah et al. (2011). This infection rate is due to the variation present in weather grazing behavior, and many other risk factors responsible for the prevalence of parasitic burden in small ruminants, as discussed earlier. Triple parasitic infections were also investigated in sheep at 45.6% by Ruhoollah et al. (2021). Female sheep showed a higher infection rate (90%) than males (85.4%). Similar findings have been reported in Bangkok, Thailand (Sangma et al. 2012), who investigated in their study that the prevalence rate in female sheep was 83.3% more than that in male sheep (79.3%). In Bangladesh, Mazid et al. (2006) recorded a 100% prevalence in female sheep, which is greater than that reported for male sheep 76%. similarly (Fatima et al., 2012) reported more prevalence in female sheep and goats in India Punjab and Pakistan Kashmir, respectively, while this observation is not agreed with (Yasmin et al., 2015), who reported that the infection rate in male sheep 81.5% were more infected than female 72.7%. The causes of low levels of parasitism in males may be the absence of pregnancy and the fetal developmental stages, which mainly affect the immunity of female ruminants. The current study revealed that grazing animals were found to be more infected 91.2% as compared to stall-feeding animals 82.5%, while in comparison with the findings of Nabi et al. 2014 the mean egg per gram for nematode infection was higher in stall-feeding animals than in grazing animals. The results of the present study are similar to those of the study conducted by Asif et al. (2008), in which they assumed that the prevalence of GI parasites in grazing sheep and goats is always greater than that in stall feed because of closed ground grassing habits that put the sheep and goats at high risk of parasitic infection. Another study, conducted by Pal et al. (1992), revealed that greater gastrointestinal parasitic infection in stall-fed livestock is due to the keeping of livestock in the form of flock with stall feeding by poor owners. The young sheep in the present study were slightly more infected (91.94%) than the adults (90.34%). The results of the present study are nearly similar to those of a study conducted by (Islam et al., 2017), which reported a prevalence rate of 78.4% in young small ruminants compared with adults (68.8%). The results of the age-wise prevalence analysis revealed the highest prevalence in the younger age groups of sheep and goats. The current study demonstrated that sheep and goats at higher altitudes showed little higher infection 91.2% as compared to lower altitudes (86.7%). ConclusionThis study calls for sheep farmers for a higher prevalence of GIT parasites. Sheep at higher altitudes, adult animals, and pregnant females were found to be more infected. The mode of nutrition, drinking water source, health status, age, and seasons were the leading factors for the incidence of parasitic infection in the sheep population. Based on the findings of the current research, proper awareness and management among sheep owners to remove parasitosis through preventive measures and deworming campaigns are suggested. Parasitic infection management, chemotherapy, and awareness programs should be launched among farmers regarding zoonotic disease transmission, unhygienic conditions, and illiteracy. AcknowledgmentsThe authors are grateful to the sheep owners for allowing the fecal samples directly from the rectum of sheep and interviewing on the information as questions related to sheep livestock. Conflict of interestNo conflict of interest was found among the authors of this study. FundingNo special funds were available for the conduction and publication of this work. This study was carried out on their own support and resources available to the authors. Authors’ contributionsAll authors contributed equally to this manuscript, including the writing, analysis, critical evaluation, and approval for publication. Data availabilityNot applicable ReferencesAliye, J. and Deressa, F.B. 2017. Prevalence and economic importance of Coenurus cerebralis in sheep and goats in and around “Legahida” district of bale zone, south eastern Ethiopia. Comp. Clin. Pathol. 26, 483–492. Al-Robaiee, I., Sabah, Z., Ahmed, K. and Salih, S.A. 2019. Diagnostic study of ovine gastrointestinal parasites in Kirkuk City, Iraq. Adv. Anim. Vet. Sci. 7(9), 727–731. Amer, S., ElKhatam, A., Fukuda, Y., Bakr, L.I., Zidan, S., Elsify, A., Mohamed, M.A., Tada, C. and Nakai, Y. 2017. Prevalence and identity of Taenia multiceps cysts “Coenurus cerebralis” in sheep in Egypt. Acta Trop. 176, 270–276. Anwar, S., Mahdy, E., El-Nesr, K.A., El-Dakhly, K.M., Shalaby, A. and Yanai, T. 2013. Monitoring of parasitic cysts in the brains of a flock of sheep in Egypt. Rev. Brasil. Parasitol. Vet. 22, 323–330. Asif, M., Azeem, S., Asif, S. and Nazir, S. 2008. Prevalence of gastrointestinal parasites in sheep and goats in and around Rawalpindi and Islamabad, Pakistan. J. Vet. Anim. Sci. 1(1), 14–17. Awaludin, A. and Nusantoro, S. 2020. Gastrointestinal parasites of sheep in Jember district (East Java–Indonesia). In IOP Conference Series: Earth and Environmental Science. IOP Publishing, vol. 411(1), pp: 012025. Ayaz, M.M., Raza, M.A., Murtaza, S. and Akhtar, S. 2013. Epidemiological survey of helminths of goats in southern Punjab, Pakistan. Trop. Biomed. 30(1), 62–71. Beskawy, M., Atwa, S., Abbas, I. and Aboelfadl, E. 2018. Clinical, some epidemiological, and risk factors studies of coenurosis in sheep at Dakahlia Governorate, Egypt. Assiut. Vet. Med. J. 64(157), 74–80. Caroprese, M., Napolitano, F., Mattiello, S., Fthenakis, G.C., Ribó, O. and Sevi, A. 2016. On-farm welfare monitoring of small ruminants. Small Ruminant Res. 135, 20–25. Deressa, A., Tilahun, T., Tadesse, A., Beyene, M., Gebrewold, G. and Pal, M. 2012. Assessment of Coenurus cerebralis and its economic impact in sheep brain harvested at Ethiopian Health and Nutrition Research Institute, Ethiopia. Int. J. Livest. Res. 2(2), 217–226. Eke, S.S., Omalu, I.C.J., Ochaguba, J.E., Urama, A.C., Hassan, S.C., Otuu, C.A. and Okafor, I.D. 2019. Prevalence of gastrointestinal parasites of sheep and goats slaughtered in Minna Modern Abattoir, Niger State, Nigeria. J. Ani. Sci. Vet. Med. 4(2), 65–70. Elemo, K.K. and Geresu, M.A. 2017. Prevalence and risk factors of gastrointestinal parasites of small ruminants in Sinana and Dinsho Districts of Bale Zone, South Eastern Ethiopia. Eur. J. Biol. Sci. 9(1), 01–08. Etter, E., Chartier, C., Hoste, H., Pors, I., Bouquet, W., Lefrileux, Y. and Borgida, L.P. 1999. The influence of nutrition on the per parturient rise in fecal egg. Revue Méd. Vét. 150 (12), 975–980. Farooq, A.A., Lashari, M.H., Akhtar., MS., Awais, M.M., Inayat, S. and Akhtar, M. 2015. Prevalence of bovine fascioliasis in different commercial and non-commercial dairy farms of District Rajanpur, Punjab, Pakistan. Pak. J. Life Soc. Sci. 13(8), 8–11. Fatima, M., Chishti, M.Z., Ahmad, F. and Lone, B.A. 2012. Epidemiological study of fasciolosis in cattle of Kashmir valley. Adv. Biol. Res. 6(3), 106–109. Fayisa, O., Duguma, A., Temesgen, M. and Lemma, F. 2020. Gastrointestinal parasites of sheep and goat in and around Gondar town, Northwest, Ethiopia. Biotechnol. Anim. Husbandry 36(3), 371–380. Gadahi, J.A., Arshed, M.J., Ali, Q., Javaid, S.B. and Shah, S.I. 2009. Prevalence of gastrointestinal parasites in sheep and goats in Rawalpindi and Islamabad, Pakistan. Vet. World 2(2), 51–61. Getachew, T., Muktar, Y., Mekonnen, N. and Tesma, F. 2016. Prevalence of gastrointestinal nematodes and efficacy of commonly used anthelmintics in different sheep breeds in Areka Agricultural Research Center, Areka, Ethiopia. Livest. Res. Rural Dev. 28, 117. Hassan, N.M., Farag, T.K., Abu El Ezz, N.M. and Abou-Zeina, H.A. 2019. Prevalence assessment of gastrointestinal parasitic infections among goats in Giza Governorate, Egypt. Bull. Nat. Res. Cen. 43, 1–7. Hughes, E.C., Kibona, T.K., De Glanville, W.A., Lankester, F., Davis, A., Carter, R.W., De Jong, R.M., Nyasebwa, O.M., Claxton, J.R., Cleaveland, S. and Allan, K.J. 2019. Taenia multiceps coenurosis in Tanzania: a major and under-recognized livestock disease problem in pastoral communities. Vet. Rec. 184(6), 191. Husen, M., Aliyi, F., Damtew, S., Negassa, T. and Abebe, H. 2018. Prevalence of small ruminant helminthiasis in and around Tullo district in western Harerghe zone, eastern Ethiopia. Austin J. Vet. Sci. Anim. Husb. 5(1), 1038–1048. Islam, M., Hossain, M., Dey, A., Alim, M., Akter, S. and Alam, M. 2017. Epidemiology of gastrointestinal parasites of small ruminants in Mymensingh, Bangladesh. J. Adv. Vet. Anim. Res. 4(4), 356–362. Juszczak, M., Sadowska, N. and Udala, J. 2019. Parasites of the digestive tract of sheep and goats from organic farms in Western Pomerania, Poland. Ann. Parasitol. 65(3), 313–319. Khajuria, J.K., Katoch, R., Yadav, A., Godara, R., Gupta, S.K. and Singh, A. 2013. Seasonal prevalence of gastrointestinal helminths in sheep and goats of middle agro-climatic zone of Jammu province. J. Parasitic Dis. 37(1), 21–25. Khan, M.P.Z., Ahmad, M., Zafar, M., Sultana, S., Ali, M.I. and Sun, H. 2015. Ethnomedicinal uses of edible wild fruits (EWFs) in Swat Valley, Northern Pak. J. Ethnophar. 173, 191–203. Koinari, M., Karl, S., Ryan, U. and Lymbery, A.J. 2013. Infection levels of gastrointestinal parasites in sheep and goats in Papua New Guinea. J. Helminth. 87(4), 409–415. Lashari, M.H., Zahida Tasawar, Z.T., Akhtar., M.S., Chaudhary., M.S. and Nuzhat Sial, N.S. 2015. Prevalence of Haemonchus contortus in local goats of DG Khan. World J. Pharm. Pharm. Sci. 4(5), 190–196. Lemma, D. and Abera, B. 2013. Prevalence of ovine gastrointestinal nematodes in and around Asella, South Eastern Ethiopia. J. Vet. Med. Ani. Heal. 5(8), 222–228. Lone, B.A., Chishti, MZ. and Ahmad Tak, H. 2012. Helminthic infestations in slaughtered Sheep and Goats of District Ganderbal, Kashmir. Liver 30(29.35), 60. Lashari, M.H. and Zahida Tasawar, Z.T., 2011. Prevalence of some gastrointestinal parasites in sheep in Southern Punjab, Pakistan. Pak. Vet. J. 31(4), 295–298. Maimadu, A.A., Akinsulie, O.C., Olabode, M.P., Bata, S.I., Waziri, I.A. and Sabo, J.A. 2020. Prevalence of gastrointestinal parasites and their impact in sheep in Riyom local government area of Plateau State. J. Bacteriol. Parasitol. 11(3), 375–380. Mazid, M., Bhattacharjee, J., Begum, N. and Rahman, M. 2006. Helminth parasites of the digestive system of sheep in Mymensingh, Bangladesh. Bangladesh J. Vet. Med. 4(2), 117–122. Mehmood, K., Ijaz, M., Durrani, A.Z., Khan, M.A., Sabir, A.J. and Saleem, M.H. 2013. Infection rate and therapeutic trials on various gastrointestinal parasites in sheep and goats in and around Lahore, Pakistan. Pak. J. Zool. 45(2), 322–326. Mengistu, S., Fayyisa, D., Belina, D. and Eshetu, A. 2017. Prevalence of Coenurus cerebralis and its economic loss in small ruminants slaughtered at Bishoftu elfora export abattoir, Ethiopia. Eur. J. Biol. Sci. 9, 101–105. Mohammed, A., Disassa, H., Kabeta, T., Zenebe, T. and Kebede, G. 2015. Prevalence of gastrointestinal nematodes in sheep in Gursum Woreda of the eastern Hararghe zone, Oromia regional state. Eth. Res. 7, 45–54. Nabi, H., Saeed, K., Shah, S.R., Rashid, M.I., Akbar, H. and Shehzad, W. 2014. Epidemiological study of gastrointestinal nematodes of goats in district Swat, Khyber Pakhtunkhwa, Pakistan. Sci. Int. 26(1), 282–299. Niguse, A., Shimelis, D. and Feyera, T. 2014. Epidemiology and chemotherapy of gastrointestinal parasites of sheep in and around Jigjiga, Eastern Ethiopia. Eur. J. Biol. Sci. 6(2), 6–53. Nwosu, C.O., Madu, P.P. and Richards, W.S. 2007. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of north-eastern Nigeria. Vet. Parasitol. 144(1–2), 118–124. Qudoos, A., Khan, M.N., Sajid, M.S. and Muhammad, G. 2017. Correlation of trace mineral profiles with gastrointestinal worm burden in rangeland sheep of Chakwal District, Punjab, Pakistan. Int. J. Agric. Biol. 19(1), 140–144. Radavelli, W.M., Pazinato, R., Klauck, V., Volpato, A., Balzan, A., Rossett, J., Cazarotto, C.J., Lopes, L.S., Kessler, J.D., Cucco, D.C., Tonin, A.A. and Silva, A.S.D. 2014. Occurrence of gastrointestinal parasites in goats from the Western Santa Catarina, Brazil. Rev. Brasil. Parasitol. Vet. 23(1), 101–104. Rafiullah, T.A., Sajid, A., Shah, S.R., Ahmad, S. and Shahid, M. 2011. Prevalence of gastrointestinal tract parasites in cattle of Khyber Pakhtunkhwa. ARPN. J. Agr. Biol. Sci. 6, 9–15. Rahman, M.A., Labony, S.S., Dey, A.R. and Alam, M.Z. 2017. An epidemiological investigation of gastrointestinal parasites of small ruminants in Tangail, Bangladesh. J. Bang. Agr. Univ. 15(2), 255–259. Rizwan, H.M., Sajid., MS., Iqbal, Z. and Saqib, M. 2017. Point prevalence of gastrointestinal parasites of domestic sheep (Ovis aries) in district Sialkot, Punjab, Pakistan. JAPS 27(3), 321–316. Rizwan, H.M., Sajid, MS., Iqbal, Z. and Saqib, M. 2019. Association of phytomineral with gastrointestinal parasites of grazing sheep in Sialkot district, Punjab, Pakistan. Pak. J. Agric. Sci. 56(2), 459–468. Raza, M.A., Younas, M. and Schlecht, E. 2014. Prevalence of gastrointestinal helminths in pastoral sheep and goat flocks in the Cholistan desert of Pakistan. J. Anim. Plant Sci. 24(1), 39–44. Ruhoollah, K.W., Al-Jabr, O.A., Khan, T., Khan, A., El-Ghareeb, W.R. and Swelum, A.A. 2021. Prevalence of gastrointestinal parasite in small ruminants of District Dir Upper Khyber Pakhtunkhwa Province of Pakistan. Brazil. J. Biol. 83, e248978. .Sangma, A., Begum, N., Roy, B.C. and Gani, M.O. 2012. Prevalence of helminth parasites in sheep (Ovis aries) in Tangail district, Bangladesh. J. Bang. Agr. Uni. 10(2), 235–244. Sarwar, M., Khan, M.A. and Iqbal, Z. 2002. Status paper feed resources for livestock in Pakistan. Int. J. Agric. Biol. 4(1), 186–192. Seyoum, Z., Getnet, K., Chanie, M., Derso, S. and Fentahun, S. 2018. Morbidity parameters associated with gastrointestinal tract nematodes in sheep in district, northwest Ethiopia. BioMed Res. Int. 1(1), 92–100. Shahnawaz, M., Shahardar, R.A. and Wani, Z.A. 2011. Seasonal prevalence of platyhelminthosis in sheep in the Ganderbal area of Kashmir valley. J. Vet. Parasitol. 25(1), 59–62. Shime, A. 2018. Helimenthe parasites of small ruminants in Gozamin Woreda. J. Nat. Sci. Res. 8, 56–62. Sivajothi, S. and Reddy, B. 2018. Seasonal prevalence of gastrointestinal parasites of small ruminants in YSR Kadapa district of Andhra Pradesh, India. Int. J. Livestock Res. 8(1), 184–189. Sohail, M., Nauman-ul-Islam, M., Shah, S.S.A., Shah, I.A. and Raziq, A. Ilyas, M. 2017. Incidence of gastrointestinal parasites in metal goats at District Peshawar, Pakistan. Adv. Anim. Vet. Sci. 5(5), 205–207. Vohra, S., Singh, S., Kumar, P. and Patil, C.S. 2018. Incidence and severity of gastrointestinal parasites in small ruminants at Hisar, Haryana. J. Anim. Res. 8(6), 1021–1025. Yadav, A., Khajuria, J.K. and Raina, A.K. 2006. Seasonal prevalence of gastrointestinal parasites in sheep and goats of Jammu. J. Vet. Parasitol. 20(1), 65–68. Yasmin, C., Otoi, T., Setiadi, M.A. and Karja, N.W. 2015. Maturation and fertilisation of sheep oocytes cultured in serum-free medium containing silk protein sericin. Acta Vet. Hungarica 63(1), 110–117. Yohannes, T. and Alemu, B. 2019. Gastrointestinal nematode infestation; prevalence and associated risk factors in small ruminants at Wolayta Soddo District, Southern Ethiopia. Int. J. Curr. Res. Med. Sci. 5(2), 31–38. | ||
| How to Cite this Article |
| Pubmed Style Aldamigh MA, Ali W, Khan W, Rahman AU, Fadladdin YAJ, Yousaf M, Ahmad Z, Ríos‑escalante PRDL. Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. Open Vet. J.. 2025; 15(10): 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 Web Style Aldamigh MA, Ali W, Khan W, Rahman AU, Fadladdin YAJ, Yousaf M, Ahmad Z, Ríos‑escalante PRDL. Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. https://www.openveterinaryjournal.com/?mno=245482 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i10.45 AMA (American Medical Association) Style Aldamigh MA, Ali W, Khan W, Rahman AU, Fadladdin YAJ, Yousaf M, Ahmad Z, Ríos‑escalante PRDL. Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. Open Vet. J.. 2025; 15(10): 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 Vancouver/ICMJE Style Aldamigh MA, Ali W, Khan W, Rahman AU, Fadladdin YAJ, Yousaf M, Ahmad Z, Ríos‑escalante PRDL. Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. Open Vet. J.. (2025), [cited January 25, 2026]; 15(10): 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 Harvard Style Aldamigh, M. A., Ali, . W., Khan, . W., Rahman, . A. U., Fadladdin, . Y. A. J., Yousaf, . M., Ahmad, . Z. & Ríos‑escalante, . P. R. D. L. (2025) Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. Open Vet. J., 15 (10), 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 Turabian Style Aldamigh, Mashael A., Wajid Ali, Wali Khan, Azizu Ur Rahman, Yousef Abdal Jalil Fadladdin, Muhammad Yousaf, Zaira Ahmad, and Patricio R. De Los Ríos‑escalante. 2025. Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. Open Veterinary Journal, 15 (10), 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 Chicago Style Aldamigh, Mashael A., Wajid Ali, Wali Khan, Azizu Ur Rahman, Yousef Abdal Jalil Fadladdin, Muhammad Yousaf, Zaira Ahmad, and Patricio R. De Los Ríos‑escalante. "Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan." Open Veterinary Journal 15 (2025), 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 MLA (The Modern Language Association) Style Aldamigh, Mashael A., Wajid Ali, Wali Khan, Azizu Ur Rahman, Yousef Abdal Jalil Fadladdin, Muhammad Yousaf, Zaira Ahmad, and Patricio R. De Los Ríos‑escalante. "Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan." Open Veterinary Journal 15.10 (2025), 5284-5293. Print. doi:10.5455/OVJ.2025.v15.i10.45 APA (American Psychological Association) Style Aldamigh, M. A., Ali, . W., Khan, . W., Rahman, . A. U., Fadladdin, . Y. A. J., Yousaf, . M., Ahmad, . Z. & Ríos‑escalante, . P. R. D. L. (2025) Prevalence of gastrointestinal parasites and their associated risk factors in sheep raised at high and low altitudes in Swat, Pakistan. Open Veterinary Journal, 15 (10), 5284-5293. doi:10.5455/OVJ.2025.v15.i10.45 |