| Research Article | ||
Open Vet. J.. 2025; 15(7): 3115-3123 Open Veterinary Journal, (2025), Vol. 15(7): 3115-3123 Original Article Effects of trehalose supplementation on Pesisir bull sperm quality during storageJaswandi*, Masrizal and AnandaDepartment of Animal Production Technology, Faculty of Animal Science, Universitas Andalas, Padang, Indonesia *Corresponding Author: Jaswandi. Department of Animal Production Technology, Faculty of Animal Science, Universitas Andalas, Padang, Indonesia. Email: jaswandij [at] ansci.unand.ac.id Submitted: 04/03/2025 Revised: 29/05/2025 Accepted: 17/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
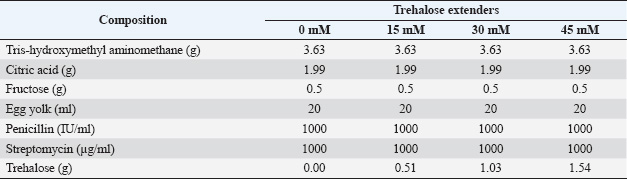
ABSTRACTBackground: Semen preservation is a critical component of artificial insemination (AI) in livestock breeding, with storage conditions playing a vital role in maintaining sperm quality. Pesisir bulls, an indigenous breed from West Sumatra, Indonesia, face reproductive challenges due to limited breeding programs and suboptimal semen preservation techniques. Trehalose, a non-reducing disaccharide, is recognized for its cryoprotective and antioxidant properties, which help preserve sperm motility, viability, and membrane integrity during storage. However, the optimal concentration of trehalose for liquid semen storage in Pesisir bulls remains undetermined. Aim: This study evaluated the effects of trehalose supplementation in a Tris–egg yolk extender on the motility, viability, plasma membrane integrity, and acrosome integrity of Pesisir bull sperm stored at 5°C for 72 hours. Methods: Ejaculates from Pesisir bulls with ≥80% individual motility were collected weekly using an artificial vagina. Semen was diluted with a Tris–egg yolk extender containing 0, 15, 30, or 45 mM trehalose to achieve a final concentration of 25 million spermatozoa per ml. Sperm quality parameters were assessed at 0, 24, 48, and 72 hours post-dilution. Results: Fresh semen had a volume of 3.5 ± 0.2 ml, a milky white color, thick consistency, a pH of 6.8 ± 0.1, and a characteristic seminal odor. Microscopically, it exhibited +++ mass movement, a concentration of 1,465 ± 50 × 106 sperm/ml, 85% ± 2.1% motility, 88% ± 1.8% viability, 5.0% ± 0.5% abnormalities, and 85.5% ± 2.0% plasma membrane integrity. Trehalose supplementation—particularly at a concentration of 30 mM—significantly (p < 0.05) enhanced sperm motility, viability, plasma membrane integrity, and acrosome integrity compared with the control group. Although storage-induced declines in these parameters were observed across all treatments, 30 mM trehalose supplementation effectively mitigated these reductions, preserving sperm quality over the 72-hour storage period. Conclusion: Trehalose supplementation in a Tris–egg yolk extender enhanced the preservation of Pesisir bull semen during liquid storage. A concentration of 30 mM trehalose was found to be optimal for maintaining sperm quality at 5°C, offering potential benefits for artificial insemination programs and genetic conservation initiatives. Keywords: Pesisir bull, Semen storage, Trehalose, Sperm quality. IntroductionArtificial insemination (AI) is a widely used reproductive technology in livestock breeding programs aimed at improving genetic quality and reproductive efficiency. The success of AI depends largely on semen quality, which is influenced by various factors, including storage conditions and the composition of semen extenders. During liquid storage and cryopreservation, spermatozoa experience physiological stress that leads to decreased motility, viability, and membrane integrity, ultimately compromising fertilization potential (Perumal et al., 2015). Therefore, the development of optimized semen preservation techniques is essential to enhance AI success rates and support the conservation of valuable livestock genetic resources. Pesisir bulls, an indigenous breed from West Sumatra, Indonesia, represent a vital genetic resource due to their adaptability and reproductive potential (Hendri et al., 2024). However, limitations in semen cryopreservation and liquid storage remain significant barriers to maximizing their reproductive efficiency. A major concern is the post-thaw decline in sperm motility and viability, which substantially impairs the fertilization potential of frozen semen (Ananda et al., 2025; Masrizal et al., 2025). In the absence of effective semen preservation strategies, the reproductive performance of Pesisir bulls may be compromised, negatively impacting both genetic conservation efforts and cattle breeding programs (Masrizal et al., 2024). Therefore, optimizing semen storage conditions is essential to improve AI outcomes and safeguard the genetic diversity of this native breed. Trehalose, a nonreducing disaccharide, has garnered considerable attention for its use in semen cryopreservation and liquid storage due to its cryoprotective and antioxidant properties. It acts as a nonpermeable cryoprotectant, helping to maintain sperm membrane integrity and enhance post-thaw motility by stabilizing cellular structures under stress conditions (Öztürk et al., 2017). Trehalose supplementation protects sperm membranes from osmotic stress, lipid peroxidation, and oxidative damage, thereby reducing overall sperm deterioration during storage (Iqbal et al., 2017; Güngör et al., 2019). Studies have shown that trehalose not only improves post-thaw motility and viability but also enhances acrosomal integrity, which is critical for successful fertilization (Zhao et al., 2020). Several studies have supported the role of trehalose in preserving sperm quality across various preservation methods. Kumar et al. (2012) reported that trehalose supplementation significantly improved post-thaw motility and membrane integrity in buffalo and Karan Fries bull spermatozoa, confirming its protective effects during freezing and thawing cycles. Additionally, Iqbal et al. (2017) found that combining trehalose with egg yolk and glycerol markedly enhanced post-thaw sperm quality and in vivo fertility in buffalo semen. Although trehalose has been widely studied in cryopreservation, its protective benefits have also been observed in liquid semen storage at 5°C. Perumal et al. (2015) demonstrated that trehalose supplementation improved the quality of mithun semen during refrigeration, highlighting its potential benefits for bull semen stored under similar conditions. Although trehalose is considered an effective sperm cryoprotectant, its efficacy is highly concentration-dependent. Research has indicated that moderate concentrations (e.g., 45–50 mM) offer optimal protection, whereas excessive levels may induce hypertonic stress, increase viscosity, and reduce sperm motility (Kamal et al., 2023). Therefore, determining the optimal trehalose concentration for liquid semen storage is essential to balance its protective properties without compromising sperm functionality. Comparative studies have also evaluated trehalose’s efficacy against conventional cryoprotectants, such as glycerol. Replacing glycerol with trehalose improved sperm motility and mitochondrial membrane potential, further underscoring trehalose’s role in enhancing sperm viability (Athurupana et al., 2015). Additionally, Bucak et al. (2021) reported that combining trehalose with fetuin provided added post-thaw protection, further supporting its application in semen extenders. Despite extensive research on the benefits of trehalose in frozen semen preservation, the optimal concentration for liquid storage of Pesisir bull semen remains uncertain. While previous studies have demonstrated its efficacy during cryopreservation, data on the impact of various trehalose concentrations during liquid storage at 5°C are limited. Given the increasing interest in liquid semen preservation as a practical alternative to cryopreservation, further investigation is needed to identify the most effective trehalose concentration for maintaining sperm motility, viability, and structural integrity over extended storage periods. This study aimed to evaluate the effects of trehalose supplementation in Tris–egg yolk extenders on the motility, viability, plasma membrane integrity, and acrosome integrity of Pesisir bull sperm stored at 5°C for 72 hours. Identifying the optimal trehalose concentration will help improve semen preservation techniques for indigenous cattle breeds. Materials and MethodsSemen collection and processingSemen samples were collected from mature Pesisir bulls (aged 3–5 years) using an artificial vagina. Only ejaculates with a minimum individual motility of 80% and a concentration of at least 800 million spermatozoa per milliliter were used for further analysis. Each ejaculate was immediately evaluated for volume (ml), color, concentration (106 sperm/ml), motility (%), viability (%), and abnormality (%) using a phase-contrast microscope (Olympus CX-23). Semen was then diluted with a Tris–egg yolk extender containing trehalose (≥99% purity, Sigma-Aldrich, USA) at concentrations of 0, 15, 30, and 45 mM (Table 1). The diluted semen samples were stored at 5°C and analyzed at four time points: 0, 24, 48, and 72 hours post-dilution. Sperm quality assessmentSperm motilitySperm motility was assessed subjectively by placing a drop of semen on a glass slide, covering it with a cover slip, and examining it under a phase-contrast microscope (Olympus CX-23) at 400× magnification. Progressive motility was defined as the percentage of sperm exhibiting forward movement, evaluated using a standard scale ranging from nonmotile to >90% motile cells. At least five random fields per slide were analyzed. Sperm viabilitySperm viability was evaluated using the eosin-nigrosin staining method (Morphisto, 12424.00100). Dead sperm cells were identified by their absorption of eosin dye, while live sperm remained unstained due to intact plasma membranes. A small drop of semen (maintained at 35°C) was mixed with 2–3 drops of eosin-nigrosin stain on a clean glass slide placed on a thermostatically controlled warm stage (34°C–35°C). After 2 minutes, the mixture was smeared onto a clean, grease-free glass slide, air-dried, and examined under a bright-field microscope using a 100× oil immersion objective. Approximately 200 spermatozoa were evaluated across five different fields per slide. Table 1. Composition of Tris–egg yolk extender with trehalose (100 ml).
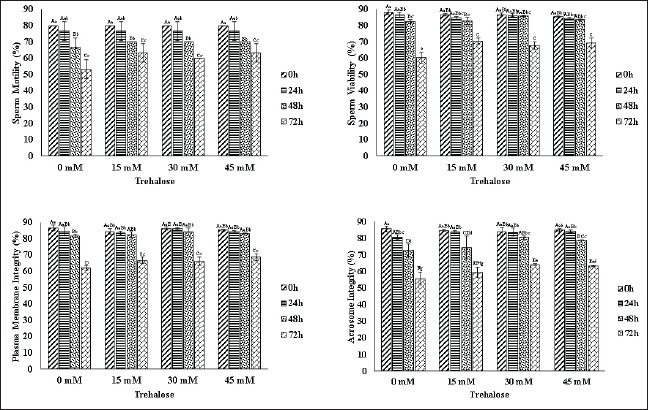
Plasma membrane integritySperm plasma membrane integrity was evaluated using the hypo-osmotic swelling test (HOST). The HOST solution was prepared by dissolving 0.735 g of sodium citrate and 1.351 g of fructose in 1,000 ml of distilled water. A 50 µl semen sample was mixed with 500 µl of the HOST solution and incubated at 37°C for 40 minutes. After incubation, 5 µl of the mixture was placed on a glass slide and examined under a phase-contrast microscope (Olympus CX-23) at 400× magnification. A total of 100 spermatozoa were counted per sample. Sperm displaying swollen tails were classified as having intact plasma membranes (normal), whereas unswollen sperm were considered to have damaged membranes (abnormal). Acrosome integrityThe acrosome integrity test (TAU) was conducted by placing a drop of semen on a clean glass slide, smearing it using another slide held at a 45° angle, and allowing it to air-dry. The smear was then fixed in 5% formalin for 30 minutes, rinsed with running water, and air-dried again. The slide was subsequently immersed in Giemsa stain (Merck, Germany) for 4 hours at 37°C. After staining, the slide was rinsed under running water and air-dried. Acrosome integrity was evaluated under a light microscope at 400× magnification, with at least 200 spermatozoa examined per slide. Sperm with intact acrosomes displayed dark purple-stained acrosomal caps, whereas those with damaged acrosomes appeared lighter in color. The acceptable threshold for acrosome integrity after cryopreservation was considered to be ≥65% (Teethol et al., 2022). Statistical analysisThis study was conducted in triplicate. Data are presented as the mean ± SEM. The average values were analyzed using a two-way ANOVA model (trehalose concentration and storage duration), followed by Duncan’s multiple range test using SPSS software. Differences were considered statistically significant at p < 0.05. Ethical approvalThis study was conducted in accordance with ethical guidelines for animal research and was approved by the Institutional Animal Ethics Committee of the Faculty of Medicine, Universitas Andalas (Approval No. 462/UN.16.2/KEP-FK/2024). ResultsFresh sperm quality of Pesisir bullsFresh semen from Pesisir bulls had an average volume of 3.5 ± 0.2 ml, a milky white color, thick consistency, and a pH of 6.8 ± 0.1 (Table 2). Microscopic evaluation showed a sperm concentration of 1465 ± 50 × 106 sperm/ml, with mass movement graded as +++. The initial sperm motility, viability, plasma membrane integrity, and acrosome integrity were 85.0% ± 2.1%, 88.0% ± 1.8%, 85.5% ± 2.0%, and 86.0% ± 1.9%, respectively. The effect of trehalose on sperm quality during liquid storageSperm motility, viability, plasma membrane integrity, and acrosome integrity declined over the 72-hour storage period at 5°C in all treatment groups (Fig. 1). At 0 hours, motility was 80% across all groups. However, a significant reduction (p < 0.05) was observed over time, with the 0 mM group showing the steepest decline, reaching 53.33% at 72 hours, whereas sperm stored with 30 mM trehalose maintained higher motility at 60%. A similar trend was observed in sperm viability: all groups began with ≥85% viability at 0 hours, but by 72 hours, the 0 mM group showed the lowest viability (60.28%), while the 30 mM trehalose group preserved the highest viability (67.72%). Plasma membrane integrity also showed a gradual decline over time. Initially, all groups exhibited membrane integrity above 85%; however, by 72 hours, sperm stored without trehalose (0 mM) demonstrated the lowest integrity (61.99%), while the 30 mM trehalose group maintained a higher percentage (66.24%). A similar pattern was observed for acrosome integrity. Although all groups started with values exceeding 85%, by 72 hours, the 0 mM group exhibited the most substantial decline, with only 55.49% intact acrosomes. In contrast, sperm in the 30 mM trehalose group retained a significantly higher acrosome integrity of 64.12%. Table 2. Macroscopic and microscopic characteristics of fresh Pesisir bull semen.
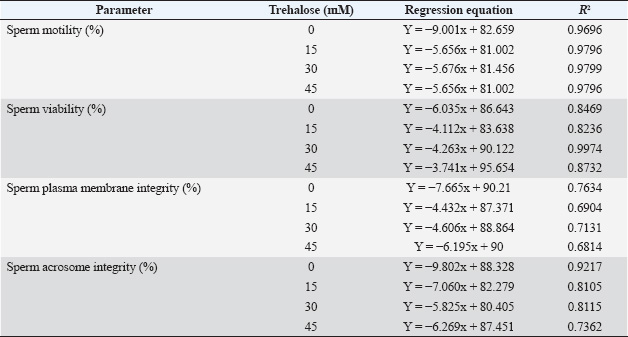
Linear regression analysis (Fig. 2 and Table 3) confirmed that sperm stored without trehalose (0 mM) exhibited the steepest decline in all sperm quality parameters, as indicated by the most negative slope values in the regression equations. In contrast, supplementation with 30 mM trehalose consistently resulted in the lowest rate of decline in sperm motility (Y=–5.676x + 81.456; R²=0.9799), viability (Y=–4.263x + 90.122; R²=0.9974), plasma membrane integrity (Y=–4.606x + 88.864; R²=0.7131), and acrosome integrity (Y=–5.825x + 80.405; R²=0.8115). The regression coefficients (R² values) further demonstrate that 30 mM trehalose provided the most stable preservation effect over the 72-hour storage period at 5 °C. These findings are illustrated in Figure 2, where the 30 mM trend lines consistently remained above those of the other treatment groups across all time points. DiscussionThe quality of fresh Pesisir bull semen is influenced by several factors, including sperm selection methods, semen processing conditions, bull age, and the composition of the semen extender. This study confirmed that Pesisir bull semen exhibits high initial quality, with an average volume of 3.5 ± 0.2 ml, a milky white color, thick consistency, and a pH of 6.8 ± 0.1 based on macroscopic and microscopic evaluations. Furthermore, the high sperm concentration (1465 ± 50 × 106 sperm/ml), progressive motility (85% ± 2.1%), and viability (88.0% ± 1.8%) indicate its suitability for AI programs. These findings are consistent with previous studies on indigenous tropical cattle, which emphasize that high sperm motility and viability are key indicators of fertility potential (Masrizal et al., 2024). Several technological advancements have been introduced to further enhance sperm motility and selection efficiency, both of which are critical determinants of semen quality. The use of computer-assisted sperm analysis (CASA) has been proposed as an effective tool for objectively quantifying sperm motility and improving the success rates of AI programs (Wahyudi et al., 2023; Hendri et al., 2024). In addition, sperm separation techniques such as albumin gradient centrifugation have been utilized to isolate X- and Y-chromosome-bearing spermatozoa, enabling gender-specific breeding programs (Afriani et al., 2022). These molecular approaches enhance the precision of sperm selection and contribute to improved herd management strategies (Mundana et al., 2023). The formulation of semen extenders plays a critical role in maintaining sperm function following collection. Supplementing Tris–egg yolk diluents with trehalose has been shown to enhance sperm motility, viability, and structural integrity (Syahminan et al., 2023). As a non-reducing disaccharide, trehalose functions as both an osmoprotectant and an antioxidant, stabilizing lipid membranes and proteins against oxidative stress and cold shock (Güngör et al., 2019; Zhao et al., 2020). This study confirmed that trehalose supplementation significantly improved sperm quality during liquid storage, as evidenced by higher motility, viability, plasma membrane integrity, and acrosome integrity in the 30 mM trehalose group compared with the 0, 15, and 45 mM groups. One of the primary protective mechanisms of trehalose is its ability to stabilize sperm membranes under stress conditions. Trehalose has been shown to interact with sperm membrane phospholipids, enhancing membrane fluidity and integrity—both of which are essential for preventing membrane rupture and maintaining sperm function during liquid storage and cryopreservation (Kumar and Atreja, 2011; Karunakaran et al., 2018). The findings of this study support these results, as sperm stored in 30 mM trehalose exhibited significantly higher plasma membrane and acrosome integrity compared with the other treatment groups. Previous research has demonstrated that trehalose supplementation improves post-thaw sperm viability and prevents premature acrosome reactions, both of which are critical for successful fertilization (Öztürk et al., 2017; Xi et al., 2018; Larasati et al., 2024). Trehalose possesses antioxidant properties that help reduce oxidative stress, which can compromise sperm viability during storage. Reactive oxygen species (ROS) induce lipid peroxidation, leading to membrane instability, DNA damage, and reduced sperm functionality (Anjos et al., 2021; Borah et al., 2021). Several studies have demonstrated that trehalose supplementation significantly reduces ROS levels, thereby preserving sperm motility and viability during storage (Olaciregui and Gil, 2016; Gholami et al., 2023). In this study, sperm stored in 30 mM trehalose retained significantly higher motility and viability than those in the control group (0 mM trehalose) throughout the 72-hour storage period, further supporting the dual function of trehalose as both a cryoprotectant and antioxidant (Güngör et al., 2017; Zhao et al., 2020).
Fig. 1. Effect of trehalose supplementation on sperm quality parameters of Pesisir bull semen during liquid storage at 5°C. The graphs represent the percentages of motility, viability, plasma membrane integrity, and acrosome integrity in semen samples supplemented with 0, 15, 30, and 45 mM trehalose at different observation times (0-, 24-, 48-, and 72-hours). Different uppercase superscript letters within the same time point indicate highly significant differences (p < 0.01), while different lowercase superscript letters indicate significant differences (p < 0.05). Interestingly, trehalose enhances the resilience of spermatozoa to desiccation, a property that may be further leveraged in freeze-drying applications (Olaciregui and Gil, 2016; Wakayama et al., 2019). Studies have shown that trehalose significantly increases sperm tolerance to extreme dehydration and temperature fluctuations, highlighting its potential for use in alternative preservation methods such as lyophilization (freeze-drying) or ambient-temperature storage (Güngör et al., 2018; Hu et al., 2019). Although this study focused on liquid semen storage, the findings suggest that the protective effects of trehalose may extend beyond traditional preservation techniques. While the findings of this study suggest that 30 mM trehalose is optimal for liquid semen storage, further research is warranted to evaluate its long-term effects beyond 72 hours and to determine whether extended storage durations can maintain acceptable sperm quality. Additionally, investigating the synergistic effects of trehalose with other antioxidants, such as glutathione and taurine, may enhance sperm protection and longevity. Molecular-level analyses examining oxidative stress biomarkers and mitochondrial function in trehalose-treated spermatozoa could provide deeper insight into the underlying protective mechanisms. Moreover, large-scale field trials are essential to evaluate the fertility outcomes of AI using trehalose-supplemented semen, ensuring that laboratory findings translate into improved reproductive performance in livestock breeding programs. Numerous studies across various animal species have confirmed the beneficial effects of trehalose supplementation on sperm preservation under both chilled and frozen conditions. In rams, supplementation with 50–100 mM trehalose significantly improved post-thaw motility, viability, and plasma membrane integrity, while concentrations exceeding 200 mM were associated with decreased sperm quality (Jia et al., 2024). Similarly, in goats, trehalose concentrations between 100 and 150 mM enhanced sperm viability and membrane stability during cryopreservation (Jia et al., 2024). In stallions, trehalose supplementation improved post-thaw sperm quality, notably preserving motility and membrane integrity during the freezing and thawing processes (Jhamb et al., 2021). In rabbits, the addition of trehalose during freezing led to better preservation of sperm motility and acrosomal integrity (Zhu et al., 2017). For human sperm, trehalose supplementation significantly enhanced post-thaw motility, reduced ROS production, and minimized DNA fragmentation (Gholami et al., 2023). In addition to mammals, studies on marine species such as Crassostrea angulata have demonstrated that trehalose supplementation at concentrations of 200–300 mM effectively protects sperm during cryopreservation by reducing oxidative stress and preserving plasma membrane integrity (Anjos et al., 2021). Collectively, these findings indicate that trehalose exerts broad-spectrum cryoprotective effects across a wide range of taxa.
Fig. 2. Linear regression analysis of sperm quality decline in Pesisir bull semen during liquid storage at 5°C. The graphs display the decline in motility, viability, plasma membrane integrity, and acrosome integrity over time (0, 24, 48, and 72 hours) in semen samples supplemented with 0, 15, 30, and 45 mM trehalose. Trehalose supplementation has been more extensively investigated under ultralow freezing conditions (−196°C) than under chilled storage at 5°C. In rams and goats, the addition of trehalose to semen extenders markedly reduced cryoinjury associated with intracellular ice formation and osmotic stress during freezing (Güngör et al., 2017). The results of the present study, which demonstrated the protective effects of 30 mM trehalose during chilled semen storage at 5°C, are consistent with previous findings highlighting the efficacy of trehalose across various preservation methods. In conclusion, trehalose exhibits robust protective effects on spermatozoa across multiple animal species, concentration ranges, and preservation strategies. The findings of this study further support the use of trehalose supplementation in semen extenders to improve sperm quality during liquid storage, particularly for indigenous cattle breeds such as Pesisir bulls. Table 3. Linear regression equations and coefficients of determination (R²) for sperm quality parameters of Pesisir bull semen supplemented with various concentrations of trehalose during 72 hours of storage.
ConclusionThis study confirmed that supplementing Tris–egg yolk extender with 30 mM trehalose effectively preserved the quality of Pesisir bull semen during liquid storage at 5 °C. Compared with other concentrations, 30 mM trehalose maintained higher levels of sperm motility, viability, plasma membrane integrity, and acrosome integrity. These benefits are attributed to trehalose’s membrane-stabilizing and antioxidant properties, which mitigate oxidative stress and structural damage. In contrast, higher concentrations (e.g., 45 mM) did not confer additional benefits, likely due to osmotic stress. These findings underscore the practical applications of trehalose in AI programs and genetic conservation efforts, particularly for indigenous cattle breeds where optimized semen storage is critical for breeding efficiency. Future research should investigate the long-term effects of trehalose beyond 72 hours, explore synergistic effects with other antioxidants, and assess fertility outcomes through field trials to further refine semen preservation strategies and improve AI success rates. AcknowledgmentsThe authors express their sincere gratitude to Dr. Azhar for his invaluable assistance and guidance in the experimental design and data analysis, which significantly contributed to the successful completion of this study. FundingThe authors express their sincere gratitude to the Institute for Research and Community Service (LPPM), Universitas Andalas, for funding this research under Grant Number T/103/UN.16.17/PT.01.03/Pangan-RPT/2022. Author’s contributionsJ contributed to the conceptualization and supervision of the research. M was responsible for methodology development, data collection, and laboratory analysis. A conducted data analysis, manuscript writing, and interpretation of the results. All authors reviewed and edited the manuscript and approved the final version for submission. Conflict of interestThe authors declare no conflict of interest. Data availabilityAll data are available in the manuscript. ReferencesAfriani, T., Pela, Y.M., Jaswandi, J., Roza, E., Rastosari, A. and Farhana, A. 2022. Addition of trehalose of duck egg yolk-tris as an extender medium on buffalo frozen semen. In Proceedings of the International Conference on Improving Tropical Animal Production for Food Security (ITAPS 2021), Advances in Biological Sciences Research, Vol. 20, pp: 23–27. Atlantis Press International B.V; doi:10.2991/absr.k.220309.005. Ananda, Gusdinal, H., Ramadhan, R., Abimanyu, A.A., Ningsih, W.H. and Jaswandi. 2025. Impact of cryopreservation on semen quality and sperm protein profiles of Pesisir bulls. Trop. Anim. Sci. J. 48(3), 189–198; doi:10.5398/tasj.2025.48.3.189. Anjos, C., Santos, A., Duarte, D., Matias, D. and Cabrita, E. 2021. Effect of trehalose and sucrose in post-thaw quality of Crassostrea angulata sperm. Front. Physiol. 12, 749735; doi:10.3389/fphys.2021.749735. Athurupana, R., Takahashi, D., Ioki, S. and Funahashi, H. 2015. Trehalose in glycerol-free freezing extender enhances post-thaw survival of boar spermatozoa. J. Reprod. Dev. 61(3), 205–210; doi:10.1262/jrd.2014-152. Borah, B., Deka, B., Biswas, R., Chakravarty, P., Sinha, S., Ahmed, K., Deori, S. and Rahman, S. 2021. Antioxidants improve the semen quality following cryopreservation in Indian yak bulls. Indian J. Anim. Sci. 91(8), 115918; doi:10.56093/ijans.v91i8.115918. Bucak, M., Akalin, P., Keskin, N., Bodu, M., Öztürk, A., İli, P., Başpınar, N., Ataman, M. and Dursun, Ş. 2021. Combination of fetuin and trehalose in presence of low glycerol has beneficial effects on freeze-thawed ram spermatozoa. Andrology, 9(3), 1000–1009; doi:10.1111/andr.12974. Gholami, D., Sharafi, M., Esmaeili, V., Nadri, T., Alaei, L., Riazi, G. and Shahverdi, A. 2023. Beneficial effects of trehalose and gentiobiose on human sperm cryopreservation. PLoS One 18(4), e0271210; doi:10.1371/journal.pone.0271210. Güngör, Ş., Ata, A. and İnanç, M. 2018. Effects of trehalose and catalase on the viability and kinetic parameters of cryopreserved ram sperm. Acta Sci. Vet. 46(1), 7; doi:10.22456/1679-9216.83865. Güngör, Ş., Ata, A., İnanç, M. and Kastelic, J. 2019. Effect of various antioxidants and their combinations on bull semen cryopreservation. Turk. J. Vet. Anim. Sci. 43(5), 590–595; doi:10.3906/vet-1907-39. Güngör, Ş., Öztürk, C. and Omur, A.D. 2017. Positive effects of trehalose and cysteine on ram sperm parameters. Vet. Med. 62(5), 245–252; doi:10.17221/131/2016-VETMED. Hendri, Jaswandi, Indriastuti, R. and Ananda. 2024. Sperm kinematics of Pesisir bull thawed at different temperatures and times. Bul. Peternak. 48(4), 233–241; doi:10.21059/buletinpeternak.v48i4.96459. Hu, H., Liu, R., Shi, X., Ji, G., Zhang, J., Zhang, H., Li, M. and Wang, Z. 2019. Comparison of rapid freezing and vitrification for human sperm cryopreservation using trehalose as a cryoprotective agent. Open J. Obstet. Gynecol. 9(10), 1407–1418; doi:10.4236/ojog.2019.910136. Iqbal, S., Naz, S., Ahmed, H. and Andrabi, S. 2017. Cryoprotectant effect of trehalose in extender on post-thaw quality and in vivo fertility of water buffalo (Bubalus bubalis) bull spermatozoa. Andrologia, 50(1), e12794; doi:10.1111/and.12794. Jhamb, D., Sharma, S., Talluri, T.R., Nirwan, S.S., Juneja, R., Kumar, V., Tanwar, A., Pargi, K., Deepak, D., Nandan, D., Kumar, P., Gaur, M. and Gautam, L.K. 2021. Effect of trehalose supplementation to semen extender on quality of cryopreserved stallion semen. Int. J. Curr. Microbiol. App. Sci. 10(1), 1342–1350; doi:10.20546/ijcmas.2021.1001.160. Jia, B., Allai, L., Li, C., Liang, J., Lv, C., Wu, G. and Quan, G. 2024. A review on the functional roles of trehalose during cryopreservation of small ruminant semen. Front. Vet. Sci. 11, 1467242; doi:10.3389/fvets.2024.1467242. Kamal, M., Alam, M., Das, S., Yeasmin, M., Ahmed, S., Rahman, M. and Masum, M. 2023. Effects of glucose and trehalose on tris-citric acid-egg yolk-fructose diluents for semen cryopreservation in goat. J. Adv. Vet. Anim. Res. 10(2), 169; doi:10.5455/javar.2023.j666. Karunakaran, M., Konyak, P., Mandal, A., Mondal, M., Bhakat, C., Bhakat, S. and Behera, R. 2018. Effect of trehalose—an impermeant cryoprotectant on cryopreservation of Black Bengal buck semen. Indian J. Anim. Res; doi:10.18805/ijar.b-3470. Kumar, R. and Atreja, S. 2011. Effect of incorporation of additives in tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm tyrosine phosphorylation during cryopreservation. Reprod. Domest. Anim. 47(3), 485–490; doi:10.1111/j.1439-0531.2011.01908.x. Kumar, R., Singh, V., Chhillar, S. and Atreja, S. 2012. Effect of supplementation of taurine or trehalose in extender on immunolocalization of tyrosine phosphoproteins in buffalo and cattle (Karan Fries) cryopreserved spermatozoa. Reprod. Domest. Anim. 48(3), 407–415; doi:10.1111/rda.12088. Larasati, M., Lestari, S., Pangestu, M., Hestiantoro, A. and Kusmardi, K. 2024. The effect of cryopreservation on the sperm ultrastructure of Mus musculus albinus strain ddy: comparison of Nakagata vs Modified vs Kitazato cryoprotectants. Pharmacogn. J. 16(3), 563–569; doi:10.5530/pj.2024.16.88. Masrizal, Afriani, T., Wahyudi, D., Saharani, S. and Ananda. 2024. The quality of frozen buffalo sperm following sexing using bovine serum albumin (BSA) column and swim-up (SU) methods. Bul. Peternak. 48(1), 55–63; doi:10.21059/buletinpeternak.v48i1.89394. Masrizal, Zaituni, U., Hendri, Afriani, T., Jaswandi. and Ananda. 2025. Optimizing equilibration time for enhanced post-thaw quality of pesisir bull frozen semen. Int. J. Vet. Sci. 14(2), 281–288; doi:10.47278/journal.ijvs/2024.239. Mundana, M., Afriani, T., Yurnalis, Rastosari, A., Oktavianti, F., Razzak, M.C., Asyraf, M. and Merdana, I.M. 2023. Molecular verification of spermatozoa sexing method in Pesisir cattle bull using bovine serum albumin column. Int. J. Vet. Sci. 13(1), 7–12; doi:10.47278/journal.ijvs/2023.074. Olaciregui, M. and Gil, L. 2016. Freeze-dried spermatozoa: a future tool?. Reprod. Domest. Anim. 52(S2), 248–254; doi:10.1111/rda.12838. Öztürk, C., Güngör, Ş., Ataman, M.B., Bucak, M.N., Başpınar, N., İli, P. and İnanç, M.E. 2017. Effects of arginine and trehalose on post-thawed bovine sperm quality. Acta Vet. Hung. 65(3), 429–439; doi:10.1556/004.2017.040. Rajkhowa, C. 2015. Effect of addition of trehalose on the liquid storage (5°C) of mithun (Bos frontalis) semen. Indian J. Anim. Res. 49(6), 7048; doi:10.18805/ijar.7048. Syahminan, A., Jaswandi. and Afriani, T. 2023. Penambahan trehalose dalam pengencer tris kuning telur terhadap kualitas semen sapi Pesisir. Wahana Peternak. 7(2), 183–192; doi:10.37090/jwputb.v7i2.1014. Teethol, A.N., Ciptadi, G., Wahjuningsih, S. and Susilawati, T. 2022. Deterioration of frozen semen of Bali cattle after cooling at 5°C. World Vet. J. 12(4), 395–404; doi:10.54203/scil.2022.wvj50. Wahyudi, D., Udin, Z. and Afriani, T. 2023. Analysis of motility characteristic of Pesisir bulls sexed semen with different pre-freezing methods based on computer-assisted sperm analyzer (CASA). Bul. Peternak. 47(3), 136; doi:10.21059/buletinpeternak.v47i3.84331. Wakayama, S., Ito, D., Kamada, Y., Yonemura, S., Ooga, M., Kishigami, S. and Wakayama, T. 2019. Tolerance of the freeze-dried mouse sperm nucleus to temperatures ranging from -196 °C to 150 °C. Sci. Rep. 9(1), 5719; doi:10.1038/s41598-019-42062-8. Xi, M., Li, P., Du, H., Qiao, X., Liu, Z. and Wei, Q. 2018. Disaccharide combinations and the expression of enolase3 and plasma membrane Ca²+ ATPase isoform in sturgeon sperm cryopreservation. Reprod. Domest. Anim. 53(2), 472–483; doi:10.1111/rda.13134. Zhao, J., Xiao, G., Zhu, W., Fang, D., Li, N., Han, C., Gao, Q. and Zhang, X. 2020. Trehalose addition to a tris-fructose egg yolk extender on quality of ram sperm preserved at 0°C. Rev. Bras. Zootec. 49, 61; doi:10.37496/rbz4920200061. Zhu, Z., Fan, X., Pan, Y., Lu, Y. and Zeng, W. 2017. Trehalose improves rabbit sperm quality during cryopreservation. Cryobiology 75, 45–51; doi:10.1016/j.cryobiol.2017.02.006. | ||
| How to Cite this Article |
| Pubmed Style Jaswandi J, Masrizal M, Ananda A. Effects of trehalose supplementation on Pesisir bull sperm quality during storage. Open Vet. J.. 2025; 15(7): 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 Web Style Jaswandi J, Masrizal M, Ananda A. Effects of trehalose supplementation on Pesisir bull sperm quality during storage. https://www.openveterinaryjournal.com/?mno=245712 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.23 AMA (American Medical Association) Style Jaswandi J, Masrizal M, Ananda A. Effects of trehalose supplementation on Pesisir bull sperm quality during storage. Open Vet. J.. 2025; 15(7): 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 Vancouver/ICMJE Style Jaswandi J, Masrizal M, Ananda A. Effects of trehalose supplementation on Pesisir bull sperm quality during storage. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 Harvard Style Jaswandi, J., Masrizal, . M. & Ananda, . A. (2025) Effects of trehalose supplementation on Pesisir bull sperm quality during storage. Open Vet. J., 15 (7), 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 Turabian Style Jaswandi, Jaswandi, Masrizal Masrizal, and Ananda Ananda. 2025. Effects of trehalose supplementation on Pesisir bull sperm quality during storage. Open Veterinary Journal, 15 (7), 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 Chicago Style Jaswandi, Jaswandi, Masrizal Masrizal, and Ananda Ananda. "Effects of trehalose supplementation on Pesisir bull sperm quality during storage." Open Veterinary Journal 15 (2025), 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 MLA (The Modern Language Association) Style Jaswandi, Jaswandi, Masrizal Masrizal, and Ananda Ananda. "Effects of trehalose supplementation on Pesisir bull sperm quality during storage." Open Veterinary Journal 15.7 (2025), 3115-3123. Print. doi:10.5455/OVJ.2025.v15.i7.23 APA (American Psychological Association) Style Jaswandi, J., Masrizal, . M. & Ananda, . A. (2025) Effects of trehalose supplementation on Pesisir bull sperm quality during storage. Open Veterinary Journal, 15 (7), 3115-3123. doi:10.5455/OVJ.2025.v15.i7.23 |